Abstract
The DJ-1 protein, known as an oxidative stress sensor, plays an important role in immunological processes. Using DJ-1 gene knock-out mice, we identified DJ-1 as a positive regulator in systemic lupus erythematosus (SLE). DJ-1 deficiency significantly alleviated skin lesion, splenomegaly, lymphadenopathy, while reducing multiple autoantibodies and immunoglobulin levels. Moreover, DJ-1 deficiency provided protection against renal hypertrophy and decreased glomerular deposition of immunoglobulin, ultimately leading to reduced albuminuria and SLE activity. These findings demonstrate that DJ-1 expression critically influences the progression of lupus-like disease.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by a global loss of immune tolerance, involving the activation of both innate and adaptive immune systems1,2,3. Key features include the accumulation of uncleared cellular debris from various forms of cell death, elevated type I interferon signaling, and increased autoantibody production4. While conventional treatments still have limitations for some SLE patients, innovative approaches such as CAR-T cell therapy and bispecific antibodies have demonstrated the potential to achieve deep remission or even functional cure, reshaping the therapeutic landscape for SLE5,6,7,8. However, a subset of patients still struggle to attain fundamental disease modification9. Recent studies have highlighted autophagy as a critical area of interest, with growing evidence suggesting its pivotal role in the pathogenesis and severity of SLE10,11,12.
DJ-1, initially identified as the protein encoded by the Park7 gene, was first linked to familial Parkinson’s disease when mutations in this gene were discovered13,14. Park7 mutations are the rarest known cause of autosomal recessive PD15. DJ-1 is a ubiquitously expressed multifunctional protein that plays pivotal roles in diverse biological processes. DJ-1-deficient bone marrow-derived cells significantly attenuated atherosclerotic lesions, with macrophage DJ-1 playing a pivotal role in this protective effect16. In recent years, emerging evidence has established DJ-1 as a critical regulator of autophagy, primarily through its modulation of oxidative stress responses and other molecular mechanisms17,18. Imberechts et al. reported that DJ-1 is an essential downstream mediator in PINK1/parkin-dependent mitophagy14. In particular, DJ-1-regulated autophagy is involved in Parkinson’s disease14,19, and plays a key role in alleviating allergic diseases, infectious diseases, and lung injuries20,21,22. This breakthrough positions DJ-1 as a promising therapeutic target for autophagy modulation, offering novel strategies for disease intervention. Therefore, in this study, we investigate the role of DJ-1 in SLE through experiments.
Method AND materials
Animals
Female C57BL/6 mice were used in this study. All animal procedures were approved by the Institutional Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine. All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines. Wild-type (WT) mice were obtained from SLAC Laboratory Animal Co., Ltd. (Shanghai, China), and global DJ-1 knock-out (Park7−/−) mice (The Jackson Laboratory, Bar Harbor, ME, stock number 006577, C57BL/6 background) were kindly provided by Dr. Xiaoni Kong. The mice were bred and housed with food and water. They were maintained under a 12-hour light/dark cycle.
Pristane-induced model of SLE
Eight-week-old female mice received a single intraperitoneal injection of 0.5 mL pristane oil (2,6,10,14-tetramethylpentadecane; Sigma-Aldrich). Control mice were administered 0.5 mL saline. Monthly morning urine samples were collected throughout the study. Twenty weeks post-injection, mice were euthanized via CO2 asphyxiation. Prior to serum collection, mice were anesthetized using isoflurane inhalation. Both kidneys were harvested, with one preserved for histological analysis and the other for immunofluorescence staining. Additionally, spleens and lymph nodes were collected for further examination.
Enzyme-linked immunosorbent assay (ELISA)
Mouse serum samples were diluted 1:100 in assay buffer prior to analysis. Complement 3 (C3) levels were quantified using a commercial ELISA kit (6270, Alpha Diagnostic Intl. Inc.) according to the manufacturer’s instructions. Urinary protein concentrations were determined using a BCA Protein Assay Kit (23225, Thermo Scientific). Serum immunoglobulin G (IgG) (E88-104, Bethyl Laboratories), anti-nuclear antibody (ANA) (E88-104, Assay Genie), Anti-ds DNA (G-AEFI01643.96, Assay Genie), and IFNα levels (E-EL-M3054.96, Elabscience) were measured using respective ELISA kits.
