Abstract
A combination of dexamethasone, ondansetron, and total intravenous anaesthesia (TIVA) is recommended as prophylaxis for preventing postoperative vomiting (POV) in high-risk children. Implementing TIVA in paediatric patients undergoing anaesthesia presents challenges due to its excessive inter-individual variability and difficult estimation. Regarding lidocaine’s antiemetic effect in paediatric patients, incorporating lidocaine can mitigate POV in high-risk children. Among 204 children undergoing elective tonsillectomy (with/without adenoidectomy), those with postoperative vomiting score ≥ 4 was randomised into Group C (saline) and Group L (lidocaine). The primary outcome was POV incidence within the first 24 h after surgery. The POV incidence differed among 15 patients in Group C (14.7%) and 5 in Group L (4.9%) presenting with one or more episodes of POV (P = 0.019). The secondary outcome was the number of coughs within the first 30 min after surgery. The number of coughs within the first 30 min after surgery significantly differed between Group L (0 [0–0.125]) and Group C (1 [0–2]) (P = 0.007). Significant between-group differences in the time to extubation were also observed, with a 3-min longer time in Group L. A lower percentage of patients experienced adverse events in Group C (2.2%) compared with Group L (1.1%) (P = 0.567); no severe events occurred. Adding intravenous lidocaine to ondansetron and dexamethasone was effective in reducing the POV incidence and extubation coughs in high-risk children following volatile anaesthesia for tonsillectomy.
Similar content being viewed by others
Introduction
Postoperative nausea and vomiting (PONV) is one of the most common complications in paediatric patients; it prolongs hospitalisation and is the most common cause of dissatisfaction in children and their parents1,2. PONV frequently delays discharge from post-anaesthesia care units (PACU) and is the leading cause of unexpected hospital admission after planned ambulatory surgery3. To address concerns about the hazards and discomfort of PONV, the combination of a 5HT3 antagonist and dexamethasone is recommended by the fourth consensus guidelines for the management of postoperative nausea and vomiting1.
Despite combining two antiemetic agents, the residual incidence of post operative vomiting (POV) remains high, approximately 30%, in high-risk patients4,5,6. In terms of the worldwide prevalence of volatile anaesthesia in paediatric patients and limited treatment efficacy with the bi-combination of antiemetics, the multimodal combination prophylactic therapy using different drug classes is more suitable for children at high risk for POV7. Although effective in high-risk adults, adding droperidol to a combination of ondansetron and dexamethasone did not further reduce POV frequency in high-risk children8.
Lidocaine is an amino-amide local anaesthetic possessing analgesic, anti-hyperalgesic, and anti-inflammatory properties, making it suitable as a general anaesthetic adjuvant9. Studies have demonstrated that intravenous lidocaine has antiemetic properties in paediatric patients10,11. We hypothesised that a combination of three drugs (ondansetron, dexamethasone, and lidocaine) could reduce the incidence of POV in paediatric patients who are at a high risk of POV. Due to the difficulty in assessing nausea in paediatric patients, we limited our trial to evaluating POV. The selection of POV as the primary outcome was guided by the need for clinically meaningful outcomes with potential benefits.
Materials and methods
Study design
This was a single-centre, parallel-group, randomised, double-blinded controlled trial conducted in accordance with the Declaration of Helsinki and its later amendments. The medical personnel responsible for the patient’s perioperative care (anaesthesiologists, surgeons, nurses) were blinded to the study. The study protocol was approved by the Institutional Review Board of Yichang Central People’s Hospital (HEC-KYJJ-2023-053-02). The trial was registered at www.chictr.org.cn (ChiCTR2300072362) on 12/06/2023. The study was conducted from 15 June 2023 to 31 August 2023 at Yichang Central People’s Hospital. Written informed consent was obtained from the parents of each child.
The patients were randomised in an equal (1:1) ratio to each group, with each patient assigned a code using computer-generated randomisation, generating the random allocation sequence.
