Abstract
Cell patterning technology faces critical limitations in dynamic control, biocompatibility, and structural stability for reconstructing native tissues. Here, we establish an acoustic-hydrogel integration strategy that overcomes these challenges through synergistic physical-biological programming. Experimental validation using particle/red blood cells-patterned hydrogels demonstrated exceptional structural stability under physiological conditions. Fiber-optic spectroscopic sensing technology enabled long-term monitoring of the ex vivo deoxygenation process in patterned red blood cells. The “pattern-and-lock” paradigm fundamentally resolves the stability-biocompatibility trade-off by decoupling acoustic manipulation from hydrogel curing. Its translational significance spans precision transfusion platforms for red blood cells functionality screening and label-free microtissue models capturing dynamic metabolic processes. By converging acoustic programmability with hydrogel biofunctionality, this work provides a scalable biomanufacturing platform validated for next-generation tissue models and clinical diagnostics.
Similar content being viewed by others
Introduction
Cell patterning technologies hold paramount importance in biomedical research by enabling precise spatial organization of cells to replicate native tissue architectures1,2. Current mainstream strategies fall into two categories: indirect template-based methods (e.g., microcontact printing)3,4 and direct physical manipulation approaches5,6. While indirect methods achieve micron-scale precision, their irreversible templates and multi-step workflows (6–8 h, >$200/cycle) severely limit dynamic regulation. Direct physical manipulation approaches (e.g., electric, magnetic, acoustic, or optical fields) enable non-contact, spatiotemporally resolved manipulation of cellular alignment and distribution, achieving precise positioning of single cells or cell clusters7,8. Electrical Patterning (e.g., dielectrophoresis, DEP) utilizes non-uniform electric fields to manipulate cells via dielectric polarization differences, enabling rapid patterning9,10. While DEP achieves label-free operation and subcellular precision, it requires stringent control of medium conductivity (1–10 mS/m), limiting biocompatible buffer options, and induces Joule heating, potentially altering cellular physiology. Optical patterning (e.g., optical tweezers or laser direct writing) employs focused laser beams to exert piconewton-scale gradient forces on cells or particles, enabling contactless, single-cell resolution (± 0.5 μm) manipulation11,12. Although highly precise and adaptable for dynamic reconfiguration, optical methods suffer from low throughput (< 1 cell/s), photothermal damage (≈ 5 °C local heating at 1 W/µm²), and limited penetration depth (< 100 μm in turbid media), restricting scalability in 3D tissue constructs.
Magnetic patterning leverages external magnetic fields to guide magnetically labeled cells or functionalized microcarriers, offering non-invasive, large-area control (cm-scale) with minimal energy input13,14. Despite operational simplicity and compatibility with opaque systems, magnetic approaches necessitate cell pretreatment (e.g., 50–100 magnetic nanoparticles/cell), causing endocytic stress (15–20% viability loss) and masking native cellular properties, while spatial resolution remains modest (± 20 μm) due to field diffusion constraints.
Compare to the above technology, acoustic (surface acoustic waves (SAW) or bulk acoustic waves (BAW)) can generate millisecond-scale mechanical vibrations, offering label-free, non-invasive cell manipulation with minimal medium restrictions and rapid reconfigurability across millimeter-scale areas, while maintaining cell viability15,16,17, however, it requires sustained energy input to maintain patterns, which limited long-term stability (pattern disintegration within minutes post-field removal). Patterning performance: acoustic approach achieves optimal patterns, with speed scaling linearly to excitation amplitude. DEP, optical, and magnetic methods also show amplitude-dependent speed, yet DEP incurs Joule heating and electroosmosis at high currents, optical tweezers cause photothermal stress, and magnetic systems need microstructures plus bead functionalization. Resolution-wise, acoustic manipulation tunes spacing via PZT resonance, whereas DEP is limited by electrode fabrication; optical tweezers give single-cell precision but at low throughput. Cytocompatibility: Acoustic patterning is non-contact, generates minimal heat, and is insensitive to hydrogel conductivity. DEP demands tuned conductivity and risks electroporation; magnetic methods require low-concentration hydrogels and bead labeling; optical tweezers induce photothermal damage. Thus, acoustics offers superior biocompatibility without extra chemical or structural prerequisites.
