Abstract
Dysfunction of Bone Marrow Derived Mesenchymal Stem Cells (BMSCs) induced by glucocorticoids has been identified as a key pathological mechanism of steroid-induced osteonecrosis of the femoral head (SONFH). Consequently, restoring the function of BMSCs is a vital strategy for treating SONFH. This study aimed to investigate the role of microRNA-576-5p (miR-576-5p) and Annexin A2 (ANXA2) in SONFH and dexamethasone (DEX) cultured BMSCs, expecting to seek new therapeutic strategies for SONFH. RNA and protein samples were extracted from the bone tissue of femoral heads and DEX treated BMSCs to verify the potential relationship among miR-576-5p, ANXA2 and SONFH. Then, the interaction between miR-576-5p and ANXA2 mRNA was investigated through dual luciferase reporter assays. Finally, BMSC proliferation, apoptosis, mineralization, and its capacity for pro-osteoclastogenesis after increasing miR-576-5p levels or reducing ANXA2 expression were detected with 10−5M DEX exposure. The results indicated that miR-576-5p levels were reduced, while ANXA2 protein was upregulated in the femoral heads of SONFH patients and in BMSCs treated with DEX. ANXA2 mRNA is a target gene of miR-576-5p, and overexpression of ANXA2 antagonized the effects of miR-576-5p on BMSCs. Interestingly, both increase of miR-576-5p and knockdown of ANXA2 mitigated the damage of DEX on BMSCs, evidenced by enhanced proliferation and mineralization and reduced apoptosis and pro-osteoclastogenesis capacity. In conclusion, elevating miR-576-5p alleviated DEX-induced BMSC injury by targeting ANXA2 mRNA, which may be a promising treatment option for SONFH.
Similar content being viewed by others
Introduction
Steroid-induced osteonecrosis of the femoral head (SONFH) has been associated with prolonged glucocorticoid (GC) exposure, resulting in compromised blood flow to the femoral head and the progressive ischemic necrosis of bone cells and tissues. Although various self-repair mechanisms are activated following necrosis, the ongoing damage inflicted by GCs often leads to structural alterations or even total collapse of the femoral head1,2. Therefore, investigating the mechanisms of blood supply and bone repair in SONFH is crucial for developing new therapeutic strategies.
Bone marrow derived mesenchymal stem cells (BMSCs) not only serve as progenitors of bone cells but also play a vital role in linking osteogenesis and angiogenesis3. Thus, their dysfunction is a key pathological factor in the development of SONFH4. GC has been reported to decrease BMSC telomerase activity, accelerate BMSC aging, diminish BMSC self-renewal capacity, and ultimately reduce BMSC proliferation5. Furthermore, GC could lower the activity of the Runx2 protein in BMSCs and inhibit their osteogenic differentiation6. Additionally, GC significantly suppresses the secretion of vascular endothelial growth factor from BMSCs, thereby impairing the critical “angiogenesis-osteogenesis coupling“7,8. Consequently, restoring the function of BMSCs against GC has emerged as a crucial strategy for the treatment of SONFH.
MicroRNAs (miRNAs) are endogenous noncoding single-stranded RNAs that influence various biological processes by regulating the expression of target mRNAs. Numerous studies have linked several miRNAs with SONFH, including miR-133a4, miR-135b9, miR-22410 and so all. MiR-576-5p has also been found to be significantly involved in bone metabolism, affecting conditions in osteosarcoma11, osteoarthritic human chondrocytes12 and atrophic nonunion13. However, the specific role and mechanism of miR-576-5p in SONFH has yet to be elucidated. Annexin A2 (ANXA2), which was predicted to be a target of miR-576-5p using TargetScan14 is part of the calcium-dependent membrane phospholipid-binding protein family and plays a crucial role in cell growth and signal transduction15. Researches have indicated that ANXA2 is involved in the development of osteoporosis, likely through mechanisms such as inhibiting osteoblast growth16, suppressing BMSC proliferation and osteogenic differentiation17, increasing osteoclast formation and bone resorption18,19. Additionally, ANXA2 has been implicated in avascular necrosis among patients with sickle cell disease20.
In this study, we have further validated the targeting regulatory relationship between miR-576-5p and ANXA2 and investigated their impact on BMSCs. Our results indicate that miR-576-5p could potentially mitigate the adverse effects of DEX on BMSC function by inhibiting the expression of ANXA2, which has been reported to influence cell growth and osteogenic activities adversely. This finding underscores the therapeutic potential of targeting miR-576-5p in treatment aimed at reducing glucocorticoid-induced complications in bone metabolism including SONFH.
Materials and methods
Study subjects
Bone tissue necrosis and subsequent repair are the pathological mechanisms of SONFH. The expression of proteins and RNAs in bone tissue of the femoral head region could provide certain value for exploring the mechanism of this disease21. Therefore, RNA and protein samples were extracted from bone tissue in femoral heads excised from patients underwent total hip arthroplasty (THA) and then preserved at −80 °C between March 2022 and December 2023. Inclusion criteria were patients diagnosed with SONFH at ACRO III/IV and underwent THA (served as the SONFH group), patients experienced femoral neck fracture and underwent THA (served as the Control group). Exclusion criteria were femoral head necrosis caused by trauma, alcohol and other unknown factors or hip osteoarthritis with THA. There were 16 males and 10 females (aged 63.04 ± 3.78 years old) in the SONFH group, and 13 males and 13 females (aged 63.89 ± 2.57 years old) in the control group. No obvious difference was documented in the general information of the patients (P > 0.05). The study received approval from the Clinical Medical Research Ethics Committee of the First Affiliated Hospital of Anhui Medical University (2022144) which allowed medical research on the excised femoral heads in THA.
