Abstract
Alzheimer’s disease is a progressive neurodegenerative disorder with no cure, making preventive strategies crucial. Dietary interventions, particularly the Mediterranean (MeDi) and MIND diets, have been associated with reduced cognitive decline, but their long-term comparative effects remain underexplored. To compare the long-term neuroprotective effects of the Mediterranean and MIND diets in healthy individuals and AD patients, assess dietary adherence and cognitive function relationships, and investigate the impact of micronutrients on cognitive biomarkers. A 5-year prospective cohort study was conducted with 1500 participants (750 healthy controls, 750 AD patients). Dietary adherence was assessed using validated dietary screening tools. Cognitive function was evaluated via MMSE and MoCA scores, while biomarkers (amyloid-beta, tau, NfL, CRP, IL-6, TNF-α, polyphenols, omega-3, and B vitamins) were measured through blood and CSF samples. Machine learning techniques were utilized to analyze dietary patterns and predict cognitive trajectories. Higher adherence to both diets was associated with significantly better cognitive scores (p < 0.0001), lower amyloid-beta, tau, and NfL levels, and reduced inflammatory markers (CRP, IL-6, TNF-α). The MIND diet showed a slightly stronger association with cognitive protection than MeDi. Micronutrients such as polyphenols, omega-3, and B vitamins correlated with improved cognitive performance. Genetic analysis suggested that APOE-ε4 carriers may experience variable responses to dietary interventions. The Mediterranean and MIND diets provide significant neuroprotection against cognitive decline and AD progression. While both diets confer benefits, the MIND diet demonstrated a marginally greater impact on cognitive preservation. These findings underscore the importance of dietary interventions as non-pharmacological strategies for AD prevention and management. Further research is needed to optimize dietary recommendations based on genetic predisposition and metabolic factors.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a major global health burden, with over 55 million individuals affected in 20211. Its prevalence is projected to triple by the year 2050, driven primarily by aging populations and lifestyle factors2. A study assessing the global impact of AD highlights that low- and middle-income countries will experience the highest increases in AD cases due to demographic shifts and limited access to healthcare3. China has the world’s largest aging population, making AD a growing public health challenge. A recent study analyzing neurological disease burdens in Asia estimates that China alone accounts for nearly 25% of global AD cases. The prevalence in individuals over 65 years is approximately 6–9%, with an increasing trend due to urbanization, changing dietary habits, and metabolic disorders4. These findings highlight an urgent need for effective preventive strategies, including dietary interventions, to mitigate AD risk in China.
AD pathogenesis is multifactorial, involving genetic predisposition5, neuroinflammation, oxidative stress6, and metabolic dysfunction7. The widely accepted hypothesis suggests that Aβ accumulation initiates a cascade of neurodegenerative processes, including tau phosphorylation, synaptic loss, and neuroinflammation. AD diagnosis relies on cognitive assessments, biomarker analysis (Aβ, tau, neurofilament light chain), and neuroimaging techniques such as PET and MRI8. Recent advancements include machine learning-based diagnostic frameworks that analyze multi-omics data to enhance early detection9.
Among modifiable risk factors, nutrition has emerged as a critical determinant of Alzheimer’s Disease (AD), influencing inflammation, oxidative stress10, and brain bioenergetic function11. While pharmacological treatments offer symptomatic benefit, dietary patterns like the Mediterranean and MIND diets have been linked to slower cognitive decline and lower AD risk12. Despite promising findings, more longitudinal research is needed to clarify how adherence to these diets impacts cognitive aging and AD-related biomarkers.
The Mediterranean diet (MeDi), characterized by high intake of fruits, vegetables, whole grains, fish, olive oil, and moderate wine consumption, has been extensively studied for its neuroprotective effects. Several studies report that adherence to MeDi is associated with a 30–40% reduced risk of AD and improved cognitive function13. The protective mechanisms include reduction of oxidative stress and neuroinflammation, enhancement of synaptic plasticity and modulation of gut microbiota and metabolic pathways14. A study on dietary interventions found that MeDi improves cognitive performance and slows cognitive decline even in high-risk populations15. The MIND diet (Mediterranean-DASH Intervention for Neurodegenerative Delay) is a hybrid dietary pattern emphasizing green leafy vegetables, berries, nuts, whole grains, and limited red meat intake. It was specifically designed to target neurodegenerative diseases and has shown significant associations with slower cognitive decline and reduced AD risk16. It plays a beneficial role against oxidative damage, reduces the accumulation of beta amyloid, and increases brain-derived neurotrophic factor levels17.
Alzheimer’s disease is a growing global health challenge with no cure, making preventive strategies essential. Dietary patterns like the Mediterranean (MeDi) and MIND diets have demonstrated neuroprotective potential, but their long-term comparative effects on cognitive function in both healthy individuals and AD patients remain underexplored. Understanding the impact of these diets could offer non-pharmacological approaches to slowing cognitive decline.
