Abstract
Cancer treatments can lead to infertility, particularly in prepubertal boys who cannot preserve sperm before therapy. In vitro spermatogenesis offers a promising strategy for fertility preservation in this population by enabling the development of sperm from immature testicular tissue under controlled conditions. This study investigates the effects of a novel culture medium containing plasma rich in growth factors (PRGF) and knockout serum replacement (KSR) on in vitro spermatogenesis. Testicular tissues from five-day-old male NMRI mice were cultured in either a medium containing 5% KSR + 5% PRGF or a control medium containing 10% KSR for 42 days. Histological analysis revealed significant degeneration of peripheral seminiferous tubules in the 5% KSR + 5% PRGF group compared to the control. Gene expression analysis showed reduced levels of spermatogenesis markers (Plzf, Tekt1, Tnp1) and the proliferation marker Ki67, alongside elevated expression of the pro-apoptotic marker Bax. Immunofluorescence confirmed fewer spermatogonial stem cells (PLZF), spermatocytes (SYCP3), and proliferating cells (Ki67), with complete absence of post-meiotic marker ACRBP in the 5% KSR + 5% PRGF group. Additionally, higher Bax and lower Bcl-2 fluorescence intensities were observed in this group. These findings indicate that a medium supplemented with 5% KSR and 5% PRGF is ineffective for supporting complete in vitro spermatogenesis and may promote apoptosis. Importantly, these results provide insights into culture system design for future applications in fertility preservation strategies for prepubertal cancer patients at risk of gonadotoxicity.
Similar content being viewed by others
Introduction
Spermatogenesis is a complex and tightly regulated process in which spermatogonia develop into mature spermatozoa. It begins shortly before puberty under the influence of pituitary gonadotropins and continues throughout life1. Cancer treatments such as chemotherapy and radiotherapy can impair spermatogenesis, often resulting in infertility. This issue is especially critical in prepubertal boys, whose testes contain only immature germ cells and therefore lack the option of sperm cryopreservation before treatment2. Approximately 200,000 children under 15 years are diagnosed with cancer annually3, with improved survival rates reaching nearly 80% due to advancements in treatments4. However, infertility following treatment remains a major quality-of-life issue, affecting around 30% of survivors5,6,7.
Adult male patients can cryopreserve sperm prior to gonadotoxic treatments, but prepubertal patients lack this option because their testes contain only immature germ cells and Sertoli cells, not mature sperm7. Therefore, developing an effective culture system that can support in vitro spermatogenesis from immature testicular tissue is a priority for fertility preservation in this population4,8. Such systems could also benefit men with non-obstructive azoospermia (NOA)9.
In response to this clinical need, significant efforts have been made over the past decades, to develop optimized testicular tissue culture media. Early attempts using Eagle’s medium supplemented with amino acids and bovine serum were unsuccessful in supporting complete spermatogenesis, as germ cells failed to progress beyond the pachytene spermatocyte stage10. Subsequent studies identified key supplements such as pyruvate, non-essential amino acids, and hormones (e.g., FSH) that improved spermatogenic progression but still did not achieve full spermatogenesis11.
Building upon these findings, in 1993, Boitani et al. cultured testicular tissues from 9-day-old rats in EMEM supplemented with various vitamins and hormones, demonstrating that FSH was essential for the progression of type A spermatogonia to pachytene spermatocytes over three weeks. In contrast, other supplements such as LH, testosterone, and vitamins A, C, and E did not promote this progression12.
A major breakthrough came with a study by Sato et al. demonstrated that supplementation with 10% Knockout Serum Replacement (KSR) could support complete spermatogenesis in mouse testicular tissue cultures, achieving functional sperm production13. Despite these advances, subsequent research highlighted that even 10% KSR media were less efficient than in vivo spermatogenesis14,15,16 and showed strain-dependent variability in outcomes17, underscoring the need for further media optimization.