Cell culture
Primary human BMSCs were purchased from ATCC (Manassas, VA, USA) and cultured in high glucose Dulbecco’s Modified Eagle Medium (DMEM) supplied by Corning (10-013-CVR), supplemented with 10% Fetal Bovine Serum (FBS) from Gibco (A3160802), and 1% penicillin/streptomycin from Beyotime (C0222). The cells were maintained at 37 °C in an atmosphere of 5% CO2. BMSCs from passages three to six were utilized for subsequent experiments. To test the effects of DEX on BMSC injury, BMSCs were incubated in full medium or osteogenic induction medium with the presence of 10−5M DEX (Sigma. St Louis, MS, USA), since 10−5M DEX has been revealed to induce BMSC injury including proliferation, apoptosis, osteogenic differentiation in previous studies22,23,24.
Cell transfection
BMSCs were transfected with miR-576-5p mimics and negative control mimics (100 pmol, Biomedical, China) using Lipofectamine 2000 (Invitrogen, USA). Additionally, ANXA2 was either knocked down using shRNA (shANXA2) or overexpressed using an overexpression fragment (OE-ANXA2), with corresponding negative controls. These constructs were incorporated into adenoviral vectors provided by OBIO SCIENTIFIC SERVICES, China, and then introduced into the BMSCs.
Osteogenic differentiation of BMSCs
The osteogenic induction medium was prepared by mixing full culture medium with 10−2M β-sodium glycerophosphate, 50 µg/mL L-ascorbic acid, and 10−7M DEX, which has been revealed to induce osteogenic differentiation of BMSCs in multiple studies5,23,24,25. The medium was changed every 3 days until sampling.
Western blot analysis
Total proteins were extracted from bone tissue and BMSCs using Radioimmunoprecipitation Assay (RIPA) Lysis Buffer (Solarbio, R0010) and their concentration was determined using a Bicinchoninic Acid (BCA) kit (Beyotime, P0010). Then, 30 micrograms of total protein were separated by Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred onto a Polyvinylidene Difluoride (PVDF) membrane. The membrane was blocked with Bovine Serum Albumin (BSA) and subsequently incubated overnight at 4 °C with primary antibodies against Osteoprotegerin (OPG) (Affinity, DF6824, 1:1000), ANXA2 (Proteintech, 11256-1-AP, 1:3000), Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL) (Proteintech, 66610-1-Ig, 1:5000), and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (Proteintech, 60004-1-Ig, 1:1000). After primary antibody incubation, the membranes were probed with goat anti-rabbit Immunoglobulin G-Horseradish Peroxidase (IgG-HRP) (absin, abs20040, 1:10000) for 1.5 h and developed using an Ultrasensitive Enhanced Chemiluminescence (ECL) Detection Kit (Proteintech, PK10002).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from bone tissue and BMSCs using Trizol. Subsequently, 1 µg of this RNA was reverse transcribed into complementary DNA (cDNA) using the Evo M-MLV RT Kit (Accurate Biology, AG11705). The expression levels of B-cell lymphoma-2 (Bcl-2), BCL-2-associated X protein (Bax), and GAPDH mRNA were then measured via qRT-PCR employing the SYBR Green Premix Pro Taq HS qPCR Kit II (Accurate Biology, AG11702). The primers used are listed in Table 1.
For microRNA detection, 1 µg of total RNA was incorporated into a reaction system containing gDNA Clean Reagent (Accurate Biology, AG11705) to eliminate genomic DNA. This RNA was then converted into cDNA using stem-loop RT primers tailored for miR-576-5p (5’-GTCGTATCCAGTGCAGGGTCCGAGGTA TTCGCACTGGATACGACACCAGA-3’) and U6 (5’-TGGAACGCTTCACGAATTTGCG-3’). qRT-PCR was performed for both miR-576-5p and U6, and the expression level of miR-576-5p was normalized to that of U6 using the 2‑ΔΔCt method. The sequences for these primers are provided in Table 1.
3-(4,5-dimethyl-2-thiazolyl)−2,5-diphenyl-2-Htetrazolium bromide (MTT) assay
Three days post-transfection, BMSCs were placed in 96-well plates at a seeding density of 2000 cells per well using 100µL of full medium. Subsequently, 10µL of MTT solution (5 mg/mL, C0009, Beyotime, China) was added to each well. The cells were then incubated for an additional 2 h at intervals of 24, 48, 72, 96, and 120 h post-seeding. After this incubation, 100µL of Formazan lysis solution was added to each well and incubated for 3 h to dissolve the formazan crystals formed. The absorbance at 570 nm was measured using a microplate spectrophotometer (Bio-Rad, Hercules, CA).
Cell apoptosis
After transfection for 3 days, BMSCs were seeded in 6-well plates and cultured in FBS-free medium for an additional 48 h. Subsequently, apoptosis in BMSCs was assessed using the Annexin V FITC/Propidium Iodide (PI) detection kit (C1062S, Beyotime, China), following the manufacturer’s protocol. Specifically, cells were resuspended in 200 µL of Annexin V-FITC and 10 µL of PI, and incubated in the dark at room temperature for 10 min. Apoptotic cells were then identified and quantified using flow cytometry, focusing on cells that were Annexin V-positive and PI-negative.
Immunofluorescence
After transfection, BMSCs were seeded in 48-well plates and subjected to osteoblast induction for two weeks. Subsequently, the BMSCs were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. After blocking with 10% FBS for 30 min, the cells were incubated with Osteocalcin Rabbit monoclonal Antibody (CST, #59757, 1:100) at 4 °C overnight. They were then incubated with Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488, Abcam, ab150077) for 1 h at 37 °C. Finally, the BMSCs were stained with 4’,6-diamidino-2-phenylindole (DAPI, Abcam, ab104139) for an additional 30 s and examined using a fluorescence microscope.