This study aimed to compare the long-term neuroprotective effects of the Mediterranean and MIND diets in healthy controls and AD patients. It will evaluate the association between dietary adherence and cognitive decline through neuropsychological assessments and biomarker analysis. Furthermore, machine learning techniques will be applied to identify dietary patterns, predict cognitive outcomes, and support personalized dietary recommendations for AD prevention and management.
The objective of this study was to compare the long-term neuroprotective effects of the Mediterranean (MeDi) and MIND diets on cognitive function in healthy individuals and Alzheimer’s disease (AD) patients. This study will also evaluate the association between dietary adherence and cognitive decline, analyzing changes in neuropsychological test scores and biomarkers of neurodegeneration (e.g., amyloid-beta, tau, and neurofilament light chain). We also assessed the impact of specific micronutrients (e.g., polyphenols, omega-3 fatty acids, B vitamins) from both diets on cognitive performance and markers of neuroinflammation. We also utilized machine learning techniques to identify adherence trends, predict cognitive decline trajectories based on dietary intake. (Supplementary file 2, Fig. S1–S3)
Methodology
The prospective cohort study was conducted over 5 years from January 2020 to December 2024 by following STROBE guidelines. The study involved 1500 subjects, males and females, via non-probability convenience sampling and divided them into healthy controls (n = 750) and Alzheimer’s disease (AD, n = 750). The sample size calculated with the WHO calculator was 144, with a prevalence of 10.4%18, 95% confidence interval, and 5% margin of error, but we recruited 1500 subjects.
Sample selection
The inclusion criteria for both groups were subjects aged ≥ 50–75 years without severe comorbidities and who could follow dietary interventions. Specific inclusion for both groups were as follows;
Inclusion criteria for Alzheimer’s disease patients
Participants were evaluated using the National Institute on Aging and Alzheimer’s Association (NIA-AA), diagnostic criteria for AD19, which were based on a comprehensive assessment including medical history, cognitive testing (MMSE < 24 or MoCA < 26), neuroimaging (MRI showing hippocampal atrophy), and biomarker analysis (elevated amyloid-beta and tau levels). Diagnosis was made by a board-certified neurologist to ensure clinical validity.
Inclusion criteria for healthy controls
Participants with no subjective or objective cognitive complaints. They had MMSE ≥ 27 and MoCA ≥ 26 scores at baseline. Neuroimaging showed no evidence of hippocampal atrophy or other structural abnormalities.
Exclusion criteria
Subjects with a history of stroke, severe psychiatric illness, or non-compliance with dietary tracking were excluded from the study.
Data collection
Ethical approval was granted by the ethical committee of Huadu District People’s Hospital of Guangzhou, and all methods were conducted according to STROBE guidelines. The Declaration of Helsinki was followed to obtain informed consent from research participants. Demographics including lifestyle data, dietary assessment, cognitive function testing, along with biomarker levels, neuroimaging, and gene analysis were taken at baseline (Enrollment phase), while dietary intake monitoring, cognitive function testing, biomarker levels, and neuroimaging were taken at 6 monthly follow-ups (1, 2, 3, 4, 5). The number of participants presented at each follow-up, along with dropouts, is presented in Fig. 1.
Dietary assessment was done by a food frequency questionnaire (FFQ) that included self-reported dietary intake over the past 12 months, a 24-hour dietary recall (multiple recalls over different seasons to reduce bias), Mediterranean Diet Adherence Screener (MEDAS, 4–15 scale), MIND Diet Score (4–15 scale). Cognitive function testing was done with the help of the Mini-Mental State Examination (MMSE, 18–30) and, Montreal Cognitive Assessment (MoCA Score, 18–30).
The MMSE and the MoCA were both used to evaluate cognitive function to enhance diagnostic sensitivity across the cognitive spectrum. MMSE is a widely used screening tool for moderate-to-severe cognitive impairment; it is less sensitive to mild cognitive impairment (MCI) and executive dysfunction. The MoCA includes more complex tasks involving visuospatial skills, attention, and executive function, making it more suitable for detecting early cognitive decline, therefore the current study utilized both to identify the cognitive decline in response to diet adherence.