In recent years, attention has turned to incorporating growth factor-rich supplements such as platelet-rich plasma (PRP) and its derivative, plasma rich in growth factors (PRGF), into culture media. PRGF provides a concentrated source of bioactive molecules, including platelet-derived growth factor (PDGF), glial cell line-derived neurotrophic factor (GDNF),vascular endothelial growth factor (VEGF), and insulin-like growth factor-1 (IGF-1), which have been shown to enhance stem cell proliferation, migration, and differentiation in various tissues18,19,20,21,22,23.
Prior studies on PRP have indicated its positive impact on the viability and differentiation of spermatogonial stem cells, with some reports highlighting the upregulation of post-meiotic gene expression, while others emphasize pre-meiotic activation24,25. Notably, optimal outcomes in such studies were generally observed at low concentrations, such as 5% PRP, suggesting a concentration-dependent effect24.
In our previous work26, we systematically evaluated various concentrations of PRGF (5%, 10%, and 20%) for testicular tissue culture. Among them, 5% PRGF was found to best preserve seminiferous tubule integrity over a 14-day period, performing comparably to 10% KSR. Furthermore, 42-day cultures supplemented with 5% PRGF supported spermatogenesis up to the formation of flagellated sperm. This was accompanied by enhanced expression of key spermatogenesis markers and reduced apoptotic signaling, indicating its potential to support germ cell development in vitro. Nevertheless, further optimization is needed to increase the efficiency of producing a higher number of mature, flagellated sperm.
In this context, combining KSR with PRGF may offer a synergistic approach by providing both a defined serum replacement and a rich mixture of growth factors to optimize the in vitro environment. Although some studies have reported beneficial effects of such combinations in germ cell cultures of other species27, their specific impact on mouse testicular tissue remains unexplored. Despite growing interest in PRGF for various regenerative purposes, its application in testicular organ culture remains largely unexplored and lacks standardized protocols. This prompted the present study, which aims to systematically evaluate the combined effects of 5% PRGF and 5% KSR on in vitro spermatogenesis in mouse testicular tissue.
Materials and methods
Ethics declarations
All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Tarbiat Modares University (Approval ID: IR.MODARES.REC.1399.043). All procedures involving animals were performed in accordance with relevant national guidelines and institutional regulations for animal welfare, and are reported in compliance with the ARRIVE guidelines.
For human sample collection, venous blood was obtained from healthy adult volunteers following written informed consent. The protocol was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, and was approved by the Ethics Committee of Tarbiat Modares University.
Animals and housing conditions
Neonatal (5-day-old) male NMRI mice were obtained from the Royan Institute (Tehran, Iran). Animals were housed under a 12-h light/dark cycle at a controlled temperature of 22 ± 2 °C and 55 ± 5% humidity, with ad libitum access to water and standard chow. All procedures were conducted in compliance with national and institutional animal care regulations.
Preparation of PRGF
A total of 300 mL of peripheral blood was collected from three healthy male adult volunteers using 50 mL tubes containing 3.8% sodium citrate. Blood samples were centrifuged at 2500 g for 4 min to separate plasma, followed by a second centrifugation at 5000 g for 5 min to isolate platelet-rich fractions. The upper buffy coat layer (Fraction F2), with the highest platelet concentration, was pooled and activated by adding 10% CaCl₂ (50 µL/mL). The mixture was incubated in a 40 °C water bath for 90 min to induce gel formation. The resulting supernatant was centrifuged at 8000 g for 10 min, aliquoted, and stored at − 80 °C. Before use, PRGF aliquots were thawed and re-centrifuged (10,000 g, 10 min, 4 °C) to remove residual clots28.
Culture media composition
The base medium consisted of α-MEM (Gibco, Thermo Fisher Scientific) supplemented with 60 ng/mL progesterone (Invitrogen, UK), 30 ng/mL β-estradiol (Pepro Tech, USA), 20 ng/mL epithelial growth factor (EGF) (Pepro Tech, USA), 10 ng/mL human basic fibroblast growth factor (bFGF) (Pepro Tech, USA), 10 ng/mL human glial cell line-derived neurotropic factor (GDNF) (Pepro Tech, USA), 10 ng/mL leukemia inhibitory factor (LIF) (Royan, I.R.I), 100 IU/mL penicillin, 100 µg/mL streptomycin, and 50 µg/mL gentamicin. For the experimental group, 5% PRGF and 5% KSR (Invitrogen) were added. The control group received 10% KSR without PRGF.