Participants
Participants aged 3–15 years (American Society of Anaesthesiologists grade I–II) scheduled for elective tonsillectomy (with or without adenoidectomy) were included. Concerning the risk factors for POV, we utilised the vomiting in the postoperative period12 score for assessing and enrolling patients with a score exceeding 4, indicating a high risk for POV8. The induction protocol included opioid administration, which conformed to the score requirement for multiple opioid doses. The enrolment criteria included the following: age 3–6 years or > 13 years with a confirmed personal history of POV, motion sickness, or familial history of POV and age 6–13 years, each contributing 2 points in the scoring system12. Children were randomised into two groups: saline (Group C) and lidocaine (Group L). The exclusion criteria included chronic cough; history of steroid or bronchodilator use; reactive respiratory tract disease; upper airway infection within the past 2 weeks; gastroesophageal reflux; morbid obesity; allergy to any study drugs; and the use of medications or nutraceuticals influencing blood pressure (BP) and heart rate. Cases of surgeries exceeding 2 h, unexpected bleeding, or more than two intubation attempts were also excluded.
Perioperative anaesthetic care
Preoperatively, each child underwent a fasting period of 6 h and abstained from clear fluid intake for 2 h. Accompanied by their parents, the children were brought into the operating room, a measure aimed to reduce their separation anxiety. Vital signs, including non-invasive BP, heart rate, electrocardiography, pulse oxygen saturation, and the Bispectral Index (BIS) were monitored using a multifunction monitor (GE Healthcare, Helsinki, Finland). The BP cuff, sized at approximately two-thirds of the upper arm length, was used in each patient. A 22-gauge intravenous catheter was placed in the dorsal veins of the hand. During the first hour, the patients received 10 ml/kg/h intravenous fluids, followed by an infusion rate using the 4-2-1 rule13.
Prior to the administration of general anaesthesia, the patients underwent preoxygenation. The anaesthesia induction protocol included sufentanyl (Yichang Renfu Pharmaceutical Co. Ltd., Yichang, China) at 0.25 µg/kg, propofol (Fresenius Kabi Deutschland GmbH, Homburg, Germany) at 2.0 mg/kg, and rocuronium (Zhejiang Xianju Pharmaceutical Co. Ltd., Taizhou, China) at 0.6 mg/kg. Ventilation through a facemask with 100% oxygen was initiated once the eyelash reflex was no longer present. A cuffed endotracheal tube was used, the size of which was selected based on a widely used formula ([3.5 + age in years]/4). Intubation was performed with a BIS score between 40 and 60. Participants were excluded from the study if any difficulty was encountered during facemask ventilation.
Anaesthesia was maintained with 2–3% of sevoflurane (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan), 0.1 µg/kg/min remifentanyl (Yichang Renfu Pharmaceutical Co. Ltd., Yichang, China), and 50% of medical air in oxygen. The delivery of sevoflurane was modified based on measurements of the depth of anaesthesia with a BIS within 40–60. Ondansetron (Qilu Pharmaceutical Co., LTD, Jinan, China) (0.1 mg/kg) and dexamethasone (Hubei Jinyao Pharmaceutical Co., LTD, Xiangyang, China) (0.125 mg/kg) were injected immediately after the induction of anaesthesia. After the injection of the combination of drugs, 1.5 mg kg− 1 lidocaine (Anhui Changjiang Pharmaceutical Co. Ltd, Wuhu, China) or 0.9% saline was injected intravenously over 5 min with activation of the pump, followed by either 2 mg kg− 1 h− 1 lidocaine or 0.9% saline, respectively, at the same rate and volume. The anaesthesia nurse who prepared the research treatment solutions and activated the pump was blinded to the study groups, strictly followed the trial protocol and was forbidden from discussing or informing others about the trial. All patients were administrated ibuprofen (Chengdu Brilliant Pharmaceutical Co., LTD, Chengdu, China) (10 mg/kg) and tramadol (Grünenthal GmbH, Aachen, Germany) (1 mg/kg) during surgery.