Alizarin red staining
After three weeks of osteogenic induction, BMSCs were fixed in 4% paraformaldehyde for 20 min and then stained with 2% Alizarin Red S Staining Solution (C0148S, Beyotime, China) for 30 min at room temperature. After being rinsed twice with PBS, the BMSCs were examined using an inverted microscope.
Dual luciferase reporter assay
The target segment of miR-576-5p on ANXA2 mRNA was predicated using TargetScan (www.targetscan.org). The 3’UTR of human ANXA2 mRNA containing the predicted binding site and its control mutant were synthesized. Subsequently, these segments were amplified and cloned into the psiCHECK-2 vector (Cat.#C8021), resulting in psiCHECK-h. ANXA2- Wild Type (WT) and psiCHECK-h. ANXA2- Mutation (MU) constructs.
293 T cells (2*104/well) were seeded in 24-well plates and transfected with either the MU or WT construct, human miR-576-5p mimics or mimic negative control (NC), and the psiCHECK-2 luciferase plasmid. The firefly and Renilla luciferase activities were measured using the Dual Luciferase Reporter Gene Assay Kit (RG027, Beyotime, China).
Statistical analysis
The data was processed using SPSS 22.0 (Microsoft, Chicago, IL, USA), and statistical analysis was conducted using either the independent-sample t-test or one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test.
Results
miR-576-5p level was reduced in femoral heads of SONFH patients
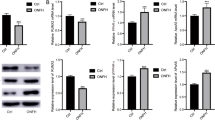
To investigate the potential role of miR-576-5p in SONFH, we analyzed femoral head bone tissue from patients who underwent THA, with and without SONFH. qRT-PCR and Western blot analyses indicated that miR-576-5p levels were decreased (Fig. 1A), while the ANXA2 protein level was increased in bone tissue with SONFH (Fig. 1B, and C). Additionally, the RANK/RANKL/OPG pathway, which is crucial for bone modeling and remodeling26 showed dysregulation in our findings. Western blot analysis confirmed reduced OPG and increased RANKL levels in bone tissue samples from SONFH patients (Fig. 1B, and C), suggesting imbalance in bone turnover.
miR-576-5p expression was reduced in SONFH patients. (A) qRT-PCR analysis of miR-576-5p relative expression in bone tissue of femoral heads with SONFH or femoral neck fracture. (B) Western blot analysis of OPG, ANXA2 and RANKL expression in bone tissue. (C) Gray analysis for the western blot results, the data between two groups were analyzed by t test. (**P < 0.01, ***P < 0.001 compared with the Control).
miR-576-5p level was reduced in DEX treated BMSCs
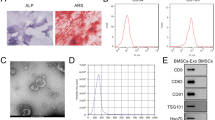
Dysfunction of BMSCs, the progenitor cells of bone, has been identified as a critical component in the pathogenesis of SONFH[3]. Consequently, we investigated the levels of miR-576-5p in BMSCs treated with DEX in vitro. Consistent with prior studies22,23,24 10−5M DEX significantly reduced osteoblast differentiation in BMSCs, as evidenced by decreased osteocalcin expression and calcification (Fig. 2A). DEX also significantly suppressed cell proliferation and enhanced apoptosis, accompanied by changes in the expression of the apoptotic genes Bax and Bcl-2 (Fig. 2B-E). Importantly, the level of miR-576-5p was also reduced in DEX treated BMSCs (Fig. 2F), paralleling observations in clinical SONFH samples, which suggests that miR-576-5p plays a significant role in glucocorticoid-induced disorders. Additionally, an increase in ANXA2 expression and an imbalance of OPG/RANKL at the cellular level were observed (Fig. 2G), further supporting the inverse relationship between miR-576-5p and ANXA2.
miR-576-5p level was reduced in glucocorticoid treated BMSCs. (A) Classical images of BMSCs with osteocalcin immunofluorescence and alizarin red staining. (B) MTT assay for BMSC proliferation. (C) The flow cytometric analysis for Annexin V-positive and PI negative apoptotic BMSCs. (D-F) qRT-PCR analysis of Bax, Bcl-2 and miR-576-5p relative expression in BMSCs. (G) Western blot analysis of OPG, ANXA2 and RANKL expression in BMSCs and the gray analysis for the western blot results. (**P < 0.01, ***P < 0.001 compared with the Control).
ANXA2 was identified as a target gene of miR-576-5p
To investigate the regulatory mechanism of miR-576-5p on ANXA2, we utilized the TargetScan website to identify potential target genes of miR-576-5p and located a binding site for miR-576-5p on the 3’UTR of ANXA2 mRNA(Fig. 3A). The results from a dual luciferase reporter gene assay demonstrated that miR-576-5p mimics significantly reduced the luciferase activity in 293 T cells transfected with psiCHECK-h. ANXA2-WT, but not with psiCHECK-h. ANXA2-MU (Fig. 3B). Additionally, further Western blot analysis supported the suppressive effect of miR-576-5p mimics on ANXA2 expression (Fig. 3C, D).