Testicular tissue culture
Testes from 5-day-old mice were dissected under sterile conditions and sectioned into fragments of 1–3 mm. Tissue fragments were placed atop 1.5% agarose gel blocks (10 × 10 × 5 mm) pre-equilibrated in culture medium. Cultures were maintained at 34 °C in a 5% CO2 incubator with medium changes every two days. The culture duration was 42 days, corresponding approximately to the full cycle of mouse spermatogenesis.
Histological evaluation
After 42 days, tissues were fixed in Bouin’s fixative (3 h), embedded in paraffin, sectioned (5 µm), and stained with hematoxylin and eosin (H&E). Morphological analysis was conducted under light microscopy to evaluate tubule structure and degeneration.
RNA extraction and real-time PCR
Total RNA was extracted using RNX-Plus™ (CinnaGen, Iran) from five pooled samples per group. RNA was quantified spectrophotometrically and treated with DNase I. First-strand cDNA synthesis was performed using the RevertAid™ kit (Thermo Fisher) with oligo(dT) primers. Gene expression of Plzf, Tekt1, Tnp1, Ki67, Bax, and Bcl-2 was assessed using SYBR Green-based qPCR (Applied Biosystems) (Table 1). β-Actin was used as the housekeeping gene. Relative expression was calculated using the 2−ΔΔCT method. The expression level of each target gene in the control group (testicular tissue cultured with 10% KSR) was set to 1, and fold changes in the experimental group were calculated accordingly. Statistical significance was considered at p < 0.05.
Immunofluorescence staining
Testicular tissues cultured for 42 days were fixed overnight at 4 °C in 4% paraformaldehyde prepared in phosphate-buffered saline (PBS). Paraffin-embedded sections (5 μm) were deparaffinized, rehydrated, and subjected to antigen retrieval by heating in 10 mM trisodium citrate buffer (pH 6.0) using a microwave at 600 W for 20 min. Sections were then washed with TBS containing 0.03% Triton X-100 (Sigma-Aldrich, Germany), and non-specific binding was blocked by incubation in 1% BSA (in TBS) for 2 h at room temperature.
Subsequently, sections were incubated overnight at 4 °C with primary antibodies against PLZF (spermatogonial stem cell marker), SYCP3 (spermatocyte marker), ACRBP (post-meiotic spermatid marker), Ki67 (proliferation marker), Bax (pro-apoptotic), and Bcl-2 (anti-apoptotic), all at 1:100 dilution (PLZF, SYCP3, ACRBP, Bax, and Bcl-2 from Santa Cruz Biotechnology, USA; Ki67 from Elabscience, China). After washing, sections were incubated with Cy3-conjugated goat anti-mouse IgG secondary antibody (1:100, Elabscience) for 1 h in the dark.
Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; 1:200, Sigma-Aldrich) for 5 min. Sections were dehydrated through graded ethanol, cleared in xylene, mounted with coverslips, and examined under a fluorescence microscope (Olympus BX50, Germany).
For quantification, four random fields per sample were captured at 400 × magnification. The number of PLZF-, SYCP3-, ACRBP-, and Ki67-positive cells per seminiferous tubule was counted, while the mean fluorescence intensity of Bax and Bcl-2 was measured using ImageJ software (NIH, Bethesda, MD, USA), following the method described by Leite et al29.
Statistical analysis
Data were analyzed using GraphPad Prism 4 (GraphPad Software, USA). Comparisons between groups were made using unpaired two-tailed t-tests. Prior to analysis, normality of data distribution was confirmed using the Shapiro-Wilk test, and equality of variances was assessed using Levene’s test. Results are reported as mean ± standard deviation (SD), and p-values < 0.05 were considered statistically significant.
Results
Histological evaluation
After 42 days of culture, histological analysis revealed that testicular tissues maintained in 5% KSR + 5% PRGF exhibited extensive central necrosis and pronounced degeneration of seminiferous tubules (Fig. 1A). The peripheral tubules also showed marked structural disorganization, with reduced germ cell cohesion, loss of epithelial layering, diminished attachment to the basement membrane, and an increased number of pyknotic and apoptotic nuclei. As a result, the outer regions of the tissue were largely depleted of viable cells (Fig. 1a).