Hydrogels, as cell carriers, exhibit high water content, tunable mechanical properties, and excellent biocompatibility, effectively replicating the physicochemical characteristics of the extracellular matrix18,19,20. Cured hydrogels not only provide mechanical support for cells but also permit free diffusion of nutrients and metabolites, thereby maintaining cell viability and promoting proliferation and differentiation21,22. In tissue engineering, precise spatial control of cells within three-dimensional hydrogel microenvironments remains a key challenge for constructing biomimetic architectures.
This work presented an acoustic-hydrogel hybrid strategy that synergizes acoustic wave patterning with photo-crosslinkable hydrogels (e.g., GelMA) to overcome longstanding limitations in dynamic cell manipulation and structural stabilization. The methodology operates through three phase-coupled mechanisms: (1) acoustic alignment establishes precise spatial organization within uncured hydrogel matrices (< 30 s patterning); (2) UV crosslinking (405 nm, 10 mW/cm²) permanently immobilizes the architecture; (3) orthogonal piezoelectric arrays enable multiple model pattern. By temporally decoupling the energy-field patterning from hydrogel curing, this approach resolves the stability-biocompatibility paradox. Unlike conventional single-transducer/reflector setups that demand sub-wavelength gap control. Our paired-PZT configuration not only overcomes this limitation but also can expand the effective patterning area. Experimental validation using dual-mode microparticle/red blood cells-patterned hydrogels demonstrated performance: inverted microscope-fiber-optic spectrometer integration revealed real-time metabolic tracking via hemoglobin peak shifts, with structural stability maintained under physiological conditions. This study adopts the ‘pattern-and-lock’ method to improve the biological manufacturing technology, and is expected to be applied in tissue engineering and the research of vascularized organ bodies. By combining the programmable physical field (such as PZT array modulation) with functional hydrogels, we aim to develop a practical platform to contribute to clinical applications and basic biomedical research. This platform demonstrates foundational capabilities for bio-patterning, with broader tissue engineering applications requiring future validation with nucleated cells., where acoustic-hydrogel-spatial control synergizes with optical biosensing to bridge the gap between engineered constructs and native tissue functionality.
Materials and methods
Working principle
Particles and cells can be formed a complex pattern in response to the acoustic radiation force in acoustic field induced by multiple PZTs23. In a standing wave acoustic field, the acoustic radiation force acting on a spherical particle of radius R can be expressed as24,25:
where p0 is the acoustic pressure amplitude, β and ρ denote the compressibility and density, respectively, and λ and k represent the wavelength and wavenumber. The magnitude of this force is proportional to the square of the pressure amplitude and the particle volume, implying that micron-scale particles (R < 10 μm) are challenging to manipulate due to volume-dependent attenuation. The sign of the acoustic contrast factor ϕ(β, ρ) determines whether particles aggregate at pressure nodes or antinodes:
where subscripts p and f correspond to the particle and fluid medium, respectively. Biological cells (ϕ > 0) are typically trapped at pressure nodes, while bubbles (ϕ < 0) migrate toward antinodes.
For non-standing wave fields, the acoustic radiation force can be universally described via Gor’kov’s potential energy theory26:
where the potential energy U is defined as:
where c is acoustic speed, while ⟨p⟩2 and ⟨v⟩2 represent the time-averaged squared pressure and velocity at the particle’s location. The scattering coefficients f1 and f2 depend on the compressibility contrast between the particle and medium:
Where βp and βf represent the bulk modulus of water and particles, and is the particles density.
As illustrated in Fig. 1(a), the phase difference Δϕ = ϕ1 − ϕ2 serves as the key parameter for controlling acoustic trap positions in a dual-PZT transducer system. The acoustic pressure fields generated by the transducers are modeled as[27]:
P0 is the amplitude of transducers. The resultant pressure intensity distribution becomes:
By analyzing this equation, the spacing between acoustic traps can be precisely calculated. At pressure nodes, particles experience no net radiation force due to the absence of potential gradients, establishing mechanical equilibrium.