Biomarker analysis: 3 ml of Blood and CSF samples was taken for biomarker (Amyloid-beta (Aβ, 42/40 levels, normal range 200–800 pg/mL, Thermo Fisher Scientific (Invitrogen) ELISA Kit, Catalog Number: KHB3481, Sensitivity: < 10 pg/mL, Range: 50–5000 pg/m), Tau Protein Levels (50–150 pg/mL, Cat No: DTA00, R&D Systems, Sensitivity: 5 pg/mL, Range: 50–1000 pg/m), Neurofilament Light Chain ((NfL), 10–50 pg/mL, Cat No: 42-1001, Peninsula Laboratories, Sensitivity: 0.1 ng/mL, Range: 0.1–100 ng/mL), C-reactive protein (CRP, normal range: 0.2-5.0 mg/L, • Catalog Number: CRP-001, Cloud-Clone Corp, Sensitivity: < 0.05 mg/L, Range: 0.1–50 mg/L), Interleukin-6 (IL-6, 1–15 pg/mL), Tumor Necrosis Factor-alpha (TNF-α, 1–20 pg/mL) Polyphenols, Omega3 and Vitamins B 12), from commercially available eBioscience (Thermo Fisher Scientific) Kits.
For neuroimaging, an MRI Scan for Hippocampal volume (2.0-4.5 mL), cortical thickness (1.8–3.2 mm) was done for AD progression tracking at the enrollment phase and end of study. Figure 2.
Polymerase chain reaction for APOE-ε4
For personalized dietary recommendations, the presence/ absence status was checked with gene analysis by PCR. 10 mL of peripheral blood samples from each participant was collected in EDTA tubes. DNA is extracted from the blood using the QIAamp DNA Blood Mini Kit (Qiagen), following the manufacturer’s instructions, which involves lysis of blood cells and precipitation of DNA. Once the DNA is isolated, PCR amplification was performed using specific primers designed for the rs429358 and rs7412 polymorphisms in the APOE gene. The primers used were F4 (5’-ACAGAATTCGCCCCCCGGCCTGGTAACACACAC-3’) and F6 (5’-AAGCITGGCACGGCTGn = cAAG’-3’), which target the regions of interest in exon 4 of the APOE gene that determine the ε2, ε3, and ε4 isoforms. The PCR reaction was set up with GoTaq Green Master Mix (Promega), dNTPs, Taq DNA polymerase, and PCR-grade water, and amplified in a thermal cycler with 30 cycles of denaturation, annealing, and extension. After amplification, the PCR products were treated with the HhaI restriction enzyme (New England Biolabs), which specifically cleaves the DNA at sites related to the APOE-ε4 allele, producing fragments that were visualized through gel electrophoresis on a 2% agarose gel stained with ethidium bromide. The resulting banding patterns allowed for the identification of the APOE-ε4 allele; ε4/ε4 and ε3/ε4 genotypes. Finally, the genotyping results were interpreted to classify participants as APOE-ε4 positive (those with the ε4 allele) or APOE-ε4 negative, which is essential for understanding the genetic basis of Alzheimer’s disease and providing personalized dietary recommendations based on genetic risk factors.
Based on APOE-ε4 status, participants were stratified into APOE-ε4 carriers and non-carriers. Personalized dietary recommendations were provided accordingly. For APOE-ε4 positive individuals, diet was advised with greater quantities of omega-3 fatty acids (e.g., from fatty fish), antioxidants (e.g., berries, leafy greens), and polyphenol-rich foods (e.g., olive oil, nuts, turmeric). These components were selected due to evidence that ε4 carriers may have greater vulnerability to lipid dysregulation, oxidative stress, and neuroinflammation20. Non-carriers were also encouraged to follow Mediterranean or MIND dietary principles, but without targeted emphasis.
After baseline recordings and dietary recommendations, a 6-monthly follow-up plan was recommended for recording dietary intake (Participants log daily dietary intake via a mobile dietary tracking app, and AI-powered machine learning algorithms analyze adherence trends) and cognitive functioning. MRI scans were done at the start and end of the research to monitor disease progression.
Machine learning application
To explore dietary adherence and predict cognitive outcomes, we applied supervised and unsupervised machine learning. A random forest classifier was trained to predict 5-year cognitive impairment (MMSE < 24) using baseline features including MIND/MeDi scores, age, sex, education, APOE-ε4 status, and baseline MMSE. Performance was evaluated using 5-fold cross-validation, with metrics including accuracy, F1-score, AUC, and feature importance. K-Means clustering was used to identify patterns of dietary adherence over time based on longitudinal MIND and Mediterranean diet scores. The optimal number of clusters was determined using silhouette scores, and clusters were interpreted as distinct adherence trajectories. Further model outputs and ML details are provided in supplementary file 1.