An experienced surgeon performed the surgery, and the surgical techniques used for tonsillectomies were standardised in all cases. The protocol included: dissection of the percapsular plane using a low-temperature plasma surgical system. Furthermore, haemostasis of the tonsillar fossa and the remaining bleeding vessels was also performed using the same system. At the end of the operation, sevoflurane, remifentanil, and the study treatment were discontinued. Neostigmine (Zhejiang Xianju Pharmaceutical Co. Ltd., Taizhou, China) at a dosage of 0.04 mg/kg and atropine (Suicheng Pharmaceutical Co. Ltd., Xinzheng, China) at a dosage of 0.02 mg/kg were administered to antagonise any residual neuromuscular blockade when the train-of-four (TOF) ratio was ≥ 0.5. Extubation was performed by an experienced anaesthetist, who was blinded to the study after confirming adequate tidal volume, regular spontaneous respiratory patterns, purposeful behaviour (eyes open upon request), and TOF ≥ 0.9. Gastric contents were suctioned via a tube before extubation. After extubation, the children were monitored for at least 5 min to resume regular spontaneous respiration and were subsequently transferred to the PACU.
In the PACU, the anaesthetic nurse who was blinded to the study assessed the pain score every 15 min; the patient was kept in the PACU for 1 h. Electrocardiography, peripheral pulse oximetry, and non-invasive BP measurements were performed. When their Steward recovery score was higher than 4, the patients were discharged from the PACU to the ward, where they remained overnight. An experienced investigator began tracking POV throughout the 24 h after surgery. We assessed pain intensity regularly during the first 24 h after surgery: at 1, 2, 4, 8, and 24 h after surgery. Different pain scales were used: the Face, Legs, Activity, Cry, and Consolability scale for children aged 3–4 years14, Wong–Baker scale for children aged 4–7 years15, and visual analogue scale for children aged ≥ 8 years16. To ensure that the side effects did not complicate the patients’ postoperative course, only acetaminophen (15 mg/kg) was administrated every 6 h for pain management during the observation period of the trial. Twenty-four hours after the end of the operation, postoperative analgesia was administered following the protocol of the local clinician, who suggested that morphine (Northeast Pharmaceutical Group Shenyang No.1 Pharmaceutical Co., LTD, Shenyang, China) be used for pain treatment with continued acetaminophen administration. Other perioperative care was provided according to the practices of the local clinicians.
Primary outcomes
The primary outcome was the incidence of any emetic episodes (retching or vomiting per the parent’s report) within 24 h postoperatively. The rescue methods for POV were intravenous ondansetron (50 µg/kg) and dexamethasone (0.1 mg/kg).
Secondary outcomes
Secondary outcomes were extubation time and the number of extubation coughs in the first 30 min after surgery. Adverse events such as drowsiness, headache, tongue numbness, tinnitus, dizziness, skin erythema, sinus arrest, hives, and tracheospasm were also recorded.
Statistical analysis
The required sample size was determined based on a preliminary experiment. To achieve 90% power (with a two-sided α risk of 0.05) for detecting a 10% of absolute risk reduction in POV incidence in Group L, we calculated a necessary sample size of 204 randomised patients. Between-group comparisons utilised the student’s t-test or Wilcoxon rank-sum test, chosen according to the Shapiro–Wilk test results. Regarding proportional inferences, we applied the χ2 test and Fisher’s exact test. Descriptive statistics are presented as mean ± standard deviation, median (interquartile range), or odds ratio (95% confidence interval [CI]). The significance threshold was set at P < 0.05. All analyses were conducted using STATA v26.0 (StataCorp LP, College Station, TX, USA).