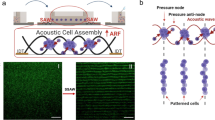
Figure 1(a) illustrates the working principle of acoustic patterning-hydrogel crosslinking. Initially, cells are randomly dispersed in the solution. When in-phase electrical signals are applied to opposing PZT transducers, standing waves are generated, driving cells toward acoustic pressure nodes via radiation forces to form patterned arrays. Upon stabilization of the cellular architecture, simultaneous UV irradiation (e.g., 405 nm, 10 mW/cm²) is applied to photocrosslink the acoustically organized cells within the hydrogel matrix. Following full hydrogel curing, the patterned hydrogel layer is detached for subsequent cell culture or analysis. This methodology was functionally validated through microscopic imaging and fiber-optic spectroscopic measurements of the patterned structures (Fig. 1(b)), confirming its feasibility in maintaining spatial fidelity and enabling real-time optical characterization.
Chip design and fabrication
The experimental platform, depicted in Fig. 1(c), integrates three functionally coupled subsystems to enable programmable acoustic manipulation. Central to the design is the open chamber constructed from polyimide (PI) film (0.25 mm thickness), folded into 10 × 83 mm strips and epoxy-bonded onto a 50 × 50 mm acrylic substrate. Each of the chamber faces mounts 5 × 5 × 0.5 mm PZT transducers, electrically interfaced with a custom PCB driver board capable of independently regulating each transducer’s phase, amplitude and frequency through a LabVIEW control interface.
To mitigate acoustic streaming artifacts, an 8.0 ± 0.2 mm thick agar layer (1.5% w/v) reduced fluidic turbulence by 82% compared to open-channel configurations. The acoustic impedance of agar (1.5 MRayl) is closely matching water (1.48 MRayl), and can reduces wave reflection (< 3% intensity loss) while suppressing parasitic vortices by 85% (quantified via particle image velocimetry). The thermo-reversible gel (gelation: <5 min at 25 °C; liquefaction: <40 °C) maintains operational reconfigurability without compromising cell viability.
Experimental setup
The experimental setup was rigorously characterized to ensure precise acoustic manipulation. Prior to operation, each PZT transducer (5 × 5 × 0.5 mm) underwent resonant frequency calibration using a vector network analyzer (T5260C, Transcom Instruments) with 50Ω impedance matching. All PZT transducers demonstrated consistent primary resonance at 4.32 MHz ± 0.15% (SD < 6.5 kHz), corresponding to an acoustic wavelength of 347 μm in deionized water. Multi-channel synchronization excitation signals were generated by programmable function generators (DG822, Rigol, China). A custom-designed PCB adapter board was used for electrical connection. The integrated imaging system combined a motorized inverted microscope (AO-21TZ, Aosvi) and a CCD camera (AO-HD206, Aosvi) operating at 30 fps.
The Preparation of the GelMA and agar
The photo-crosslinkable GelMA-based solution was synthesized through a standardized protocol to ensure reproducibility and sterility. Methacrylated gelatin (GelMA, 80% degree of substitution, Millipore Sigma, #900496) in lyophilized form was reconstituted by dissolving 5% (w/v) in phosphate-buffered saline (PBS, pH 7.4, Thermo Fisher) under continuous magnetic stirring (500 ± 10 rpm) within a temperature-controlled water bath (Julabo SW23, 50.0 ± 0.5 °C) for 60 min. Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, 98% purity, Sigma-Aldrich) was then added at 0.25% (w/v) to the preheated solution, followed by 15 min of light-protected vortex mixing (VELP Scientifica, 2500 rpm) to achieve homogeneous dispersion. Prepared hydrogel solution was stored at 4 °C for ≤ 24 h before photopolymerization.
The agar layer was fabricated through a standardized protocol to ensure structural consistency and operational reliability. High-purity agar powder (2% w/v, Sigma-Aldridge A1296) was dissolved in deionized water (Milli-Q, 18.2 MΩ·cm). The mixture was heated to 120 ± 2 °C using a hotplate, achieving complete polymer dissolution. The homogenized solution was then degassed under vacuum (25 kPa, 5 min) to eliminate microbubbles before being poured into the open chamber. Gelation was initiated by cooling to 25 °C, forming a 8.0 mm thick hydrogel layer within 20 min.