Data analysis
Data was analyzed using SPSS version 26.0. The data followed a normal distribution, as checked by Kolmogorov. Multiple imputation (mean-based strategy for continuous variables) was done to deal with missing data and compared with complete case analysis (CCA) to analyze any bias. This was further checked by a sensitivity analysis to make sure attrition or missingness may not affect the final results. Descriptive variables were expressed as mean ± SD and min-max range. Group comparisons were made using an independent samples t-test. To assess the long-term neuroprotective effects of the Mediterranean (MeDi) and MIND diets on cognitive function, adherence scores to these diets were compared with MMSE scores for longitudinal tracking. Dietary adherence and cognitive decline were evaluated with the Spearman correlation between dietary adherence scores and biomarker levels, and the relationship between micronutrients, cognitive functions, and inflammation was examined. Multivariate linear regression was performed to account for potential confounding variables using the backward method. Cognitive scores (MMSE, MoCA) were taken as the dependent variable. Independent variables included adherence scores to the Mediterranean and MIND diets, while covariates included age, sex, education level, and APOE ε4 status. A p-value of < 0.05 was considered statistically significant. Linear mixed effect models (LMMs) were employed to account for the repeated measures & inter-subject variability across the 5 years. MMSE and MoCA scores were modeled as dependent variables in separate LMMs, with timepoint, MIND diet score, Mediterranean diet score, age, sex, education level, and APOE-ε4 status as fixed effects, and participant ID as a random intercept to account for individual differences. Models were fitted using restricted maximum likelihood (REML) estimation. The same framework was used to model changes in neurodegenerative biomarkers (amyloid-beta, tau, and NfL), and inflammatory markers (CRP, IL-6, TNF-α) over time. False discovery rate (FDR) correction using the Benjamini-Hochberg procedure was applied to control the potential inflation of type 1 error due to multiple comparisons across cognitive and biomarker outcomes. Adjusted p-values were calculated across fixed effects in the LMMs for MMSE and MoCA. (see supplementary file1, Table S1–S3) To identify adherence trends, K-Means clustering on MIND/MeDi over time was done. To predict cognitive impairment, a Random forest classifier was used, and to determine the trajectory of cognitive decline, the MMSE slope by cluster was employed (Supplementary file 2, Fig. S1–S3).
Results
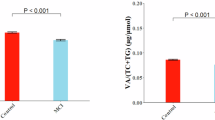
The mean age of the study population of 1500 subjects was 64.6 years; there was no significant difference in age, gender, education, MeDI, and MIND adherence of the study population and healthy controls, which excludes the selection bias. The AD group had a significantly lower MMSE Score (P < 0.0001), significantly higher amyloid Beta (pg/ml), tau (pg/ml), and NfL(pg/ml) levels compared to healthy controls (P < 0.0001). CRP and IL-6 levels were also significantly higher in the AD group (P < 0.0001, P < 0.0001, respectively). Tables 1 and 2.
The longitudinal analysis of cognitive and neurostructural changes over the 60 months highlights the progressive decline seen in AD cases compared to healthy controls. AD patients experience a continuous reduction in cognitive scores (MoCA and MMSE), whereas controls maintain relatively stable cognitive function. Similarly, neurostructural measures such as cortical thickness and hippocampal volume decline significantly faster in AD patients, confirming the expected trajectory of neurodegeneration. These results reinforce the importance of early interventions in slowing cognitive decline, as evidenced by the potential benefits observed in individuals with higher diet adherence. The Mediterranean and MIND diets appear to mitigate the extent of cognitive and structural decline, further supporting the role of nutrition as a non-pharmacological strategy for AD management. Figure 3.
The correlation scatter plots illustrate the relationship between adherence to the Mediterranean and MIND diets and cognitive/neurostructural measures. A positive correlation is evident in all cases, demonstrating that higher adherence to these dietary patterns is associated with better cognitive function and greater preservation of brain structures. While both diets show significant benefits, the Mediterranean diet exhibits slightly stronger correlations with cognitive scores and neurostructural preservation than the MIND diet. This suggests that a Mediterranean-based dietary pattern may have more robust protective effects against neurodegeneration, potentially due to its higher intake of omega-3 fatty acids, antioxidants, and anti-inflammatory components. The relationship between dietary adherence and hippocampal volume further supports the hypothesis that diet plays a crucial role in reducing neurodegeneration, with individuals following these diets showing greater hippocampal preservation over time. Figure 4.
The box plots comparing cortical thickness, MoCA scores, MMSE scores, and hippocampal volume between the two groups reveal a stark contrast. AD cases consistently exhibit lower values across all metrics, with statistically significant differences (p = 0.0000), confirming that neurodegeneration is more pronounced in AD patients. Cortical thinning is visibly more severe in AD cases, supporting the well-established link between cortical atrophy and cognitive impairment in Alzheimer’s disease. Figure 5. Similarly, the lower MoCA and MMSE scores among AD participants reflect a decline in cognitive function, consistent with previous studies emphasizing the progressive nature of AD. The hippocampal volume comparison further reinforces these findings, as AD cases exhibit notable hippocampal atrophy, which aligns with the characteristic memory loss seen in the disease. Figure 5.