Meanwhile, we explored the antagonism of ANXA2 overexpression on miR-576-5p. Our proliferation assays indicated that overexpressing ANXA2 not only reduced proliferation (Fig. 4A), but also increased apoptosis in BMSCs transfected with miR-576-5p mimics, accompanied by significant changes in the expression levels of apoptotic genes Bax and Bcl-2 (Fig. 4B-D). Furthermore, overexpression of ANXA2 decreased OPG protein levels and increased RANKL protein levels, while also enhancing ANXA2 protein expression in BMSCs transfected with miR-576-5p mimics(Fig. 4E). ANXA2 overexpression also impaired the osteogenic differentiation of BMSCs (Fig. 4F), suggesting that increased levels of ANXA2 could negate the beneficial effects of miR-576-5p on BMSCs.
miR-576-5p directly targets ANXA2 mRNA. (A) Construction of h. ANXA2−3’UTR-WT and h. ANXA2−3’UTR-MU. (B) Values of the firefly luminescence normalized to the Renilla luminescence. (C) Western blot analysis of ANXA2 expression in BMSCs transfected with miR-576-5p mimic and the gray analysis for the western blot results. (***P < 0.001 compared with NC).
ANXA2 overexpression antagonize the effect of miR-576-5p elevation on BMSCs. (A) MTT assay for BMSC proliferation. (B) The flow cytometric analysis for Annexin V-positive and PI negative apoptotic BMSCs. C, D. qRT-PCR analysis of Bax, Bcl-2 relative expression in BMSCs. E. Western blot analysis of OPG, ANXA2 and RANKL expression in BMSCs and the gray analysis for the western blot results. F. Classical images of BMSCs with osteocalcin immunofluorescence and alizarin red staining. (**P < 0.01, ***P < 0.001 compared with the mimic + OE-NC group).
Elevated miR-576-5p attenuated the injury of DEX on BMSCs
Since DEX decreased miR-576-5p level in BMSCs, we hypothesized that regulating miR-576-5p expression would influence BMSCs under DEX treatment. To test this, we upregulated miR-576-5p expression using microRNA mimics (Fig. 5A) and conducted subsequent analyses. The MTT assay demonstrated that elevated miR-576-5p significantly enhanced BMSC proliferation compared to DEX treatment alone (Fig. 5B). Annexin V FITC/PI analysis further revealed that miR-576-5p upregulation mitigated DEX-induced apoptosis in BMSCs, accompanied by a reduction in Bax levels and an increase in Bcl-2 levels (Fig. 5C-F). Regarding osteogenic differentiation, DEX notably suppressed osteocalcin expression and calcification in BMSCs, while elevated miR-576-5p partially reversed these effects, though not to the level of the control group (Fig. 5H). Moreover, Western blot analysis showed that miR-576-5p upregulation improved the expression of bone formation-related proteins, OPG and RANKL, in BMSCs after DEX treatment (Fig. 5G). Elevated miR-576-5p also restrained the DEX-induced upregulation of ANXA2 (Fig. 5G), which further confirmed the regulation relationship between miR-576-5p and ANXA2.
Elevated miR-576-5p attenuated the injury of DEX on BMSCs. (A) qRT-PCR analysis of miR-576-5p relative expression. (B) MTT assay for BMSC proliferation. (C, D) The flow cytometric analysis for Annexin V-positive and PI negative apoptotic BMSCs. (E, F) qRT-PCR analysis of Bax, Bcl-2 relative expression. G Western blot analysis of OPG, ANXA2 and RANKL expression in BMSCs and the gray analysis for the western blot results. H. Classical images of BMSCs with osteocalcin immunofluorescence and alizarin red staining. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
ANXA2 knockdown also protected BMSCs against DEX
As a target gene of miR-576-5p, the regulation of ANXA2 is also expected to influence BMSCs after DEX treatment. To explore this, we conducted experiments on BMSCs transfected with shANXA2 or its control and then treated with 10−5 M DEX. The results revealed that ANXA2 knockdown significantly improved proliferation, reduced apoptosis, decreased Bax expression, and increased Bcl-2 expression in BMSCs exposed to DEX (Fig. 6A-E). Furthermore, ANXA2 knockdown enhanced OPG expression and reduced RANKL expression, along with promoting the mineralization capacity of BMSCs treated with DEX (Fig. 6F and G). The above results confirmed that ANXA2 knockdown ameliorated the negative effect of DEX on BMSCs.
ANXA2 knockdown protected BMSCs against DEX. (A) qRT-PCR analysis of ANXA2 relative expression. (B) MTT assay for BMSC proliferation. (C) The flow cytometric analysis for Annexin V-positive and PI negative apoptotic BMSCs. (D, E) qRT-PCR analysis of Bax, Bcl-2 relative expression. (F) Western blot analysis of OPG, ANXA2 and RANKL expression in BMSCs and the gray analysis for the western blot results. (G) Classical images of BMSCs with osteocalcin immunofluorescence and alizarin red staining. (**P < 0.01, ***P < 0.001 compared with the shCtrl).
Discussion
Several studies have demonstrated that restoring the function of BMSCs in the presence of GC could be an effective strategy for treating SONFH4,27,28. MicroRNAs, as key regulators of gene expression, have emerged as promising tools for modulating BMSCs fate and improving their resilience against glucocorticoid-induced damage29. The findings of this study revealed that glucocorticoid-induced BMSC injury may occur through the suppression of miR-576-5p. The downregulation of miR-576-5p was associated with increased expression of ANXA2, a target gene implicated in promoting apoptosis and reducing osteogenic differentiation of BMSCs. Importantly, the upregulation of miR-576-5p or the downregulation of ANXA2 demonstrated a protective effect against glucocorticoid-induced damage, as evidenced by enhanced proliferation, reduced apoptosis, improved osteogenic differentiation, and the restoration of the OPG/RANK.
miR-576-5p has been reported to play an important role in different human cancers, for example, miR-576-5p facilitated aggressive cell behaviors in colon adenocarcinoma via targeting NEGR130, miR-576-5p promoted endometrial cancer cell growth and metastasis by targeting ZBTB431. However, the role of miR-576-5p in bone metabolism was unclear until now. Although miR-576-5p has been reported to be downregulated in atrophic non-union after fractures13, the specific mechanism has not been illustrated. The results of this study suggested that miR-576-5p plays a crucial role in promoting BMSC proliferation and osteoblast differentiation while inhibiting BMSC apoptosis, likely through its regulation of the target gene ANXA2.