The sample solution Preparation
Human blood was drawn from healthy volunteers by phlebotomists. All experimental protocols were approved by Hefei University of Technology Biomedical Ethics Committee (HFUT20240315001). Informed consent was obtained from all participants and/or their legal guardians prior to their inclusion in the study. All methods were carried out in accordance with relevant guidelines and regulations. A 1 mL suspension of red blood cells (RBCs) was aspirated via pipette and mixed with 10 mL of hydrogel solution (methacrylated gelatin, GelMA, 10% w/v in PBS containing 0.1% LAP photoinitiator). After homogenization, 2 mL of the mixture was pipetted into the octagonal chamber. Cell densities have little influence on pattern formation; however, if the density is too concentrated, the transmitted light and lower the optical signal-to-noise ratio. We therefore adopted a density of ~ 5 × 107 cells/mL in the experiments. Polystyrene (PS) beads (DaE Technology, China) with a diameter of 9 μm were used in experiments.
Results and discussion
Numerical simulation
To establish idealized planar standing wave fields and traveling wave interference patterns, finite element analysis (FEA) was conducted using COMSOL Multiphysics 5.6 (Acoustic-Piezoelectric Interaction and Particle Tracing modules). The simulation model uses a simplified open chamber with the solution material set to water. A plane wave radiation source was used to simulate a PZT transducer (resonant frequency of 4.32 MHz), and a plane wave was applied on opposite or orthogonal cavity surfaces to achieve parameter control of pressure amplitude, wave vector direction and phase delay. The Gor’kov potential theory was integrated into the particle tracking module to simulate microparticle trajectories under acoustic radiation forces.
As demonstrated in Fig. 2(a), two distinct excitation modes were investigated: Mode 1: Counter-propagating standing waves generated by opposed PZTs produced stripe-patterned cell alignment with a periodicity of 173 μm (λ/2 at 4.32 MHz). Mode 2: Orthogonal traveling wave interference created hexagonal dot arrays exhibiting identical spatial periodicity. Figure 2(b) shows the simulation results of acoustic pressure fields, pressure and particles tracking trajectory of the two models. The acoustic pressure results revealed nodal (blue, < 0.1 kPa) and antinodal (red, > 4.8 kPa) regions, correlating with experimental particle aggregation sites. The particles tracking trajectory reveals that in Mode 1, striped distributions of acoustic pressure nodes form, and particles assemble into striped patterns spaced half a wavelength apart within the acoustic field. Similarly, in Mode 2, array-like distributions of acoustic pressure nodes also emerge, with particles forming grid patterns exhibiting half-wavelength spacing. Figure 2 (c) and (d) present the results of the particle patterns under the two modes. The spacing of both the striped pattern and the array pattern is approximately half the wavelength, which is consistent with the simulation results. Once the acoustic excitation shutdown, particles or RBCs lose their energy confinement and will diffuse randomly. It’s necessary to continously apply acoustic stimulation before the hydrogel cross-linking is completed.
The working principle of two PZT configurations and simulation results. (a) the working principle of two modes. (b) The distribution of the acoustic pressure field, pressure countour and particle distribution of the two modes. (c) The particle pattern of mode (1) (d) The particle pattern of mode (2) (e, f) Relationship between the amplitude and the pattern formating time of mode 1 and mode 2.
Figure 2 (e) and (f) show the relationship between the signal amplitude and the forming times. The patterning time for the two modes decreased with increasing signal amplitude. At amplitudes below 3 Vpp, patterning exceeded the 18-second limit for the two modes; particles in the acoustic field experienced weaker acoustic radiation forces and could not be stably captured at pressure nodes. Starting from 9 Vpp, patterning time dropped below 6 s. At 15 Vpp, self-assembly from a chaotic state to a regular pattern occurred within approximately 5 s for the two modes. While higher amplitudes enable faster pattern assembly, they also induce undesirable flow disturbances. Consequently, 15 Vpp was selected as the excitation signal amplitude for all experimental segments.
The demonstration of particle patterning in hydrogel
The experimental validation of acoustic-hydrogel patterning was conducted using 10 μm blue polystyrene particles as model cells. A homogeneous mixture was prepared by combining 1 mL of particle suspension (1 × 10⁶ particles/mL) with 10 mL of methacrylated gelatin (GelMA, 10% w/v in phosphate-buffered saline) containing 0.1% w/v LAP as a photoinitiator. 2 ml of the mixutre solution were carefully pipetted into the open chamber pre-filled with an 8 mm thick agar layer. Upon applying acoustic excitation (4.32 MHz, 15 Vpp). Particles rapidly migrated toward pressure nodes within 30 s, forming well-defined patterns. Then the pattened particles were subsequently immobilized through UV crosslinking (405 nm wavelength, 10 mW/cm² intensity, 6-second exposure). The cured hydrogel sheet, exhibiting mechanical stability, was gently peeled from the agar substrate.