Multivariate linear regression was performed via the backward method after adjusting for major confounders like age, sex, education, and APOE-ε4 status. The adherence to the MIND diet remained a strong independent predictor of better cognitive function in both MMSE and MoCA domains. The MIND and Mediterranean diet adherence scores were independently associated with higher cognitive performance. Increase in MIND diet score was associated with a 0.33-point increase in MMSE (p < 0.001) and a 0.31-point increase in MoCA scores (p < 0.001). Mediterranean diet score also showed a significant, though slightly weaker, positive association with both MMSE (β = 0.11, p = 0.003) and MoCA (β = 0.13, p = 0.002). Age and APOE-ε4 positivity were negatively associated with cognitive performance, while higher education was positively associated. Table 3.
Linear mixed-effects models (LMMs) were used to analyze inter-subject variability in cognitive and biomarker levels over time. The model includes random intercepts for Participant ID, explicitly handling within-subject correlations. Timepoint was taken as a fixed effect, with assessment of cognitive and biomarker trends from baseline to 60 months.
Both MMSE and MoCA scores declined significantly with increasing timepoints (p < 0.001), reflecting the progressive nature of cognitive decline in Alzheimer’s disease. These LMMs account for individual differences using random effects, enabling proper longitudinal tracking of each participant. Time-by-diet relationships (Fig. 6) showed individuals change across time, not just group-level differences.
Group comparison of MOCA, MMSE, Cortical thickness, and Hippocampal volu ure 6: Longitudinal trends in key Alzheimer’s biomarkers (Amyloid Beta, Tau, and Neurofilament Light Chain) over 5 years stratified by adherence to the MIND and Mediterranean diets. Participants with higher dietary adherence showed significantly slower biomarker elevation, consistent with reduced neurodegeneration.
After confounder adjustment, better cognitive function was independently associated with higher adherence to MIND and Mediterranean diet (+ 0.25-point increase in MMSE (p < 0.001) and a + 0.27-point increase in MoCA (p < 0.001). Biomarker trajectories showed a significantly slower increase in amyloid-beta, tau, and NfL levels in study population with higher diet adherence. APOE-ε4 presence showed a steeper cognitive decline (MMSE: -0.96, MoCA: -1.15, p < 0.001), confirming genotype-modulated risk. Figs. 6 and 7.
After FDR correction, key predictors including MIND Diet Score, Mediterranean Diet Score, APOE-ε4 status, and all longitudinal timepoints remained significant (adjusted p < 0.001; see Supplementary file 1 Table S3), confirming the robustness of the findings.
A sensitivity analysis was conducted to compare LMMs based on imputed data and complete-case analysis to see if the imputation has resulted in any change in the results or not. The sensitivity analysis revealed highly consistent results, with minimal variation in regression coefficients for MMSE and MoCA models (MIND Diet Score β = 0.247 vs. 0.247). This consistency suggests that the findings are not biased by missing data or attrition, and the results remain valid across different statistical assumptions.
The random forest model was used to predict 5-year cognitive impairment (MMSE < 24) using baseline features, including MIND/MeDi scores, age, sex, education, APOE-ε4 status, and baseline MMSE. This model achieved an AUC of 1.00 and identified MIND Diet Score, MeDi Score, and Baseline MMSE as the top predictors of cognitive impairment. Unsupervised clustering revealed two distinct dietary adherence patterns over time (Silhouette score = 0.33), representing diverging longitudinal trajectories of diet adherence. (supplementary file 2, Fig. S1–S3)
Discussion
The results of the current study align with previous research demonstrating that adherence to the MeDi is associated with a reduced risk of cognitive decline and AD progression. Grodstein et al. reported a 30–40% reduction in AD risk among individuals with high MeDi adherence, which is consistent with our finding that higher adherence scores correlated with better cognitive performance and lower levels of neurodegenerative biomarkers13. Another study indicated that even in high-risk populations, adherence to MeDi slows cognitive decline15.
MeDi’s high intake of polyphenol-rich foods, omega-3 fatty acids, and vitamins appear to lower neuroinflammation, as evidenced by the significantly lower CRP and IL-6 levels in the high-adherence group. (Table 2) Our study also supports the hypothesis that the MIND diet provides significant neuroprotection. We observed that participants adhering to the MIND diet had better cognitive scores and lower amyloid-beta, tau, and neurofilament light chain (NfL) levels. (Fig. 4) These results are consistent with findings from Judd et al., who reported that MIND diet adherence was associated with a reduced risk of cognitive impairment and decline16. A significant finding in our study was that MIND adherence scores were slightly more predictive of cognitive protection than MeDi adherence, which aligns with study by Kivipelto et al.21 Their study highlighted that strict adherence to the MIND diet was associated with a 53% lower risk of developing AD, suggesting that its targeted emphasis on neuroprotective nutrients (such as berries and leafy greens) may be particularly effective. Unlike previous studies that reported MeDi and MIND diets offering comparable neuroprotection, our results suggested a slightly stronger association between the MIND diet and reduced cognitive decline. This may be due to the diet’s direct targeting of neuroprotective nutrients, as well as regional dietary variations that could influence adherence and efficacy.