Results
Patients
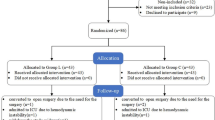
Between 15 June 2023 and 31 August 2023, 204 patients were included in the study and divided into two groups; the characteristics and operative data did not differ between the groups (Table 1). The study flow chart is shown in (Fig. 1).
Primary outcomes
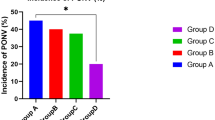
The incidence of POV was 14.7% and 4.9% in Groups C (15/102) and L (5/102), respectively. A significant difference between Groups C and L (P = 0.019) (95%CI: 1.13, 7.94) (Fig. 2) was observed.
Secondary outcomes
Twelve times higher doses of rescue antiemetics were used in group C and 4 times in group L. The total average dose per kg of body weight was 7.49 µg/kg of ondansetron and 0.015 mg of dexamethasone in group C vs. ondansetron (2.51 µg/kg) and 0.005 mg/kg of dexamethasone in group L. Statistically significant differences were also observed in the time to extubation, where Group L exhibited an extubation time 3 min longer than did Group C (Table 1).The number of extubation coughs was 1 [0–2] in group C vs. 0 [0–0.125] in group L, respectively. A significant difference between the groups (P = 0.007) (95%CI: 0.000, 0.0001) (Fig. 3). Furthermore, no severe complications were reported in either group (Table 2).
The figure illustrates the logistic regression analysis of the risk factor for childhood post-extubation cough. Small rectangles denote the OR for this factor, while the short line represents the 95% confidence interval of the OR. The green dashed line signifies the threshold of OR = 1, and the 95% confidence interval that spans OR = 1 indicates no statistical significance for this factor. BMI stands for body mass index, and OR represents the risk ratio. The specific numbers are marked to the right of the corresponding figure. BMI body mass index, OR odds ratio; Group C, saline; group L, lidocaine.
Discussion
A key finding of our study is that adding lidocaine to the prophylactic mixture of ondansetron and dexamethasone decreased POV incidence in high-risk children. Additionally, incorporating lidocaine reduced the number of coughs within 30 min post-surgery. Notably, the extubation time was 3 min longer in Group L, which was deemed clinically insignificant.
Previous studies have attempted to assess the relative benefits of triple-combined prophylaxis in children combined with droperidol or metoclopramide; however, they were unsuccessful8,17. Since marked differences in pharmacological, electroencephalographic, and neurological responses to anaesthesia occur between adult and paediatric patients, which could influence the efficacy of the treatments, these differences explain why some studies showed successful findings in adults but failed in children. Our results demonstrated a positive effect of lidocaine combined with 5-HT3 antagonist and dexamethasone for the treatment of PONV in high-risk paediatric patients and a reduction in the requirement for rescue medicine postoperatively. Different mechanisms may account for the efficacy of lidocaine and other drugs. One mechanism for the low incidence of POV in our trial would be the analgesic effect and opioid-sparing properties of lidocaine, as postoperative long-acting opioid administration is another risk factor1. Postoperative pain is a risk factor for POV and consists of a mixture of inflammatory and neuropathic pain, often manifesting as an increased sensitivity to pain. These are targets of intravenous lidocaine administration18. With pain resolution, postoperative requirements for opioids are reduced; opioid use represents another risk factor of PONV. Moreover, studies have demonstrated the efficacy of lidocaine in reducing the requirement of inhaled anaesthetics during the anaesthesia and surgery, which is another risk factor for PONV19. A possible explanation is the ability of lidocaine to prompt postoperative gastrointestinal recovery. Furthermore, the combined efficacy of analgesics uses, opioid-sparing effects, and the reduced requirement for inhale anaesthetics, may prompt the postoperative gastrointestinal recovery18,20.