As a multifunctional member of the annexin protein, there has been increasing recognition of the significant role of ANXA2 in inhibiting apoptosis and promoting immune evasion in tumor cells32, in neurodegenerative processes33, and in immune response34. In addition, ANXA2 has been extensively studied in the context of BMSC-mediated bone remodeling. However, its effects on BMSCs remain controversial. Under high-glucose conditions, ANXA2 was shown to restore osteogenic differentiation and mitigate cellular senescence in mesenchymal stem cells (MSCs)35. Conversely, Chen et al. reported that ANXA2 promoted apoptosis, suppressed proliferation, and reduced osteocalcin expression in tumor necrosis factor (TNF)-treated MSCs17. This study provides additional evidence of the negative effects of ANXA2 on BMSCs, demonstrating that ANXA2 inhibits BMSC proliferation and osteoblast differentiation. These findings contribute to a better understanding of the dual role of ANXA2 in BMSC biology and highlight its context-dependent effects.
Apoptosis, the most common form of programmed cell death, is tightly regulated by various cellular responses36. The Bcl-2 protein family, exemplified by Bcl-2 and Bax, plays a critical role in the initiation of irreversible mitochondrial outer membrane permeability37. High Bax expression sensitizes cells to death signals and promotes apoptosis, whereas high Bcl-2 expression forms heterodimers with Bax, thereby inhibiting apoptosis37. Previous studies have demonstrated that GC can induce BMSC apoptosis by increasing Bax levels and decreasing Bcl-2 levels38, findings that are consistent with the results of this study. Notably, miR-576-5p was found to counteract these effects by reducing Bax mRNA expression and enhancing Bcl-2 mRNA expression in DEX-treated BMSCs. This modulation of apoptotic gene expression may underlie the protective role of miR-576-5p in promoting BMSC survival under DEX-induced stress.
The RANK/RANKL/OPG signaling pathway is a critical regulator of bone resorption and formation39. RANK and its ligand RANKL, members of the TNF ligand family, are key factors in the survival and differentiation of osteoclasts. Through a signaling cascade, RANKL activates osteoclast maturation and differentiation, promoting bone resorption, disrupting the balance between osteoblasts and osteoclasts, accelerating apoptosis, and contributing to bone metabolism disorders40. In contrast, OPG competes with RANK to bind RANKL, thereby inhibiting osteoclast activation, differentiation, and maturation. This action prevents excessive bone resorption and maintains bone homeostasis41. RANK/RANKL/OPG signaling also plays a central role in the molecular mechanism of SONFH42,43,44. GC can promote bone resorption and osteoclastogenesis by increasing RANKL expression and decreasing OPG expression in osteoblast lineage cells45. This study corroborated these findings by demonstrating that DEX can suppress OPG expression while promoting RANKL expression in BMSCs during osteoblast differentiation. Interestingly, miR-576-5p was shown to counteract the effects of DEX, as evidenced by upregulated OPG and downregulated RANKL levels. These results suggest that miR-576-5p exerts an inhibitory effect on bone resorption in SONFH, highlighting its potential as a therapeutic target.
This study has several limitations. Firstly, except for the role of exogenous signals, miRNAs and their targets were also regulated by other RNAs, which was defined as competitive endogenous RNA (ceRNA) mechanism46,47. The role of miR-576-5p could also be regulated by other RNAs, as reported previously, long non-coding RNAs (lncRNA) including Linc0113348, LincPINT49 and LincCASC950 could directly bind to microRNA-576-5 to regulate its target mRNAs. Circular RNAs (circRNA), such as circ_008821211, circ_000144551, circ_003707852, could also act as ‘ceRNAs’ of miR-576-5p to affect the downstream signaling pathways. The ceRNA mechanism also exists in the fate regulation of BMSCs, For example, the lncRNA-PART1 contributed to osteogenic differentiation and inhibited the hBMSCs apoptosis via targeting miR-185-5p to influence RUNX3 level53, the circRNA CircCOX6A154 negatively regulated BMSC osteogenic differentiation through the miR-512-3p/DYRK2 axis. This study just focused on the role of miR-576-5p and its target ANXA2 in DEX-induce BMSC injury, and did not investigate the role of other endogenous factors, which provided limited information for SONFH. Secondly, as a commonly used long-acting glucocorticoid drug, DEX is often employed in cellular research to explore the effect of glucocorticoids. In terms of BMSCs, many reports have demonstrated the time-dependent and dose-dependent variability with DEX induction25. For example, continuous treatment with low dose Dex(10−8M,10−7M) facilitated osteogenic differentiation of BMSCs in the proper induction medium, while long exposure to higher concentration (10−6M and 10−5M) inhibited osteogenic differentiation obviously25,55,56. Therefore, in accordance with previous studies, this study just used 10−7M DEX in the osteogenic induction medium and 10−5M DEX to explore BMSC injury by glucocorticoids, without study on continuous gradient DEX concentration. Thirdly, this study just explored the role of miR-576-5p and ANXA2 on DEX treated BMSCs invitro, further invivo study should be focused to detect the protective role of miR-576-5p against GC-induced damage in SONFH.