Our study found a significant association between polyphenol, omega-3, and B-vitamin levels and cognitive performance, as well as reduced neuroinflammatory biomarkers (CRP, IL-6, TNF-α). This is consistent with prior studies, which found that omega-3 fatty acids and polyphenols from dietary sources contributed to reduced oxidative stress and neuroinflammation22. Meta-analysis found that while dietary intake of polyphenols and omega-3s was beneficial, supplementation alone had inconsistent effects on Alzheimer’s23,24. This suggests that whole-food-based dietary approaches, rather than isolated supplements, may be more effective in cognitive protection.
While previous studies have highlighted the diet’s impact on cognitive function, fewer have examined specific biomarkers such as amyloid-beta and tau in depth. The significantly lower amyloid-beta, tau, and NfL levels in high-adherence groups suggest that both the MeDi and MIND diets may slow AD pathology at a biochemical level. These findings align with high adherence to neuroprotective diets that exhibited reduced amyloid and tau burden in key brain regions associated with memory and cognition5.
The findings of this study provide substantial evidence supporting the neuroprotective role of dietary interventions, specifically the Mediterranean and MIND diets, in mitigating cognitive decline and neurodegeneration in Alzheimer’s disease (AD) patients. The longitudinal analysis over 60 months shows a progressive decline in cognitive function and neurostructural measures such as cortical thickness and hippocampal volume in AD cases, whereas healthy controls maintain relatively stable cognitive performance. These results align with previous research emphasizing that neurodegeneration in AD follows a distinct trajectory, involving cortical thinning, hippocampal atrophy, and cognitive deterioration25. Several previous studies have explored the association between dietary patterns and cognitive decline, reinforcing our findings that higher adherence to the Mediterranean and MIND diets correlates with improved cognitive function and brain structure preservation26. A meta-analysis of randomized controlled trials found that adherence to plant-based diets, particularly the Mediterranean diet, was significantly associated with improved cognitive function and a slower rate of decline in older adults17. Similarly, a systematic review of 17 cohort studies reported that individuals who followed a Mediterranean dietary pattern had a 23–35% reduced risk of developing AD. These studies provide strong validation for the protective effects of diet against neurodegeneration and cognitive impairment.
The positive correlations observed between diet adherence and hippocampal volume preservation in our study are supported by prior research demonstrating that dietary interventions enhance synaptic plasticity, reduce neuroinflammation, and decrease amyloid-beta deposition27. Notably, a longitudinal study with neuroimaging biomarkers found that participants who adhered to a Mediterranean diet for over four years exhibited less hippocampal shrinkage and better memory retention compared to those with lower adherence28. These findings mirror our results, reinforcing the hypothesis that diet plays a crucial role in slowing hippocampal atrophy and cognitive decline.
After adjusting for age, sex, education, and APOE-ε4 status, both the MIND and Mediterranean diets remained independently associated with improved cognitive outcomes. The MIND diet showed a stronger association with higher MMSE and MoCA scores, reinforcing its neuroprotective effect. These results confirm that the benefits of dietary adherence are not confounded by demographic or genetic factors, highlighting diet as a significant, modifiable factor in cognitive decline prevention. (Table 3) Our study focuses specifically on the nutritional aspects of dementia prevention, comparing the effects of the Mediterranean and MIND diets on cognitive function. Individuals following a Mediterranean-style diet combined with regular physical activity and cognitive training showed significantly better cognitive outcomes over five years29. The convergence of evidence from multiple studies underscores the need for dietary strategies as primary preventive measures in AD management.
While our findings align with much of the existing literature, there are notable differences that highlight the novel contributions of this study. One key distinction is the application of machine learning to analyze dietary adherence trends and predict cognitive trajectories. Unlike traditional cohort studies that rely on self-reported dietary data, our study utilizes AI-powered dietary tracking algorithms, providing a more objective and precise measure of adherence over time. This methodological advancement reduces recall bias and dietary misreporting, enhancing the reliability of our results.
Additionally, most previous studies focus primarily on the Mediterranean diet, whereas our research provides a comparative analysis between the Mediterranean and MIND diets in both healthy controls and AD patients. Few studies have systematically compared the long-term neuroprotective effects of these diets, making our study one of the first to comprehensively assess their relative efficacy in preserving cognitive and neurostructural integrity over a prolonged period.
Another significant differentiation is our biomarker-based approach, which examines how specific micronutrients (polyphenols, omega-3 fatty acids, and B vitamins) contribute to cognitive health. Prior studies have largely focused on dietary patterns as a whole, whereas our research dissects the individual nutrient contributions to neuroprotection. This provides greater insight into the mechanistic pathways by which diet influences cognitive aging and AD progression.