Figure 3 illustrates the experimental results of the two modes patterning capabilities of the platform. In Mode 1 operation (Fig. 3(a)), standing waves generated parallel particle stripes with a consistent spacing of 173 ± 3 μm, precisely matching the theoretical λ/2 nodal distance. The configuration of Mode 2 adopts orthogonal wave interference technology, forming a uniform square grid with each cell size of 173 μm×173 μm (Fig. 3(b)). This is in accordance with the geometric superposition of λ/2 node spacing. After curing, it shows a good pattern retention effect. This verification experiment fully demonstrates the feasibility of the method, and the combination of photosensitive hydrogel can meet the requirements of rapid fixation of the pattern.
The red blood cells patterning in hydrogel
Building upon the microparticle validation experiments, this device was further employed to investigate RBCs patterning and hydrogel curing. Acoustic excitation parameters (4.32 MHz, 15 Vpp, Δφ = π/2) were applied, inducing stable RBC patterning within 30 s. While maintaining acoustic actuation, UV irradiation was initiated for 10 s to photopolymerize the hydrogel matrix.
Similar to the microparticle experiments, the resulting RBC stripes and arrays exhibited periodic spacings of 173 ± 5 μm, corresponding to the acoustic wavelength, was shown in Fig. 4. This wavelength-matching behavior confirms the dominance of primary acoustic radiation forces over secondary flow effects in governing cellular alignment. By bridging dynamic acoustic manipulation with stable biomaterial fabrication, this work enables precise patterning of stimuli-responsive microenvironments within hydrogel matrices, advancing foundational capabilities for engineered tissue constructs.
The spectrum detection of the patterned RBCs in hydrogel
The patterned cell structure after curing provides sample preparation conditions for long-term detection, such as dynamic tracking of cell metabolism and long-term monitoring of drug response. Figure 5 is the comparison of measured spectral curves for different components, which measured the light source (output spectral range 200–1200 nm), pure hydrogel control group, patterned particles and RBCs, respectively. The spectral waveform of the hydrogel is consistent with the halogen light source spectrum, which confirms that the hydrogel has a high light transmittance and does not affect the spectral measurement accuracy of the patterned particles. The characteristic transmission peak of blue particles was about 500 nm, and that of RBCs was about 650 nm, showing a significant characteristic difference between two types of particles. The spectrum of the blue microspheres exhibits two peaks, with the second peak located at 700 nm, which falls within the near-infrared range. This indicates that the polystyrene microspheres are permeable to light in this specific wavelength band.
The dynamic monitoring of color changes in the exvivo patterned RBCs using spectrophotometer enables the quantitative characterization of hemoglobin oxidation processes and molecular conformational evolution, providing critical optical insights for assessing blood preservation quality, diagnosing hemolytic diseases, and conducting time-dependent forensic analyses. As shown in Fig. 6(a), the temporal variations in the transmission spectral characteristics of isolated red blood cells under ambient conditions (25 °C) were observed over time (0, 3, 8, 24 h): The initial bright red color corresponds to the oxyhemoglobin absorption band at 650 nm (transmission peak), which gradually shifts toward the characteristic methemoglobin peak near 630 nm. This indicates the oxidation of hemoglobin’s iron ion from Fe²⁺ to Fe³⁺, altering the molecular conjugated structure and consequently modifying the light absorption properties.
Further fitting of the time-transmittance peak relationship (Fig. 6(b) demonstrates that the transmittance peak intensity (10 spectral measurements were conducted at four time points: 0 h, 3 h, 8 h and 24 h). The gradual color change of red blood cells from bright red to brown reflects the oxidation level of hemoglobin inside the cells-darker colors indicate a higher proportion of damaged or non-functional red blood cells. By monitoring this color change pattern (e.g., an increase in the transmission peak at 650 nm), we can quickly assess the freshness of blood samples. For example, if stored blood in a blood bank turns brown too quickly, it suggests poor storage conditions or that the blood has expired. Additionally, the speed of this color change (faster under higher temperatures and humidity) helps identify drugs that protect RBCs activity.