Representative histological sections of testicular tissues cultured for 42 days in two different medium conditions. (A) Cross-sectional overview of tissue cultured with 5% KSR + 5% PRGF, showing extensive central necrosis and structural degeneration of seminiferous tubules. (a) Higher magnification of the peripheral region shown in (A), revealing loss of germ cell layers, detachment from basement membrane, and increased pyknotic nuclei. (B) Cross-sectional overview of tissue cultured with 10% KSR, demonstrating relatively preserved seminiferous tubule architecture. (b) Higher magnification of the peripheral region shown in (B), demonstrating relatively preserved epithelial organization and partial maintenance of germ cell layering, despite mild disorganization in some tubules. All sections were stained with hematoxylin and eosin (H&E); scale bars = 50 μm (A and B), 20 μm (a and b).
In contrast, tissues cultured in 10% KSR exhibited relatively preserved morphology (Fig. 1B). A torus-like peripheral zone of seminiferous tubules was maintained, with partially retained germ cell stratification and intact basement membranes, despite the presence of central necrosis and structural disorganization in the core region (Fig. 1b).
Gene expression analysis of spermatogenesis, proliferation, and apoptosis markers
Quantitative real-time PCR analysis revealed significant alterations in the expression of genes associated with spermatogenesis, proliferation, and apoptosis between the two groups (Fig. 2). In the 5% KSR + 5% PRGF group, the expression levels of Plzf (spermatogonia), Tekt1 (spermatocytes), Tnp1 (post-meiotic spermatids), and Ki67 (proliferation marker) were all significantly downregulated compared to the 10% KSR group (P < 0.0001 for all; Fig. 2). This pattern indicates a broad suppression of germ cell development and mitotic activity under KSR + PRGF treatment.
Relative mRNA expression levels of spermatogenesis- and apoptosis-related genes in testicular tissues cultured for 42 days in either 10% KSR or 5% KSR + 5% PRGF media. Genes analyzed included Plzf (spermatogonia marker), Tekt1 (spermatocyte marker), Tnp1 (spermatid marker), Ki67 (proliferation marker), Bax (pro-apoptotic), and Bcl-2 (anti-apoptotic). Gene expression was measured by real-time PCR, and data were normalized to the housekeeping gene β-actin. Expression in the 10% KSR group was set as the calibrator (fold change = 1). Statistical comparisons were performed using unpaired two-tailed t-tests. Significance is indicated as: P < 0.01, **P < 0.0001 for 10% KSR vs. 5% KSR + 5% PRGF.
Conversely, expression of the pro-apoptotic gene Bax was significantly increased in the KSR + PRGF group (P < 0.01), while Bcl-2, an anti-apoptotic gene, showed no significant difference between the two conditions. The elevated Bax expression, in the absence of a compensatory rise in Bcl-2, suggests a shift in the apoptotic balance toward cell death in the KSR + PRGF-treated tissues.
Quantitative immunofluorescence analysis
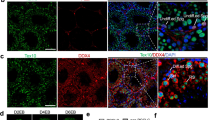
Immunofluorescence staining further supported the molecular findings, as shown in Figs. 3A and 4A. Tissues cultured in 5% PRGF + 5% KSR exhibited significantly fewer PLZF-positive spermatogonial stem cells per seminiferous tubule (p = 0.0285), as well as a notable reduction in SYCP3-positive spermatocytes (p = 0.0034) compared to the 10% KSR group (Fig. 3B). These data confirm early-stage germ cell depletion at both premeiotic and meiotic levels.