Histological staining and immunofluorescence staining
Kidney and spleen tissues were harvested from mice and fixed in 4% paraformaldehyde. Following fixation, tissues were paraffin-embedded and stained with either hematoxylin-eosin (HE) or periodic acid-Schiff (PAS). The contralateral kidney from each mouse was snap-frozen in O.C.T. compound (Tissue-Tek) at −80 °C for immune complex deposition analysis. For immunofluorescence staining, 6-µm cryosections of kidney tissue were blocked for 1 h using a buffer containing 100 mM Tris-HCl (pH 8.0), 0.3% Triton X-100, 2% BSA, and 50 µg/ml goat non-specific IgG. Sections were then incubated with the following fluorescent conjugates: anti-mouse IgG Alexa Fluor 594, anti-mouse IgM Alexa Fluor 647, or anti-C1q antibody followed by anti-rabbit IgG Alexa Fluor 488. After staining, coverslips were mounted using an anti-fade mounting medium containing DAPI. All samples were imaged using a Nikon A1 HD25/A1R HD25 confocal laser scanning microscope (Nikon, Japan). Image processing and optimization were performed using Adobe Photoshop CC 2017 and Illustrator CC 2017 software.
RNA-seq analysis
Total RNA from fresh tissues (spleen and kidney) was extracted using TRIzol reagent and subjected to RNA-seq analysis. RNA sequencing was performed by the Novogene Experimental Department using the Illumina HiSeq 4000 platform. The raw reads in FASTQ format were first processed through in-house Perl scripts, and all downstream analyses were based on high-quality clean data. The reference genome index was built using Bowtie2 v2.2.8, and paired-end clean reads were aligned to the reference genome using HISAT2 v2.0.4. The mapped reads of each sample were assembled using StringTie (v1.3.1). Cuffdiff (v2.1.1) was employed to calculate FPKMs (Fragments Per Kilobase of transcript per Million mapped reads) of coding genes in each sample and provided statistical routines for determining differential expression based on a negative binomial distribution model. Transcripts with an adjusted P-value < 0.05 were identified as differentially expressed.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Normality of distribution was assessed using Kolmogorov-Smirnov and Shapiro-Wilk tests (P > 0.05 considered normally distributed). Normally distributed continuous variables were analyzed using Student’s t-test. For data meeting assumptions of both normality and homogeneity of variance, one-way analysis of variance (ANOVA) and multiple testing correction were performed. All statistical analyses were conducted using SPSS version 22.0 (IBM Corp., Chicago, IL, USA).
Results
DJ-1 deficiency alleviates skin lesions, splenomegaly, and lymphadenopathy in lupus-prone mice
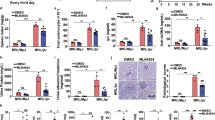
The WT-pristane group exhibited significantly more severe skin lesions compared to the WT-PBS group, and these changes were attenuated by DJ-1 knock-out (KO) (Fig. 1A). Similarly, the WT-pristane group showed increased spleen length and weight relative to the WT-PBS group, both of which were reduced by DJ-1 KO (Fig. 1B and D). Lymph node enlargement was also significantly greater in the WT-pristane group compared to the WT-PBS group, and this effect was similarly mitigated by DJ-1 KO (Fig. 1E and F). Importantly, at the treatment endpoint, no significant differences in skin lesions, splenomegaly, or lymphadenopathy were observed between the WT-PBS group and DJ-1-KO-PBS group (Fig. 1A and F).
DJ-1 deficient alleviates skin lesion, splenomegaly, lymphadenopathy. (A) skin lesion, (B) spleen, and (C)lymph node from DJ-1-KO and wild-type (WT) mice with or without pristane induction. (D, E) Length and weight of spleens. (F) Length of axillary lymph nodes. n = 7. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns p > 0.05.