Tonsillectomy (with/without adenoidectomy) remains one of the most common surgical procedures performed in children worldwide and is a high-risk surgery for POV1. With the combination of a 5-HT3 antagonist and dexamethasone, the incidence of PONV/POV in high-risk children decreased to 30%1. However, we observed a lower incidence of POV (14.7%) with the antiemetic prophylaxis of ondansetron and dexamethasone. Different anaesthetic techniques (intravenous induction and parental accompaniment to treat anxiety in children) might have partly accounted for the efficacy observed in our trial, considering that anxiety and inhaled anaesthetics are risk factors for PONV1.
In children, coughing during emergence following tonsillectomy may cause bleeding and laryngospasm and pose risks in children21. A meta-analysis indicated that a single intravenous bolus of lidocaine decreased the incidence of cough in children dose-dependently22. We observed similar results in our trial. At the time of extubation, the duration in Group L exceeded that in Group C by 3 min, was deemed clinically insignificant; no substantial difference (P < 0.001) was observed in the baseline characteristics of patients (Table 1). Our trial reported no severe adverse events, aligning with the findings of similar studies12.
Several strengths of our study should be highlighted. First, the baseline characteristics of the participants revealed a homogeneous study population. Second, the same anaesthetic protocol was strictly performed for all participants. Third, no differences were observed due to confounding factors, such as age, risk factor of PONV, type of surgery, time of surgery, or anaesthesia. Fourth, the opioid used in the study was carefully selected. The use of only sufentanyl, and no other opioid, allowed the dose to be strictly controlled to avoid its efficacy on PONV.
The limitations of this study include the lack of testing for lidocaine plasma concentration. Although lidocaine application was advised and its safety and plasma concentration correlation was confirmed in prior studies9,11,23, direct measurements were not conducted in this trial. Second, the children who underwent tonsillectomy needed hospitalisation in China, which was different from patients in the United States, who were inpatients and ambulatory patients. Third, we maintained the minimum alveolar concentration value between 0.7 and 1.2 as the anaesthesia depth in our trial. Despite the study’s randomised design and similar baseline characteristics among enrollers, we acknowledge the potential variability in anaesthesia depth across the groups. Fourth, the generalisability of the results might be limited given this was a single-centre study. Fifth, we did not record the time of initiation of drinking and oral feeding which may have affected the results of our trial.
In conclusion, adding lidocaine to ondansetron and dexamethasone reduced the incidence of POV following volatile anaesthesia in high-risk children undergoing tonsillectomy and lowered the incidence of extubation cough during the first 30 min postoperative. however, further research is needed to establish the efficacy of lidocaine in high-risk paediatric populations.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Gan, T. J. et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 131, 411–448 (2020).
Kermode, J., Walker, S. & Webb, I. Postoperative vomiting in children. Anaesth. Intensive Care. 23, 196–199 (1995).
Gold, B. S., Kitz, D. S., Lecky, J. H. & Neuhaus, J. M. Unanticipated admission to the hospital following ambulatory surgery. JAMA 262, 3008–3010 (1989).
Bharti, N. & Shende, D. Comparison of anti-emetic effects of Ondansetron and low-dose Droperidol in pediatric strabismus surgery. J. Pediatr. Ophthalmol. Strabismus. 40, 23–26 (2003).
Bolton, C. M., Myles, P. S., Carlin, J. B. & Nolan, T. Randomized, double-blind study comparing the efficacy of moderate-dose Metoclopramide and Ondansetron for the prophylactic control of postoperative vomiting in children after tonsillectomy. Br. J. Anaesth. 99, 699–703 (2007).
Klockgether-Radke, A., Neumann, S., Neumann, P., Braun, U. & Mühlendyck, H. Ondansetron, Droperidol and their combination for the prevention of post-operative vomiting in children. Eur. J. Anaesthesiol. 14, 362–367 (1997).
Kovac, A. L. Postoperative nausea and vomiting in pediatric patients. Paediatr. Drugs. 23, 11–37 (2021).
Bourdaud, N. et al. Addition of Droperidol to prophylactic Ondansetron and dexamethasone in children at high risk for postoperative vomiting. A randomized, controlled, double-blind study. Br. J. Anaesth. 118, 918–923 (2017).