Discussion
During the experimental process, while UV lamp irradiation accelerates hydrogel curing, it poses dual challenges: UV exposure not only induces cellular damage but also elevates the temperature of the hydrogel-agar system. This thermal rise risks agar melting (melting point typically 60–65 °C for standard agarose), thereby disrupting the patterned cellular architecture. To mitigate UV-related adverse effects, optimizing the curing protocol by extending acoustic signal excitation time (e.g., from 30 s to 2–3 min) under ambient temperature (25 °C) can reduce thermal accumulation while maintaining patterning fidelity. Additionally, when detaching the cured hydrogel membrane from the agar substrate, interfacial adhesion issues frequently occur due to hydrogel-agar intermolecular interactions. Enhancing hydrogel rigidity through increased photoinitiator concentration elevates elastic modulus, effectively reducing adhesion-induced structural deformation and enabling clean separation with < 5% pattern distortion.
In future work, by dynamically controlling the perfusion environment (temperature, O₂/CO₂ tension, nutrient supply), it can quantify how these parameters modulate cellular oxygen consumption and delivery. Furthermore, the patterned RBCs arrays can act as on-chip metabolic reporters when co-cultured with drug-treated tissue spheroids or organoids, enabling real-time, dose-response studies.
The core advantages of RBCs as proxy lie in their dimensional and metabolic properties. With a diameter of 5–8 μm closely matching capillary dimensions and an anuclear structure avoiding paracrine interference, RBCs offer distinct benefits for foundational vascular model construction. However, the absence of ion channels presents a critical limitation for studying electroactive tissues (e.g., neural or muscular systems). Furthermore, as contemporary tissue engineering increasingly prioritizes intercellular communication, RBCs’ inability to secrete growth factors constrains their utility in complex tissue constructs. Nevertheless, this work validates their fundamental feasibility, demonstrating significant translational potential for nucleated cell applications in vascularized tissue engineering.
Conclusion
This study developed an innovative approach for fabricating patterned cell samples by integrating acoustofluidic technology with a photo-crosslinkable hydrogel crosslinking system. The method achieves long-term stability of cellular patterns through the synergistic effects of acoustic field-induced spatial arrangement of cells and photo-cured hydrogel immobilization, effectively addressing technical bottlenecks in traditional methods for cell fixation, long-term culture, and dynamic observation. The feasibility of this strategy was validated by acoustically patterning microparticles and red blood cells followed by hydrogel photopolymerization, while systematic verification based on characteristic transmission spectra of patterned erythrocytes confirmed its reliability. The established acoustofluidic-spectroscopic integrated platform enables transition from spatial cell manipulation to localized detection. This integration of physical manipulation and optical sensing technologies holds significant potential as a multidisciplinary research tool for investigating biological challenges such as tumor heterogeneity analysis and antibiotic resistance mechanisms.
Data availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
References
Hannachi, I. E., Yamato, M. & Okano, T. Cell sheet technology and cell patterning for biofabrication. Biofabrication 1, 022002 (2009).
Guillotin, B., & Guillemot, F. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 29, 183–190 (2011).
Sun, M., Zhang, J., Xuanyuan, T., Liu, X. & Liu, W. Facile and rapid microcontact printing of additive-free polydimethylsiloxane for biological patterning diversity. ACS Appl. Mater. Interfaces. 16, 20132–20142 (2024).
Foncy, J. et al. Dynamic Inking of large-scale stamps for multiplexed microcontact printing and fabrication of cell microarrays. PLoS One. 13, e0202531 (2018).
Mizuhara, M. S. & Berlyand, L. Aranson.Minimal model of directed cell motility on patterned substrates. Phys. Rev. E. 96, 052408 (2017).
Vasilopoulos, G. & Painter, K. J. Pattern formation in discrete cell tissues under long range filopodia-based direct cell to cell contact. Math. Biosci. 273, 1–15 (2016).
Lee, K. B., Kelbauskas, L., Brunner, A. & Meldrum, D. R. A versatile method for dynamically controlled patterning of small populations of epithelial cells on substrates via non-contact piezoelectric inkjet printing. PLoS One. 12, e0176079 (2017).
Liang, S. et al. A versatile optoelectronic tweezer system for micro-objects manipulation: transportation, patterning, sorting, rotating and storage. Micromachines 12, 271 (2021).