DJ-1 deficiency alleviates autoantibodies and proteinuria in lupus-prone mice
As shown in Fig. 2, pristane-induced lupus mice exhibited decreased serum C3 levels along with elevated serum levels of IFNα, anti-ds DNA, ANAs, and IgG, and increased proteinuria. Notably, DJ-1 KO in pristane-induced lupus mice significantly reduced proteinuria and serum levels of FNα, anti-ds DNA, ANAs, and IgG (Fig. 2A, C, E and F). Furthermore, DJ-1 KO restored serum C3 levels in these mice (Fig. 2D).
DJ-1 deficient alleviates autoantibodies and proteinuria. ELISA assessment of (A) serum IFNα, (B) serum anti-ds DNA, (C) serum ANA, (D) serum complement 3, (E) and serum immunoglobulin G in different groups. (F) Bicinchoninic acid (BCA) assay of urine proteins. n = 7. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns p > 0.05.
DJ-1 deficiency alleviates renal hypertrophy and glomerular deposition of immunoglobulin in lupus-prone mice
Histopathological evaluation of pristane-induced lupus mice demonstrated significant renal pathology. HE staining revealed glomerular hypercellularity featuring prominent leukocyte infiltration, thickened capillary walls (Fig. 3A), while PAS staining identified mesangial matrix expansion, irregular glomerular basement membrane thickening, and cortical abnormalities including extensive tubular atrophy in dilated tubules accompanied by interstitial inflammation (Fig. 3B). Strikingly, these pathological changes were restored by the inhibition of DJ-1 (Fig. 3A and B). Immunofluorescence analysis further confirmed robust IgG/IgM immune complex deposition along capillary walls with strong C1q positivity in these mice (Fig. 4A and D). Similarly, DJ-1 inhibition ameliorated these pathological features (Fig. 4A and D).
DJ-1 deficient alleviates glomerular deposition of IgG, IgM and C1q. (A) Representative immunofluorescent images of glomeruli stained with C1q (red), IgM (yellow), IgG (green), and DAPI (blue). Quantitative analysis of mean fluorescence intensity (MFI) for IgM (B), IgG (C), and C1q (D). The scale bar represents 50 μm. n = 5. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns p > 0.05.
DJ-1 deficiency alleviates splenomegaly and its lymphoid hyperplasia in lupus-prone mice
Pristane-induced lupus mice exhibited significant pathological alterations characterized by marked splenomegaly resulting from combined lymphoid hyperplasia and vascular congestion, along with expanded pulp featuring prominent germinal centers, periarteriolar lymphoid sheath (PALS) proliferation. Histological examination revealed disrupted splenic follicular organization with substantial lymphocytes expansion (Fig. 5). Notably, these pathological changes were ameliorated by the inhibition of DJ-1 (Fig. 5).
Exploring the potential mechanisms of DJ-1 in lupus
Through comprehensive RNA sequencing analysis of kidney and spleen tissues, we identified multiple potential targets associated with DJ-1’s regulatory role in SLE pathogenesis (Supplementary Fig. 1A-1D, Supplementary Fig. 2A-2D). Notably, our transcriptomic data revealed that TRIM10 expression in kidney tissue may represent a key downstream effector mediating DJ-1’s involvement in SLE development.
Discussion
In this study, we investigated the role of DJ-1 in SLE pathogenesis and demonstrated that DJ-1 inhibition protects against lupus onset and disease severity. DJ-1, a highly conserved antioxidant protein originally identified through mutations causing familial early-onset Parkinson’s disease15, performs multiple functions including transcriptional regulation, protein stabilization, and antioxidative defense23,24. Our findings reveal that DJ-1 deficiency exerts protective effects against lupus-induced tissue injury despite the altered immune status and multi-organ damage characteristic of SLE. Specifically, DJ-1-deficient mice showed significant resistance to pristane-induced SLE, as evidenced by reduced autoantibody production, attenuated skin lesions, diminished splenomegaly and lymphadenopathy, decreased proteinuria, and lower glomerular deposition of IgG, IgM and C1q compared to WT controls.