Immunofluorescence staining and quantitative analysis of germ cell markers in testicular tissues cultured for 42 days in media containing either 5% KSR + 5% PRGF or 10% KSR. (A) Representative immunofluorescence images showing PLZF-positive (PLZF⁺) spermatogonial stem cells, SYCP3⁺ spermatocytes, and ACRBP⁺ post-meiotic spermatids (red). Cell nuclei were counterstained with DAPI (blue). Yellow arrowheads indicate ACRBP⁺ spermatids. Dashed white lines indicate the basal lamina of seminiferous tubules. Scale bar = 20 μm. (B) Quantification of the average number of PLZF⁺, SYCP3⁺, and ACRBP⁺ cells per tubule. Tissues cultured in 10% KSR showed significantly higher counts compared to the 5% KSR + 5% PRGF group. Data are presented as mean ± standard deviation (SD). Significance is indicated by *P ≤ 0.05, **P ≤ 0.01.
Immunofluorescence staining and quantitative analysis of Ki67, Bax, and Bcl-2 protein expression in testicular tissues cultured for 42 days in media containing either 5% KSR + 5% PRGF or 10% KSR. (A) Representative images showing immunofluorescence staining for the proliferation marker Ki67 (red), the pro-apoptotic marker Bax (green), and the anti-apoptotic marker Bcl-2 (green). Cell nuclei were counterstained with DAPI (blue). Scale bar = 20 μm. (B) Quantitative analysis revealed significantly higher Ki67 expression in the 10% KSR group, indicating enhanced proliferative activity. Conversely, tissues cultured with 5% KSR + 5% PRGF exhibited a significant increase in Bax expression and a decrease in Bcl-2 expression, suggesting enhanced apoptotic activity. Data are presented as mean ± standard deviation (SD). Significance levels: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Importantly, ACRBP-positive post-meiotic spermatids were entirely absent in the KSR + PRGF group, indicating that germ cell development was arrested prior to completion of meiosis. In contrast, 10% KSR-supported cultures showed robust ACRBP expression within multiple tubules (p = 0.0011), demonstrating successful progression to the spermatid stage under this condition (Fig. 3B).
Proliferative activity, as measured by Ki67-positive cell counts, was also significantly diminished in the KSR + PRGF group (p = 0.0011), consistent with the observed downregulation of Ki67 transcript levels (Fig. 4B).
Fluorescence intensity analysis of apoptotic markers revealed that tissues in the KSR + PRGF group exhibited significantly elevated Bax signal intensity (p = 0.0005), alongside reduced Bcl-2 expression (p = 0.027), confirming increased apoptotic stress at the protein level in the KSR + PRGF-treated cultures (Fig. 4B).
Discussion
In this study, we assessed whether a combination of 5% PRGF and 5% KSR could support in vitro spermatogenesis in neonatal mouse testicular tissue. Our results demonstrate that this formulation was inadequate for preserving seminiferous tubule architecture and sustaining germ cell differentiation throughout the extended culture duration.
Histological examination revealed extensive degeneration, characterized by central necrosis, peripheral disorganization of seminiferous tubules, and loss of cell adhesion. These architectural disruptions were paralleled by molecular alterations, including reduced expression of spermatogenic markers (Plzf, Tekt1, Tnp1) and the proliferation marker Ki67, alongside elevated Bax expression. Immunofluorescence analysis further confirmed depletion of PLZF + spermatogonia, SYCP3 + spermatocytes, and a complete absence of ACRBP + post-meiotic cells. Increased Bax and decreased Bcl-2 and Ki67 labeling indicate that this formulation may trigger an apoptotic shift through molecular imbalance, compromising germ cell viability and progression.
The observed detrimental effects may arise from the interaction between biologically distinct components: PRGF, a human-derived plasma concentrate rich in growth factors30, and KSR, an animal-derived chemically defined supplement optimized for embryonic stem cell maintenance31. While each component supports cell survival under specific conditions, their combined presence may disrupt the finely balanced testicular microenvironment.
An excessive or imbalanced supply of bioactive compounds from both PRGF and KSR—including lipids, vitamins, hormones, and particularly growth factors—may overwhelm endogenous signaling pathways32. Examples include PDGF, VEGF, IGF-1, HGF, bFGF, and TGF-β1. These factors, though beneficial at physiological concentrations, can elicit paradoxical responses when overabundant, such as oxidative stress, aberrant intracellular signaling, and apoptosis33,34,35,36,37,38. Notably, TGF-β and HGF can activate stress-associated pathways (e.g., p38 MAPK) and upregulate cell cycle inhibitors (e.g., p21Waf1, p27Kip1), leading to cell cycle arrest and apoptosis39,40,41,42. Previous studies have also reported that supraphysiological levels of LIF or bFGF impair spermatogonial proliferation43. These molecular pathways may underlie the increased Bax expression and decreased Bcl-2 levels observed in our study, and help explain the degeneration of testicular architecture in the KSR + PRGF-treated group.