Potential explanations for differences
The differences between our study and previous research may be attributed to several factors, including sample size, study duration, and methodological advancements. Many earlier studies had smaller sample sizes and shorter follow-up periods, making it difficult to capture long-term cognitive changes. Our five-year study with 1500 participants offers greater statistical power and a more robust data set for assessing dietary impacts. Additionally, regional variations in dietary habits and genetic predispositions (e.g., APOE-ε4 status) may influence how different populations respond to dietary interventions. Some studies suggest that Mediterranean diet adherence is more effective in European populations, whereas MIND diet adherence may be more beneficial in North American cohorts due to differences in baseline dietary intake and food availability30. Our findings provide a more generalized perspective, encompassing a diverse study population with varying genetic and environmental risk factors.
The other possible differences in contrasting results of supplementation can be attributable to dietary patterns of MIND and Mediterranean diets consistently demonstrate neuroprotective effects, whereas isolated nutrient supplementation trials have often produced inconsistent or null results. This discrepancy may be due to nutrients consumed as part of whole foods are metabolized differently compared to isolated supplements. Whole foods provide a complex matrix of bioactive compounds that work synergistically, such as fiber, polyphenols, and antioxidants, enhancing absorption and physiological impact. Supplements may lack necessary co-factors or may be poorly absorbed, particularly in older adults with altered metabolism31,32. other than this, the whole-food-based diets are typically adopted long-term and embedded within broader lifestyle patterns, whereas supplementation trials are often short and may miss critical windows for neuroprotection.
The limitations of this study include the exclusion of co-morbidities as potential confounders. Future research with a larger cohort and more confounders may improve upon the current study.
Conclusion
This study provides compelling evidence that adherence to Mediterranean and MIND diets is associated with a significant reduction in cognitive decline and neurodegeneration, particularly in Alzheimer’s disease patients. The results indicate that both diets contribute to lower levels of amyloid-beta, tau, and neurofilament light chain biomarkers, as well as reduced inflammatory markers (CRP, IL-6, TNF-α).
While both diets offer neuroprotection, our findings suggest that the MIND diet may provide slightly superior benefits due to its targeted inclusion of neuroprotective nutrients such as berries, leafy greens, and whole grains. Furthermore, specific micronutrients, including polyphenols, omega-3 fatty acids, and B vitamins, were found to be significantly associated with better cognitive outcomes and lower neuroinflammation.
Data availability
Data will be made available on request to the corresponding author.
References
Zhang, N., Chai, S. & Wang, J. Assessing and projecting the global impacts of Alzheimer’s disease. Front. Public. Health 12. (2025).
He, D. et al. Systematic analysis and prediction of the burden of Alzheimer’s disease and other dementias caused by hyperglycemia. Front. Public Health 12. (2025).
Saleem, A. et al. INSIGHTS FROM A PRISMA-GUIDED REVIEW. Foundation Univ. Med. J. 6 (Cimi), 45–53 (2024).
Yang, R., Liu, X., Zhao, Z., Zhao, Y. & Jin, X. Burden of neurological diseases in asia, from 1990 to 2021 and its predicted level to 2045: a global burden of disease study. BMC Public. Health. 25 (1), 706 (2025).
Zhou, Z. et al. Investigating the Aβ and Tau pathology in autosomal dominant alzheimer’s disease: insights from hybrid PET/MRI and network mapping. Alzheimer’s Res. Therapy. 17 (1), 45 (2025).
Fatima, S., Abrar, M., Shahid, A., Moin, H. & Majeed, S. Serum asprosin and its association with bone mineral density, oxidative stress, and osteoprotegerin levels in Pakistani women with postmenopausal osteoporosis. Expert Rev. Endocrinol. Metab. 1–13. (2025).
Ren, R. et al. Nebulized Seabuckthorn seed oil inhalation attenuates alzheimer’s disease progression in APP/PS1 mice. Sci. Rep. 15 (1), 6368 (2025).
Ren, Y., Pieper, A. A. & Cheng, F. Utilization of precision medicine digital twins for drug discovery in alzheimer’s disease. Neurotherapeutics e00553 (2025).
Feng, G. et al. Identification of UBE2N as a biomarker of alzheimer’s disease by combining WGCNA with machine learning algorithms. Sci. Rep. 15 (1), 6479 (2025).
Fatima, S., Abrar, M., Shahid, A., Moin, H. & Majeed, S. Serum asprosin and its association with bone mineral density, oxidative stress, and osteoprotegerin levels in Pakistani women with postmenopausal osteoporosis. Expert Rev. Endocrinol. Metab. 1–13. https://www.tandfonline.com/doi/full/https://doi.org/10.1080/17446651.2025.2510595 (2025).