In conclusion, GC can cause significant damage to BMSCs, leading to suppressed cell proliferation and osteogenic differentiation, increased apoptosis, and enhanced pro-osteoclastogenic activity. These detrimental effects disrupt the balance between bone formation and resorption, contributing to bone metabolism disorders of SONFH. This study highlights the protective role of miR-576-5p in mitigating glucocorticoid-induced BMSC injury. Elevating miR-576-5p levels not only improved BMSC proliferation and osteogenic differentiation but also reduced apoptosis and inhibited pro-osteoclastogenesis by targeting ANXA2. In addition, miR-576-5p effectively restored the balance of the RANK/RANKL/OPG signaling pathway via downregulating ANXA2, further reducing bone resorption, and preserving bone homeostasis. These findings suggest that miR-576-5p upregulation represents a promising therapeutic strategy for counteracting GC-induced damage in SONFH. Future research should focus on validating these results in vivo and exploring the translational potential of miR-576-5p-based interventions, including their efficacy, safety, and delivery mechanisms in clinical settings.
Data availability
The data of this study is available from the corresponding author on reasonable request.
Abbreviations
- SONFH:
-
Steroid-induced osteonecrosis of the femoral head
- ANXA2:
-
Annexin A2
- GC:
-
Glucocorticoid
- BMSC:
-
Bone marrow derived mesenchymal stem cell
- miRNA:
-
microRNA
- THA:
-
Total hip arthroplasty
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- FBS:
-
Fetal Bovine Serum
- OPG:
-
Osteoprotegerin
- RANKL:
-
Receptor Activator of Nuclear Factor Kappa-Β Ligand
- GAPDH:
-
Glyceraldehyde 3-Phosphate Dehydrogenase
- ceRNA:
-
Competitive endogenous RNA
References
Huang, C., Qing, L., Xiao, Y., Tang, J. & Wu, P. Insight into Steroid-Induced ONFH: the molecular mechanism and function of epigenetic modification in mesenchymal stem cells. Biomolecules 14, 4. https://doi.org/10.3390/biom14010004 (2023).
Zhang, J., Cao, J., Liu, Y. & Zhao, H. Advances in the pathogenesis of Steroid-Associated osteonecrosis of the femoral head. Biomolecules 14, 667. https://doi.org/10.3390/biom14060667 (2024).
Chen, L. et al. STAT3 activation by Catalpol promotes osteogenesis-angiogenesis coupling, thus accelerating osteoporotic bone repair. Stem Cell. Res. Ther. 12, 108. https://doi.org/10.1186/s13287-021-02178-z (2021).
Wang, G., Wang, F., Zhang, L., Yan, C. & Zhang, Y. miR-133a Silencing rescues glucocorticoid-induced bone loss by regulating the MAPK/ERK signaling pathway. Stem Cell. Res. Ther. 12, 215. https://doi.org/10.1186/s13287-021-02278-w (2021).
Zhang, Y. L. et al. Vitamin K2 prevents Glucocorticoid-induced osteonecrosis of the femoral head in rats. Int. J. Biol. Sci. 12, 347–358. https://doi.org/10.7150/ijbs.13269 (2016).
Zhang, S. et al. Recent advances in osteonecrosis of the femoral head: a focus on mesenchymal stem cells and adipocytes. J. Transl Med. 23, 592. https://doi.org/10.1186/s12967-025-06564-6 (2025).
Guzmán-Morales, J. et al. Effect of Chitosan particles and dexamethasone on human bone marrow stromal cell osteogenesis and angiogenic factor secretion. Bone 45, 617–626. https://doi.org/10.1016/j.bone.2009.06.014 (2009).
Ma, T. et al. Research progress in the pathogenesis of hormone-induced femoral head necrosis based on microvessels: a systematic review. J. Orthop. Surg. Res. 19, 265. https://doi.org/10.1186/s13018-024-04748-2 (2024).
Zhang, X., You, J. M., Dong, X. J. & Wu, Y. Administration of mircoRNA-135b-reinforced exosomes derived from MSCs ameliorates glucocorticoid-induced osteonecrosis of femoral head (ONFH) in rats. J. Cell. Mol. Med. 24, 13973–13983. https://doi.org/10.1111/jcmm.16006 (2020).
Cao, Y. et al. Reciprocal effect of microRNA-224 on osteogenesis and adipogenesis in steroid-induced osteonecrosis of the femoral head. Bone 145, 115844. https://doi.org/10.1016/j.bone.2021.115844 (2021).
Yang, X. & Sun, P. Circ_0088212 targeting miR-576-5p/FKBP1A axis inhibits osteosarcoma progression. Ann. Clin. Lab. Sci. 53, 548–561 (2023).
Díaz-Prado, S. et al. Characterization of MicroRNA expression profiles in normal and Osteoarthritic human chondrocytes. BMC Musculoskelet. Disord. 13, 144. https://doi.org/10.1186/1471-2474-13-144 (2012).
Chen, H., Ji, X., She, F., Gao, Y. & Tang, P. miR-628-3p regulates osteoblast differentiation by targeting RUNX2: possible role in atrophic non-union. Int. J. Mol. Med. 39, 279–286. https://doi.org/10.3892/ijmm.2016.2839 (2017).
Mondal, S., Rathor, R., Singh, S. N. & Suryakumar, G. MiRNA and leptin signaling in metabolic diseases and at extreme environments. Pharmacol. Res. Perspect. 12, e1248. https://doi.org/10.1002/prp2.1248 (2024).
Christofidis, K. et al. Annexin A2 in tumors of the Gastrointestinal tract, liver, and pancreas. Cancers (Basel). 16, 3764. https://doi.org/10.3390/cancers16223764 (2024).