In our previous study26, we demonstrated that 5% PRGF alone was effective in supporting spermatogenic progression and maintaining seminiferous structure. However, the addition of 5% KSR to this concentration negated its beneficial effects. This observation aligns with previous reports indicating that excessive or unbalanced supplementation with serum components such as PRP can exert detrimental effects on cell viability. The dose-dependent cytotoxicity of PRP has been documented in various systems. For example, PRP at 40–60% reduces proliferation in adipose-derived mesenchymal stem cells, with 20% being optimal38. Similarly, high concentrations (≥ 50%) suppress periodontal cell viability44, and complete PRP (100%) induces cytotoxicity in osteoblasts and ligament cells45. These findings highlight the necessity of carefully titrating bioactive supplements in culture systems.
Parallel evidence exists for KSR. A study by Liu et al. demonstrated that 5% KSR provided insufficient support for testicular cultures, while 15% induced atrophy after initial growth. At 15% KSR, rapid initial growth slowed after two weeks, with shrinkage observed by the fourth week—likely due to cellular toxicity. In contrast, 10% KSR yielded optimal outcomes, underscoring the importance of concentration balance and highlighting the dose-dependent risk of cellular toxicity associated with excessive KSR supplementation46.
Collectively, our findings underscore the sensitivity of in vitro spermatogenesis to the precise composition and dosage of culture medium components. A mechanistic understanding of how specific growth factor interactions affect spermatogonial stem cell fate is essential for developing optimized protocols that enable complete spermatogenic progression. This knowledge will be critical for advancing in vitro fertility preservation strategies.
Despite providing critical insights, this study has certain limitations. The absence of mechanistic pathway validation and intermediate time-point assessments limits our understanding of the dynamic cellular responses to PRGF and KSR. Future studies incorporating transcriptomic or proteomic profiling, alongside time-course analyses, are warranted to unravel these complex interactions.
Conclusion
This study demonstrated that culturing testicular tissues with a combination of 5% KSR and 5% PRGF for 42 days resulted in significant structural and functional deterioration compared to the standard 10% KSR condition. The observed degeneration was marked by central necrosis, disrupted tubule organization, downregulation of spermatogenesis- and proliferation-related genes, and increased pro-apoptotic signaling (Bax). Quantitative immunofluorescence further confirmed reduced numbers of spermatogonial stem cells, spermatocytes, and proliferating cells, alongside increased Bax and decreased Bcl-2 expression. These findings underscore the need for careful optimization of growth factor supplementation in culture systems aiming to support complete spermatogenesis in vitro. This is particularly critical for developing personalized fertility preservation strategies in prepubertal patients who are vulnerable to gonadotoxic therapies.
Data availability
The data supporting the findings of this study are available from the corresponding authors upon reasonable request.
References
Guerriero, G., Trocchia, S., Abdel-Gawad, F. K. & Ciarcia, G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 5, 56 (2014).
Delessard, M. et al. Exposure to chemotherapy during childhood or adulthood and consequences on spermatogenesis and male fertility. Int. J. Mol. Sci. 21(4), 1454 (2020).
Rodriguez-Galindo, C., Friedrich, P., Morrissey, L. & Frazier, L. Global challenges in pediatric oncology. Curr. Opin. Pediatr. 25(1), 3–15 (2013).
Mertens, A. C. et al. Conditional survival in pediatric malignancies: analysis of data from the Childhood Cancer Survivor Study and the Surveillance, Epidemiology, and End Results Program. Cancer 121(7), 1108–1117 (2015).
Pasten González, A. et al. Current status of fertility preservation in pediatric oncology patients. Children (Basel). 11(5), 537 (2024).