Yahya, W. N. W., Ibrahim, F., Thiha, A., Jamaluddin, N. F. & Madou, M. Fabrication of a 3D carbon electrode for potential dielectrophoresis-based hepatic cell patterning application using carbon micro-electrical-mechanical system (CMEMS). J. Micromech Microeng. 32, 055005 (2022).
Yahya, N. W., Kadri, N. A. & Ibrahim, F. Design and numerical analysis of interdigitated radiating-strips electrode for uniform 3D dielectrophoretic patterning of liver cells. Microsyst. Technol. 25, 3037–3045 (2019).
Yan, X. & Sun, D. Multilevel-based topology design and cell patterning with robotically controlled optical tweezers. IEEE T Contr Syst. T. 23, 176–185 (2015).
Hu, S., Ye, J., Zhao, Y. & Zhu, C. Advanced optical tweezers on cell manipulation and analysis. Eur. Phys. J. Plus. 137, 1024 (2022).
Shen, F. et al. Microscale magnetic field modulation using rapidly patterned soft magnetic microstructures. RSC Adv. 11, 34660–34668 (2021).
Lin, Z. et al. Magnetically actuated peanut colloid motors for cell manipulation and patterning. ACS Nano. 12, 2539–2545 (2018).
Devendran, C., Albrecht, T., Brenker, J., Alan, T. & Neild, A. The importance of travelling wave components in standing surface acoustic wave (SSAW) systems. Lab. Chip. 16, 3756–3766 (2016).
Vinita & Singh, J. Bulk acoustic wave (BAW) resonator-based MEMS magnetic field sensor. J. Mater. Sci: Mater. Electron. 35, 720 (2024).
Ger, T. R. et al. Cell patterning using magnetic concentric rectangular thin films for biochip application. IEEE T Magn. 49, 3496–3499 (2013).
Sagdic, K., Fernández-Lavado, E., Mariello, M. & Akouissi, O. Lacour. Hydrogels and conductive hydrogels for implantable bioelectronics. MRS Bull. 48, 495–505 (2023).
Zhao, H. et al. Development of a novel nanoclay-doped hydrogel adsorbent for efficient removal of heavy metal ions and organic dyes from wastewater. Gels 11, 287 (2025).
Xu, X., Jerca, V. V. & Hoogenboom, R. Bio-inspired hydrogels as multi-task anti-icing hydrogel coatings. Chem 6, 820–822 (2020).
Göckler, T. et al. Schepers. Tuning superfast curing thiol-norbornene-functionalized gelatin hydrogels for 3D Bioprinting. Adv. Health Mater. 10, e2100206 (2021).
Cruz-Medina, R. et al. Zaragoza-Contreras. Curing of cellulose hydrogels by UV radiation for mechanical reinforcement. Polymers 13, 2342 (2021).
Bernassau, A. L., Courtney, C. R. P., Beeley, J. & Drinkwater, B. W. Cumming. Interactive manipulation of microparticles in an octagonal sonotweezer. Appl. Phys. Lett. 102, 164101 (2013).
Ding, X. et al. Surface acoustic wave microfluidics. Lab. Chip. 13, 3626–3649 (2013).
Huang, L., Tang, D., Qian, J. & Xia, H. An octagonal acoustofluidic device for multimode cell and particle patterning and dynamical manipulation. IEEE Sens. J. 23, 6589–6595 (2023).
Andrade, M. A. B. et al. Contactless acoustic manipulation and sorting of particles by dynamic acoustic fields. IEEE T Ultrason. Ferr. 63, 1593–1600 (2016).
Courtney, C. R. P. et al. Manipulation of particles in two dimensions using phase controllable ultrasonic standing waves. Proc. Roy. Soc. A, Math., Phys. Eng. Sci. 468, 337–360 (2012).
Funding
This research was funded by the Fundamental Research Funds for the Central Universities (JZ2022HGTB0258).
Author information
Authors and Affiliations
Contributions
C.S.: Conceptualization, Investigation, Validation, Writing-original draft. B.X.: Validation and Methodology. L.H.: Methodology and Writing-review & editing. W.Z.: Supervision and Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, C., Xu, B., Huang, L. et al. A pattern and lock strategy integrating acoustic patterning and hydrogel crosslinking for stable cell architectures. Sci Rep 15, 30885 (2025). https://doi.org/10.1038/s41598-025-16296-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16296-8