Zhou, X. et al. Anxa2 attenuates osteoblast growth and is associated with hip BMD and osteoporotic fracture in Chinese elderly. PLoS One. 13, e0194781. https://doi.org/10.1371/journal.pone.0194781 (2018).
Chen, G. et al. MicroRNA-425-5p modulates osteoporosis by targeting Annexin A2. Immun. Ageing. 18 https://doi.org/10.1186/s12979-021-00256-7 (2021).
Menaa, C. et al. Annexin II increases osteoclast formation by stimulating the proliferation of osteoclast precursors in human marrow cultures. J. Clin. Invest. 103, 1605–1613. https://doi.org/10.1172/jci6374 (1999).
Takahashi, S. et al. Cloning and identification of Annexin II as an autocrine/paracrine factor that increases osteoclast formation and bone resorption. J. Biol. Chem. 269, 28696–28701 (1994).
Leandro, M. P. et al. Polymorphisms and avascular necrosis in patients with sickle cell disease - A systematic review. Rev. Paul Pediatr. 40, e2021013. https://doi.org/10.1590/1984-0462/2022/40/2021013in (2022).
Li, Z. et al. Emerging roles of long non-coding RNAs in osteonecrosis of the femoral head. Am. J. Transl Res. 12, 5984–5991 (2020).
Zhao, Y. et al. Effects of Puerarin-Loaded tetrahedral framework nucleic acids on osteonecrosis of the femoral head. Small 19, e2302326. https://doi.org/10.1002/smll.202302326 (2023).
Zhou, D. et al. Valproic acid prevents glucocorticoid–induced osteonecrosis of the femoral head of rats. Int. J. Mol. Med. 41, 3433–3447. https://doi.org/10.3892/ijmm.2018.3534 (2018).
Zeng, C. et al. Galangin mitigates glucocorticoid-induced osteoporosis by activating autophagy of BMSCs via triggering the PKA/CREB signaling pathway. Acta Biochim. Biophys. Sin (Shanghai). 55, 1275–1287. https://doi.org/10.3724/abbs.2023063 (2023).
Li, T., Xu, Y., Wang, Y. & Jiang, Y. Differential expression profiles of long noncoding RNAs and mRNAs in human bone marrow mesenchymal stem cells after exposure to a high dosage of dexamethasone. Stem Cell. Res. Ther. 12, 9. https://doi.org/10.1186/s13287-020-02040-8 (2021).
Amin, N., Boccardi, V., Taghizadeh, M. & Jafarnejad, S. Probiotics and bone disorders: the role of RANKL/RANK/OPG pathway. Aging Clin. Exp. Res. 32, 363–371. https://doi.org/10.1007/s40520-019-01223-5 (2020).
Li, W. et al. Exogenous melatonin ameliorates steroid-induced osteonecrosis of the femoral head by modulating ferroptosis through GDF15-mediated signaling. Stem Cell. Res. Ther. 14, 171. https://doi.org/10.1186/s13287-023-03371-y (2023).
Yang, N. et al. MAGL Blockade alleviates steroid-induced femoral head osteonecrosis by reprogramming BMSC fate in rat. Cell. Mol. Life Sci. 81, 418. https://doi.org/10.1007/s00018-024-05443-5 (2024).
Li, Z. et al. Emerging roles of MicroRNAs in osteonecrosis of the femoral head. Cell. Prolif. 51, e12405. https://doi.org/10.1111/cpr.12405 (2018).
Jin, L., Li, X., Zhao, Y., Zhu, G. & Shen, W. miR-576-5p facilitates aggressive cell behaviors in colon adenocarcinoma via targeting NEGR1. Crit. Rev. Eukaryot. Gene Expr. 32, 25–33. https://doi.org/10.1615/CritRevEukaryotGeneExpr.2022043160 (2022).
Chen, C., Zhang, Q. & Kong, B. miRNA-576-5p promotes endometrial cancer cell growth and metastasis by targeting ZBTB4. Clin. Transl Oncol. 25, 706–720. https://doi.org/10.1007/s12094-022-02976-8 (2023).
Huang, L. et al. ANXA2 in cancer: aberrant regulation of tumour cell apoptosis and its immune interactions. Cell. Death Discov. 11, 174. https://doi.org/10.1038/s41420-025-02469-x (2025).
Partevian, S. A., Slominsky, P. A., Shadrina, M. I. & Alieva, A. K. ANXA2 protein and its role in neurodegeneration processes. Life (Basel). 15, 402. https://doi.org/10.3390/life15030402 (2025).
Huang, Y. et al. Annexin A2: the diversity of pathological effects in tumorigenesis and immune response. Int. J. Cancer. 151, 497–509. https://doi.org/10.1002/ijc.34048 (2022).
Klabklai, P. et al. Annexin A2 improves the osteogenic differentiation of mesenchymal stem cells exposed to High-Glucose conditions through lessening the senescence. Int. J. Mol. Sci. 23, 12521. https://doi.org/10.3390/ijms232012521 (2022).
Liang, Z. et al. Bone-Differentiation-Associated Circ-Spen regulates death of mouse bone marrow mesenchymal stem cells by inhibiting apoptosis and promoting autophagy. Int. J. Mol. Sci. 25, 3034. https://doi.org/10.3390/ijms25053034 (2024).
Shan, H. et al. Effects of Astragaloside IV on glucocorticoid-induced avascular necrosis of the femoral head via regulating Akt-related pathways. Cell. Prolif. 56, e13485. https://doi.org/10.1111/cpr.13485 (2023).
Jia, B. et al. Baicalin attenuates dexamethasone-induced apoptosis of bone marrow mesenchymal stem cells by activating the Hedgehog signaling pathway. Chin. Med. J. (Engl). 136, 1839–1847. https://doi.org/10.1097/cm9.0000000000002113 (2023).