Recent years have witnessed significant advances in SLE research, with numerous high-quality findings emerging. Notably, Li et al.3 redirected SLE mechanistic investigations from traditional adaptive immunity to innate immune system involvement, while Caielli et al.25 identified erythrocytes as potential key players in lupus pathogenesis. In Li et al.’s study, autoantibodies and interferon-α present in the serum induce neutrophil ferroptosis through enhanced binding of the transcriptional repressor CREMα to the glutathione peroxidase 4 promoter, which leads to suppressed expression of Gpx4 and subsequent elevation of lipid-reactive oxygen species3. In Caielli et al.’s study, during human erythroid cell maturation, a hypoxia-inducible factor-mediated metabolic switch is responsible for the activation of the ubiquitin-proteasome system, which precedes and is necessary for the autophagic removal of mitochondria. A defect in this pathway leads to accumulation of red blood cells carrying mitochondria in SLE patients and in correlation with disease activity25. Dijkstra et al.26 demonstrated that phagocytic state alterations critically influence SLE pathogenesis, showing that anti-C1q autoantibodies specifically bind solid-phase C1q to enhance phagocytosis without activating complement. Previous studies have further highlighted oxidative stress’s crucial role in SLE progression27. These studies have collectively enriched our multidimensional understanding of SLE complexity3,25,28. Building on this foundation, our study investigates potential SLE therapeutic targets through the dual perspectives of phagocytosis and oxidative stress, we selected DJ-1 as our research focus through preliminary screening.
To our knowledge, this study represents the first investigation of DJ-1’s systemic in vivo role in an SLE mouse model. However, DJ-1’s functions in immune disorders have been explored in other contexts22,29,30. For example, in rheumatoid arthritis, DJ-1 exhibits therapeutic potential by modulating the Th17/Treg balance, suppressing proinflammatory cytokine production, inhibiting fibroblast-like synoviocyte activation, and impairing osteoclast differentiation30. Additionally, DJ-1 deficiency or dysregulated activity may promote mast cell–mediated allergic disease pathogenesis22. Together, these findings underscore DJ-1’s complex, multifaceted roles across immune-related disease models. In this study, the pivotal role of DJ-1 in SLE further substantiates that phagocytosis/oxidative stress may constitute a determinant factor in SLE pathogenesis. This discovery transcends previous understanding of SLE, shifting the paradigm from a singular cellular focus to a holistic comprehension of disease mechanisms.
Our investigation of DJ-1’s role in SLE pathogenesis has yielded novel mechanistic insights through next-generation transcriptome sequencing of renal and splenic tissues. We identified tripartite motif-containing protein 10 (TRIM10) as a potentially critical mediator of DJ-1’s effects in SLE, supported by both sequencing data and existing literature evidence. While studies suggest that TRIM family-mediated acetylation-ubiquitination crosstalk may promote DJ-1 degradation31, the precise molecular mechanisms require further investigation. As an E3 ubiquitin ligase within the TRIM family, TRIM10 regulates innate immunity, inflammatory responses, and autoimmune disorders through multiple pathways32,33. In SLE specifically, TRIM10 appears to modulate disease progression by regulating type I interferon signaling, mediating inflammasome activation, and influencing autoantigen clearance34. Clinical observations show significantly reduced serum TRIM10 levels in SLE patients versus healthy controls, consistent with its role as a negative regulator of JAK/STAT signaling. Mechanistically, TRIM10 binds the intracellular domain of IFNAR1, disrupting its interaction with TYK2 and thereby attenuating IFN/JAK/STAT pathway activation34. Although preliminary evidence indicates potential TRIM-mediated regulation of DJ-1 stability, the exact nature of DJ-1-TRIM10 interactions in SLE pathogenesis demands further exploration. These findings establish TRIM10 as both a key molecular target for understanding DJ-1’s role in SLE and a potential therapeutic candidate for autoimmune disease intervention.