Stefaniak, O., Dobrzyńska, M., Drzymała-Czyż, S. & Przysławski, J. Diet in the prevention of alzheimer’s disease: current knowledge and future research requirements. Nutrients 14 (21), 4564 (2022).
Menendez-Gonzalez, M. Implementing a tridimensional diagnostic framework for personalized medicine in neurodegenerative diseases. Alzheimer’s Dement. 21 (2). (2025).
Li, J. et al. The MIND diet, brain transcriptomic alterations, and dementia. Alzheimer’s Dement. 20 (9), 5996–6007 (2024).
Khandayataray, P. & Murthy, M. K. Dietary interventions in mitigating the impact of environmental pollutants on alzheimer’s disease – A review. Neuroscience 563, 148–166 (2024).
Agarwal, P. et al. Association of MIND diet with cognitive decline among black and white older adults. Alzheimer’s Dement. 20 (12), 8461–8469 (2024).
Sawyer, R. P., Blair, J., Shatz, R., Manly, J. J. & Judd, S. E. Association of adherence to a MIND-Style diet with the risk of cognitive impairment and decline in the REGARDS cohort. Neurology 103 (8). (2024).
O’Reilly, S. et al. Onset of cognitive impairment, diet quality and adherence to dietary guidelines over 12 years: the personality and total health cohort study. Br. J. Nutr. 1–8. (2024).
Liu, Y. et al. Projection for dementia burden in China to 2050: a macro-simulation study by scenarios of dementia incidence trends. Lancet Reg. Health - Western Pac. 50, 101158 (2024).
Jack, C. R. et al. Revised criteria for diagnosis and staging of alzheimer’s disease: alzheimer’s association workgroup. Alzheimer’s Dement. 20 (8), 5143–5169 (2024).
Raulin, A. C. et al. ApoE in alzheimer’s disease: pathophysiology and therapeutic strategies. Mol. Neurodegeneration. 17 (1), 72 (2022).
Vu, T. et al. Adherence to MIND diet, genetic susceptibility, and incident dementia in three US cohorts. Nutrients 14 (13), 2759. (2022).
Fekete, M. et al. Improving cognitive function with nutritional supplements in aging: A comprehensive narrative review of clinical studies investigating the effects of vitamins, minerals, antioxidants, and other dietary supplements. Nutrients 15 (24). (2023).
Arora, S., Santiago, J. A., Bernstein, M. & Potashkin, J. A. Diet and lifestyle impact the development and progression of alzheimer’s dementia. Front. Nutr. 10. (2023).
Chen, X., Walton, K., Brodaty, H. & Chalton, K. Polyphenols and diets as current and potential nutrition senotherapeutics in alzheimer’s disease: findings from clinical trials. J. Alzheimer’s Disease. 101 (s1), S479–501 (2024).
Seago, E. R., Davy, B. M., Davy, K. P. & Katz, B. Neuroprotective dietary patterns and longitudinal changes in cognitive function in older adults. J. Acad. Nutr. Dietetics (2024).
Levak, N. et al. Nutrition guidance within a multimodal intervention improves diet quality in prodromal alzheimer’s disease: multimodal preventive trial for alzheimer’s disease (MIND-ADmini). Alzheimer’s Res. Therapy. 16 (1), 147 (2024).
Cheatham, C. L., Nieman, D. C., Neilson, A. P. & Lila, M. A. Enhancing the cognitive effects of flavonoids with physical activity: is there a case for the gut microbiome?? Front. NeuroSci. 16. (2022).
Gregory, S. et al. Mediterranean diet and structural neuroimaging biomarkers of alzheimer’s and cerebrovascular disease: A systematic review. Exp. Gerontol. 172, 112065 (2023).
Wen, B. et al. Nutrition: A non-negligible factor in the pathogenesis and treatment of alzheimer’s disease. Alzheimer’s Dement. (2025).
Timlin, D., McCormack, J. M., Kerr, M., Keaver, L. & Simpson, E. E. A. The MIND diet, cognitive function, and well-being among healthy adults at midlife: a randomised feasibility trial. BMC Nutr. 11 (1), 59 (2025).
Govindugari, V. L. et al. Thwarting alzheimer’s disease through healthy lifestyle habits: hope for the future. Neurol. Int. 15 (1), 162–187 (2023).
Xu, W., Xu, Z., Guo, Y. & Wu, J. Two decades of research on the role of diet in alzheimer’s disease (2003–2023): a bibliometric and visual analysis based on CiteSpace. J. Health Popul. Nutr. 43 (1), 9 (2024).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the conception of the study design, data acquisition, data analysis, initial drafting, and final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, X., Yang, B., Liu, Q. et al. The long-term neuroprotective effect of MIND and Mediterranean diet on patients with Alzheimer’s disease. Sci Rep 15, 32725 (2025). https://doi.org/10.1038/s41598-025-17055-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17055-5