Estebe, J. P. Intravenous Lidocaine. Best Pract. Res. Clin. Anaesthesiol. 31, 513–521 (2017).
Nakajima, D., Kawakami, H., Mihara, T., Sato, H. & Goto, T. Effectiveness of intravenous Lidocaine in preventing postoperative nausea and vomiting in pediatric patients: a systematic review and meta-analysis. PLoS One. 15, e0227904 (2020).
Echevarría, G. C. et al. Intra-operative Lidocaine in the prevention of vomiting after elective tonsillectomy in children: a randomised controlled trial. Eur. J. Anaesthesiol. 35, 343–348 (2018).
Bourdaud, N. et al. Development and validation of a risk score to predict the probability of postoperative vomiting in pediatric patients: the VPOP score. Paediatr. Anaesth. 24, 945–952 (2014).
Vallet, B. et al. Guidelines for perioperative haemodynamic optimization. Ann. Fr. Anesth. Reanim. 32, e151–158 (2013).
Redmann, A. J., Wang, Y., Furstein, J., Myer, C. M., de Alarcón, A. & 3rd & The use of the FLACC pain scale in pediatric patients undergoing adenotonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 92, 115–118 (2017).
Garra, G. et al. Validation of the Wong-Baker FACES pain rating scale in pediatric emergency department patients. Acad. Emerg. Med. 17, 50–54 (2010).
Bijur, P. E., Silver, W. & Gallagher, E. J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. 8, 1153–1157 (2001).
Gunter, J. B. et al. A. M. A factorial study of ondansetron, metoclopramide, and dexamethasone for emesis prophylaxis after adenotonsillectomy in children. Paediatr. Anaesth. 16, 1153–1165 (2006).
Weibel, S. et al. Efficacy and safety of intravenous Lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br. J. Anaesth. 116, 770–783 (2016).
Batko, I., Kościelniak-Merak, B., Tomasik, P. J. & Kobylarz, K. Lidocaine reduces Sevoflurane consumption and improves recovery profile in children undergoing major spine surgery. Med. Sci. Monit. 26, e919971 (2020).
Dai, Y., Huang, J. & Liu, J. Effects of intravenous Lidocaine on postoperative pain and Gastrointestinal function recovery following Gastrointestinal surgery: a meta-analysis. Minerva Anestesiol. 90, 561–572 (2024).
Afshan, G., Chohan, U., Qamar-Ul-Hoda, M. & Kamal, R. S. Is there a role of a small dose of Propofol in the treatment of laryngeal spasm? Paediatr. Anaesth. 12, 625–628 (2002).
Clivio, S., Putzu, A. & Tramèr, M. R. Intravenous Lidocaine for the prevention of cough: systematic review and meta-analysis of randomized controlled trials. Anesth. Analg. 129, 1249–1255 (2019).
Beaussier, M., Delbos, A., Maurice-Szamburski, A., Ecoffey, C. & Mercadal, L. Perioperative use of intravenous Lidocaine. Drugs 78, 1229–1246 (2018).
Funding
Support was provided solely from institutional and/or departmental sources.
Author information
Authors and Affiliations
Contributions
Jin-fei Xu and Ming-cheng Du helped with the data curation. Jin-fei Xu and Yi Chen helped with the software and formal analysis. Yang Hu, Ming-cheng Du, and Yi Chen helped with project administration. Xiang Long and Jing-jing Jiang helped with resources and investigation. Yuan Gong helped with the methodology, supervision, and validation. All authors have contributed equally to the manuscript and have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Jf., Du, Mc., Chen, Y. et al. Intravenous lidocaine enhances the efficacy of ondansetron and dexamethasone in postoperative vomiting prophylaxis among high-risk children. Sci Rep 15, 30168 (2025). https://doi.org/10.1038/s41598-025-16367-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16367-w