While this study elucidates DJ-1’s role in SLE, several limitations must be addressed. There are significant differences in environmental triggers between pristane-induced SLE and human SLE. The former is primarily induced by pristane through specific molecular mechanisms (e.g., the TLR7/MyD88 pathway) that drive interferon (IFN) signatures, while the latter is triggered by diverse environmental exposures (e.g., biochemical stimuli) and is currently recognized as resulting from multifactorial interactions. At the genetic level, the pristane model demonstrates relatively well-defined gene pathway dependencies (e.g., type I interferon receptor signaling), whereas human SLE exhibits complex polygenic involvement (encompassing multiple susceptibility genes including HLA, IRF5, and STAT4). Therefore, the absence of human SLE samples restricts the clinical translatability of our findings, underscoring the need for future validation in patient cohorts. Secondly, while we provide preliminary insights into DJ-1’s regulatory mechanisms, deeper validation of key molecular pathways—particularly its interplay with TRIM10 and downstream immune cascades—remains incomplete. Thirdly, the specific cell populations responsible for mediating DJ-1’s effects in SLE pathogenesis (e.g., myeloid vs. lymphoid lineages) remain unresolved, necessitating lineage-specific studies. Fourthly, the pristane-induced lupus model does not fully recapitulate the pathogenesis of SLE, and this study was unable to conduct further validation using other established models such as MRL/lpr or Sle1/Sle2/Sle3 mice. Together, these gaps highlight critical avenues for future research to fully exploit DJ-1’s therapeutic potential in SLE.
Conclusion
Inhibition of DJ-1 demonstrates efficacy in ameliorating lupus phenotypes. Further exploration of its underlying mechanisms may provide novel therapeutic targets and a theoretical foundation for lupus treatment.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation. The datasets generated and analysed during the current study are available in the CNCB repository, CRA026290, https://bigd.big.ac.cn/gsa/browse/CRA026290.
References
Bentham, J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47 (12), 1457–1464 (2015).
Lazar, S. & Kahlenberg, J. M. Systemic lupus erythematosus: new diagnostic and therapeutic approaches. Annu. Rev. Med. 74, 339–352 (2023).
Li, P. et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 22 (9), 1107–1117 (2021).
Kato, Y. et al. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann. Rheum. Dis. 77 (10), 1507–1515 (2018).
Askanase, A. et al. New and future therapies: changes in the therapeutic armamentarium for SLE. Best Pract. Res. Clin. Rheumatol. 37 (4), 101865 (2023).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28 (10), 2124–2132 (2022).
Zhang, M. et al. Development of an ICOSL and BAFF bispecific inhibitor AMG 570 for systemic lupus erythematosus treatment. Clin. Exp. Rheumatol. 37 (6), 906–914 (2019).
Zhou, J. et al. CAR T-cell therapy for systemic lupus erythematosus: current status and future perspectives. Front. Immunol. 15, 1476859 (2024).
Jesus, D. et al. Derivation and validation of the SLE disease activity score (SLE-DAS): a new SLE continuous measure with high sensitivity for changes in disease activity. Ann. Rheum. Dis. 78 (3), 365–371 (2019).
Frangou, E. et al. REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann. Rheum. Dis. 78 (2), 238–248 (2019).
Qi, Y. Y. et al. Increased autophagy is cytoprotective against podocyte injury induced by antibody and interferon-α in lupus nephritis. Ann. Rheum. Dis. 77 (12), 1799–1809 (2018).
Tanaka, T. et al. LAMP3 inhibits autophagy and contributes to cell death by lysosomal membrane permeabilization. Autophagy 18 (7), 1629–1647 (2022).
Heremans, I. P. et al. Parkinson’s disease protein PARK7 prevents metabolite and protein damage caused by a glycolytic metabolite. Proc. Natl. Acad. Sci. U S A 119 (4), e2111338119 (2022).
Imberechts, D. et al. DJ-1 is an essential downstream mediator in PINK1/parkin-dependent mitophagy. Brain 145 (12), 4368–4384 (2022).
Bonifati, V. et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299 (5604), 256–259 (2003).
Sivasubramaniyam, T. et al. Dj1 deficiency protects against atherosclerosis with anti-inflammatory response in macrophages. Sci. Rep. 11 (1), 4723 (2021).