Thomson, A. B. et al. Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: a case-control study. Lancet 360(9330), 361–367 (2002).
Meistrich, M. L. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil. Steril. 100(5), 1180–1186 (2013).
Chen, L., Dong, Z. & Chen, X. Fertility preservation in pediatric healthcare: A review. Front Endocrinol (Lausanne). 14, 1147898 (2023).
Lee, J. H. et al. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 87(4), 824–833 (2007).
Steinberger, E., Steinberger, A. & Perloff, W. Studies on growth in organ culture of testicular tissue from rats of various ages. Anat. Rec. 148(4), 581–589 (1964).
Steinberger, A., Steinberger, E. & Perloff, W. Mammalian testes in organ culture. Exp. Cell Res. 36(1), 19–27 (1964).
Boitani, C., Politi, M. G. & Menna, T. Spermatogonial cell proliferation in organ culture of immature rat testis. Biol. Reprod. 48(4), 761–767 (1993).
Sato, T. et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 471(7339), 504–507 (2011).
Chapin, R. E. et al. Lost in translation: The search for an in vitro screen for spermatogenic toxicity. Birth Defects Res. B 107(6), 225–242 (2016).
Yokonishi, T. et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat. Commun. 5, 4320 (2014).
Amirkhani, Z., Movahedin, M., Baheiraei, N. & Ghiaseddin, A. Mini bioreactor can support in vitro spermatogenesis of mouse testicular tissue. Cell J. 24(5), 277–284 (2022).
Portela, J. M. et al. Strains matter: Success of murine in vitro spermatogenesis is dependent on genetic background. Dev. Biol. 456(1), 25–30 (2019).
Santos, L. C. et al. The Biological role of platelet derivatives in regenerative aesthetics. Int. J. Mol. Sci. 25(11), 5604 (2024).
Dos Santos, R. G. et al. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 144, 155560 (2021).
Ruzafa, N. et al. Plasma rich in growth factors (PRGF) increases the number of retinal Müller Glia in culture but not the survival of retinal neurons. Front. Pharmacol. 12, 606275 (2021).
Anitua, E., Mdl, F., Troya, M., Zalduendo, M. & Alkhraisat, M. H. Autologous platelet rich plasma (PRGF) preserves genomic stability of gingival fibroblasts and alveolar osteoblasts after long-term cell culture. Dentist. J. 10(9), 173 (2022).
Turajane, T., Cheeva-Akrapan, V., Saengsirinavin, P. & Lappaiwong, W. Composition of platelet-rich plasma prepared from knee osteoarthritic patients: platelets, leukocytes, and subtypes of leukocyte. Cureus. 15(3), e36399 (2023).
Santos, S., Sigurjonsson, Ó. E., Custódio, C. A. & Mano, J. Blood plasma derivatives for tissue engineering and regenerative medicine therapies. Tissue Eng. Part B Rev. 24(6), 454–462 (2018).
Khadivi, F. et al. Application of platelet-rich plasma (PRP) improves self-renewal of human spermatogonial stem cells in two-dimensional and three-dimensional culture systems. Acta Histochem. 122(8), 151627 (2020).
Salem, M. et al. Differentiation of human spermatogonial stem cells using a human decellularized testicular scaffold supplemented by platelet-rich plasma. Artif. Organs 47(5), 840–853 (2023).
Moradian, S. A. & Movahedin, M. In vitro sperm generation from immature mouse testicular tissue using plasma rich in growth factors. Stem Cell Res. Ther. 16(1), 17 (2025).
Fayaz, M. A. & Honaramooz, A. Culture media and supplements affect proliferation, colony-formation, and potency of porcine male germ cells. Theriogenology 187, 227–237 (2022).
Chiu, C. H., Lei, K. F. & Yeh, W. L. Development of a co-culture device for the study of human tenocytes in response to the combined stimulation of electric field and platelet rich plasma (PRP). Biomed. Microdevices 19(3), 69 (2017).
Leite, C. et al. Differentiation of human umbilical cord matrix mesenchymal stem cells into neural-like progenitor cells and maturation into an oligodendroglial-like lineage. PLoS ONE 9(10), e111059 (2014).