Kenkre, J. S. & Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 55, 308–327. https://doi.org/10.1177/0004563218759371 (2018).
Cheon, Y. H. et al. Pitavastatin prevents ovariectomy-induced osteoporosis by regulating osteoclastic resorption and osteoblastic formation. Biomed. Pharmacother. 139, 111697. https://doi.org/10.1016/j.biopha.2021.111697 (2021).
Cheng, C. H., Chen, L. R. & Chen, K. H. Osteoporosis due to hormone imbalance: an overview of the effects of Estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci. 23, 1376. https://doi.org/10.3390/ijms23031376 (2022).
Fu, D. et al. Proper mechanical stress promotes femoral head recovery from steroid-induced osteonecrosis in rats through the OPG/RANK/RANKL system. BMC Musculoskelet. Disord. 21, 281. https://doi.org/10.1186/s12891-020-03301-6 (2020).
Zhou, Z. et al. IL-15 deficiency alleviates steroid-induced osteonecrosis of the femoral head by impact osteoclasts via RANKL-RANK-OPG system. Immun. Ageing. 17 https://doi.org/10.1186/s12979-020-00190-0 (2020).
Sun, M. et al. DNA methylation in the OPG/RANK/RANKL pathway is associated with steroid-induced osteonecrosis of the femoral head. BMC Musculoskelet. Disord. 22, 599. https://doi.org/10.1186/s12891-021-04472-6 (2021).
Wang, L. T., Chen, L. R. & Chen, K. H. Hormone-Related and Drug-Induced osteoporosis: A cellular and molecular overview. Int. J. Mol. Sci. 24, 5814. https://doi.org/10.3390/ijms24065814 (2023).
An, F. et al. Research progress on the role of lncRNA-miRNA networks in regulating adipogenic and osteogenic differentiation of bone marrow mesenchymal stem cells in osteoporosis. Front. Endocrinol. (Lausanne). 14, 1210627. https://doi.org/10.3389/fendo.2023.1210627 (2023).
Salmena, L., Poliseno, L., Tay, Y., Kats, L. & Pandolfi, P. P. A CeRNA hypothesis: the Rosetta stone of a hidden RNA language? Cell 146, 353–358. https://doi.org/10.1016/j.cell.2011.07.014 (2011).
Ghafouri-Fard, S., Khoshbakht, T., Hussen, B. M., Taheri, M. & Mokhtari, M. A review on the role of LINC01133 in cancers. Cancer Cell. Int. 22, 270. https://doi.org/10.1186/s12935-022-02690-z (2022).
Li, D. & Hu, A. LINC-PINT suppresses breast cancer cell proliferation and migration via MEIS2/PPP3CC/NF-κB pathway by sponging miR-576-5p. Am. J. Med. Sci. 367, 201–211. https://doi.org/10.1016/j.amjms.2023.08.013 (2024).
Liu, H. Z., Shan, T. D., Han, Y. & Liu, X. S. Silencing long non-coding RNA CASC9 inhibits colorectal cancer cell proliferation by acting as a competing endogenous RNA of miR-576-5p to regulate AKT3. Cell. Death Discov. 6, 115. https://doi.org/10.1038/s41420-020-00352-5 (2020).
Wu, Y. et al. Hsa_circ_0001445 works as a cancer suppressor via miR-576-5p/SFRP1 axis regulation in ovarian cancer. Cancer Med. 12, 5736–5750. https://doi.org/10.1002/cam4.5317 (2023).
Zou, H. & Mao, Q. Circ_0037078 promotes trophoblast cell proliferation, migration, invasion and angiogenesis by miR-576-5p/IL1RAP axis. Am. J. Reprod. Immunol. 87, e13507. https://doi.org/10.1111/aji.13507 (2022).
Zhang, J., Xu, N., Yu, C., Miao, K. & Wang, Q. LncRNA PART1/miR-185-5p/RUNX3 feedback loop modulates osteogenic differentiation of bone marrow mesenchymal stem cells. Autoimmunity 54, 422–429. https://doi.org/10.1080/08916934.2021.1966771 (2021).
He, D. et al. CircCOX6A1 suppresses osteogenic differentiation and aggravates osteoporosis via miR-512-3p/DYRK2 axis. Mol. Biol. Rep. 51, 636. https://doi.org/10.1007/s11033-024-09532-3 (2024).
Ma, L. et al. Dexamethasone promotes mesenchymal stem cell apoptosis and inhibits osteogenesis by disrupting mitochondrial dynamics. FEBS Open. Bio. 10, 211–220. https://doi.org/10.1002/2211-5463.12771 (2020).
Xiao, Y., Peperzak, V., van Rijn, L., Borst, J. & de Bruijn, J. D. Dexamethasone treatment during the expansion phase maintains stemness of bone marrow mesenchymal stem cells. J. Tissue Eng. Regen Med. 4, 374–386. https://doi.org/10.1002/term.250 (2010).
Funding
This work was funded by the Natural Science Foundation of Anhui Province (2308085MH248); Anhui Province University Research Project (2022AH051188).
Author information
Authors and Affiliations
Contributions
YZ and YD designed and executed the experiments, WY, LZ and KX helped in statistical analysis and cell experiments, and contributed towards writing manuscript, GW is the corresponding author, contributed in conceiving and designing the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study received approval from the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (2022144) and adhered to the World Medical Association Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual patients with THA operation included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Deng, Y., Yao, W. et al. microRNA-576-5p ameliorates dexamethasone-induced BMSC injury by suppressing ANXA2. Sci Rep 15, 30612 (2025). https://doi.org/10.1038/s41598-025-16883-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-16883-9