Janda, E., Isidoro, C., Carresi, C. & Mollace, V. Defective autophagy in parkinson’s disease: role of oxidative stress. Mol. Neurobiol. 46 (3), 639–661 (2012).
Zhao, P. et al. Atox1 protects hippocampal neurons after traumatic brain injury via DJ-1 mediated anti-oxidative stress and mitophagy. Redox Biol. 72, 103156 (2024).
Skou, L. D., Johansen, S. K., Okarmus, J. & Meyer, M. Pathogenesis of DJ-1/PARK7-mediated Parkinson’s disease. Cells 13 (4), 296 (2024).
Bahmed, K. et al. DJ-1 modulates nuclear erythroid 2-Related Factor-2-Mediated protection in human primary alveolar type II cells in smokers. Am. J. Respir Cell. Mol. Biol. 55 (3), 439–449 (2016).
Kim, D. K., Beaven, M. A., Metcalfe, D. D. & Olivera, A. Interaction of DJ-1 with Lyn is essential for IgE-mediated stimulation of human mast cells. J. Allergy Clin. Immunol. 142 (1), 195–206e8 (2018).
Kim, D. K. et al. DJ-1 regulates mast cell activation and IgE-mediated allergic responses. J. Allergy Clin. Immunol. 131 (6), 1653–1662 (2013).
Urano, Y. et al. 6-Hydroxydopamine induces secretion of PARK7/DJ-1 via autophagy-based unconventional secretory pathway. Autophagy 14 (11), 1943–1958 (2018).
Zhang, L. et al. Role of DJ-1 in immune and inflammatory diseases. Front. Immunol. 11, 994 (2020).
Caielli, S. et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 184 (17), 4464–4479e19 (2021).
Dijkstra, D. J. et al. Human anti-C1q autoantibodies bind specifically to solid-phase C1q and enhance phagocytosis but not complement activation. Proc. Natl. Acad. Sci. U S A. 120 (50), e2310666120 (2023).
Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 9 (11), 674–686 (2013).
Crow, M. K. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann. Rheum. Dis. 82 (8), 999–1014 (2023).
Lev, N. et al. Experimental encephalomyelitis induces changes in DJ-1: implications for oxidative stress in multiple sclerosis. Antioxid. Redox Signal. 8 (11–12), 1987–1995 (2006).
Min, H. K., Kim, S. H., Lee, J. Y., Lee, S. H. & Kim, H. R. DJ-1 controls T cell differentiation and osteoclastogenesis in rheumatoid arthritis. Sci. Rep. 12 (1), 12767 (2022).
Sun, Z. et al. Acetylation-ubiquitination crosstalk of DJ-1 mediates microcalcification formation in diabetic plaques via collagen-matrix vesicles interaction. Cardiovasc. Res. 121 (2), 296–310 (2024).
Blaybel, R., Théoleyre, O., Douablin, A. & Baklouti, F. Downregulation of the Spi-1/PU.1 oncogene induces the expression of TRIM10/HERF1, a key factor required for terminal erythroid cell differentiation and survival. Cell. Res. 18 (8), 834–845 (2008).
Kong, L. et al. The ubiquitin E3 ligase TRIM10 promotes STING aggregation and activation in the golgi apparatus. Cell. Rep. 42 (4), 112306 (2023).
Guo, M. et al. TRIM10 binds to IFN-α/β receptor 1 to negatively regulate type I IFN signal transduction. Eur. J. Immunol. 51 (7), 1762–1773 (2021).
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82201970, Awarded to: Mengdi Jiang; NO. 82400661, Awarded to: Shuangshuang Li), and the Key Program of the Zhejiang Provincial Natural Science Foundation of China (Grant No. LHDMZ23H310001; Awarded to: Heng Cao).
Author information
Authors and Affiliations
Contributions
M.J., M.W.,L.W., and S.L. conducted the experiments. M.J., H.C., C.S., and J.L. drafted the manuscript. All authors approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The animal study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, M., Wang, M., Wan, L. et al. Inhibition of DJ-1 protects from lupus onset and severity. Sci Rep 15, 31217 (2025). https://doi.org/10.1038/s41598-025-15670-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-15670-w