Perussolo, J., Calciolari, E., Dereka, X. & Donos, N. 2025 Platelet-rich plasma and plasma rich in growth factors in extra-oral wound care. Periodontol 97, 320–341 (2000).
Sakurai, M., Suzuki, C. & Yoshioka, K. Effect of knockout serum replacement supplementation to culture medium on porcine blastocyst development and piglet production. Theriogenology 83(4), 679–86.e1 (2015).
Arigony, A. L. et al. The influence of micronutrients in cell culture: A reflection on viability and genomic stability. Biomed Res Int. 2013, 597282 (2013).
Tan, H. et al. Engineering a favourable osteogenic microenvironment by heparin mediated hybrid coating assembly and rhBMP-2 loading. RSC Adv. 7(19), 11439–11447 (2017).
Bhakta, G. et al. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 33(26), 6113–6122 (2012).
Kakudo, N. et al. Proliferation-promoting effect of platelet-rich plasma on human adipose–derived stem cells and human dermal fibroblasts. Plast. Reconstr. Surg. 122(5), 1352–1360 (2008).
Graziani, F. et al. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral. Implants Res. 17(2), 212–219 (2006).
Choi, B. H. et al. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: An in vitro study. Int J. Oral. Maxillofac Surg. 34(4), 420–424 (2005).
Atashi, F., Jaconi, M. E., Pittet-Cuénod, B. & Modarressi, A. Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng. Part C Methods. 21(3), 253–262 (2015).
Jin, R. et al. Effects of concentrated growth factor on proliferation, migration, and differentiation of human dental pulp stem cells in vitro. J Tissue Eng. 9, 2041731418817505 (2018).
Battegay, E. J., Raines, E. W., Seifert, R. A., Bowen-Pope, D. F. & Ross, R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 63(3), 515–524 (1990).
Liu, W., Du, L., Li, J., He, Y. & Tang, M. Microenvironment of spermatogonial stem cells: A key factor in the regulation of spermatogenesis. Stem Cell Res. Ther. 15(1), 294 (2024).
Rodrigues, M., Griffith, L. G. & Wells, A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res. Ther. 1(4), 32 (2010).
Kubota, H., Avarbock, M. R. & Brinster, R. L. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol. Reprod. 71(3), 722–731 (2004).
Tavassoli-Hojjati, S., Sattari, M., Ghasemi, T., Ahmadi, R. & Mashayekhi, A. Effect of platelet-rich plasma concentrations on the proliferation of periodontal cells: An in vitro study. Eur. J. Dent. 10(4), 469–474 (2016).
Creeper, F., Lichanska, A. M., Marshall, R. I., Seymour, G. J. & Ivanovski, S. The effect of platelet-rich plasma on osteoblast and periodontal ligament cell migration, proliferation and differentiation. J. Periodontal Res. 44(2), 258–265 (2009).
Liu, F. et al. Effect of KnockOut serum replacement on germ cell development of immature testis tissue culture. Theriogenology 85(2), 193–199 (2016).
Acknowledgements
The authors are grateful for the support and assistance provided by Tarbiat Modares University (TMU) Research Deputy, the Ministry of Science, Research and Technology, and Modares Science and Technology Park.
Author information
Authors and Affiliations
Contributions
Seyyed Amir Moradian conducted the in vitro experiments and drafted the manuscript. Mansoureh Movahedin conceived and designed the study, secured funding, supervised the research, and revised the manuscript. Sajed Khaledi contributed to animal experiments and assisted with data analysis. Zahra Amirkhani was responsible for data interpretation, validation, statistical analysis, and critical manuscript review. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors have reviewed and understood the journal’s policies on conflict-of-interest disclosure and authorship. They declare no competing interests related to this work and confirm that they have made significant contributions to the research and manuscript preparation. All authors have reviewed and approved the final version of the manuscript for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moradian, S.A., Khaledi, S., Amirkhani, Z. et al. Combination of knockout serum replacement and plasma rich in growth factors does not support in vitro spermatogenesis in mice. Sci Rep 15, 31506 (2025). https://doi.org/10.1038/s41598-025-16502-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16502-7