Abstract
Animal behavior is dynamically shaped by internal states and external social contexts. This study examines how internal states and social buffering influence aggression and social behavior. Food deprivation and mirror stimuli amplified aggression and reduced exploratory activity, highlighting a state-dependent shift in behavioral priorities. However, the presence of conspecific cues significantly mitigated aggression, irrespective of hunger, underscoring the regulatory role of social interaction. These results demonstrate the critical interplay between metabolic and social factors in modulating stress-related behaviors and position zebrafish as a powerful model for unraveling the neurobiological mechanisms underlying social modulation and adaptive behavioral strategies.
Similar content being viewed by others
Introduction
Social interactions involve more than just a preference for proximity to conspecifics, conferring multiple fitness benefits, including predator avoidance, improved foraging efficiency, reduced energetic costs, and opportunities for social learning1,2,3. These affiliations arise from individual behavioral rules shaped by conspecific signals4and are further modulated by the broader social context, which influences aggression, anxiety, and interaction patterns5,6,7. Distinct neuronal populations in flies, zebrafish and mice play causal roles in social tasks such as social recognition, affiliation and aggression4,8,9demonstrating how brains evolved to process social stimuli into adaptive responses10. Importantly, goal-directed behaviors like aggression are underpinned by scalable and persistent internal states of emotion, motivation or drive9,11,12. Aggression serves as a vital strategy for resource acquisition, and its expression is modulated by the animal’s internal energy state. Across species, animals display increased aggression when in energy-deficient states such as hunger13,14,15. Hunger has been found to alter decision-making, increasing impulsivity and biasing behavioral choices toward the acquisition of resources16. However, how internal states interact with social context to modulate behavioral decision-making and aggression remains poorly understood.
Food deprivation represents a potent homeostatic stressor, fundamentally threatening survival. Animals adapt to nutritional scarcity through complex physiological regulation, including hormonal and metabolic changes mediated by the central nervous system17,18. In addition to physiological adaptations, animals also exhibit notable behavioral changes, such as increased aggression15,19heightened risk-taking behaviors like foraging in areas associated with potential threats20 and shifts in decision-making, including a transition from avoidance to approach behaviors21,22. At the molecular level, regulators such as Pcp4a in zebrafish have been shown to directly link feeding state to behavioral prioritization23and “hunger” in zebrafish is encoded by alternative activity states in opposing brain regions depending on food availability24. Neural systems governing caloric intake and social behavior exhibit significant overlap, suggesting an intrinsic link between these fundamental drives25. Despite the established link between energy deficiency and elevated aggression, the potential for social cues to buffer this specific form of stress-induced aggression remains unexplored. Understanding how aggression is influenced by social context requires an experimental system in which both aggressive triggers and social cues can be precisely manipulated. The zebrafish is an ideal model in this regard, as it is highly social and exhibits robust and measurable aggressive behaviors in the laboratory towards both conspecifics and their own mirror image26,27,28,29,30. While subtle neurophysiological differences exist between responses to real and mirror opponents, the overt levels of aggression remain comparable31,32. The mirror test reliably captures aggressive behavior and serves as a practical tool for studying aggression dynamics33,34. Additionally, zebrafish also display social buffering, which is the attenuation of stress responses in the presence of peers35,36,37underscoring the capacity of social cues to mitigate stress. The intersection of hunger-induced internal states and external aggression triggers creates a potent stress paradigm in which social buffering may serve as a key modulator.
Social buffering is a widely observed phenomenon in which the presence of conspecifics ameliorates the effects of aversive experience38,39,40. This effect has been documented in mammals, with growing evidence on underlying neural mechanisms41,42,43. Recent studies in zebrafish provide robust evidence that social buffering may share a common evolutionary origin in vertebrates and elicits a specific co-activation pattern marked by significant functional connectivity among Dm, Vs, and POA, nuclei implicated in mammalian buffering responses36. Moreover, functional connectivity within this network has been observed not only in fear contexts but also during aggression paradigms, indicating that distinct social behaviors may be supported by a distributed activation profile rather than by isolated nodes44. While social buffering of fear is well studied in zebrafish35,36its capacity to specifically modulate aggression remains significantly less explored. We hypothesize that the presence of conspecifics suppresses aggression elicited by both hunger and mirror exposure, implying a novel social buffering mechanism dedicated to aggression modulation. To understand the influence of social interaction on aggression, we focused on social maintenance, the capacity of zebrafish not only to initiate but to persist in close social interactions over time. Social maintenance is distinct from social approach; while the latter reflects the initial drive to seek conspecifics, social maintenance embodies the ongoing, cohesive interactions that underpin effective social buffering. Building on emerging evidence that these two components are differentially regulated45our study employed a modified social buffering paradigm to assess recovery from aggression induced by mirror stimuli and food deprivation. This allowed us to isolate the role of social maintenance in reducing aggressive and stress-related behaviors. Our findings demonstrate that zebrafish exposed to aggressive stimuli exhibit reduced aggression and stress-related behaviors when accompanied by familiar conspecifics.
Materials and methods
Experimental animals
Zebrafish were maintained at the University of Calicut using standard husbandry protocols and in accordance with institutional guidelines for animal welfare. Adult wild-type zebrafish of the AB strain, both males and females, aged 6–8 months, were procured from a government-approved aquaculture facility (KL/04/OH/264/2021) in Alappuzha, Kerala, India, for this study. The fish were maintained at 27 ± 1 °C, with a pH of 6.5–7, 14-hour light/10-hour dark cycle, and were fed twice daily. All animals were kept at the same stocking density of 5–6 adults per liter to ensure uniform environmental conditions. For experiments requiring familiar conspecifics, subjects and conspecifics were raised together. This study adhered to the ARRIVE guidelines46and all animal experiments were approved by the Committee for Control and Supervision of Experiments on Animals (CCSEA) under the 2021 regulation and conducted in accordance with the institutional guidelines of the University of Calicut (426/GO/Re/S/01/CCSEA).
Feeding protocol
In the non-starved group (CNTRL), fish were fed daily with brine shrimp and commercial pellets, while the starved group (HGRY) was completely deprived of food for six days. No mortality was observed in the starved group during the food deprivation period, and these fish exhibited normal aggressive behavior47. Prior to behavioral experiments, we verified that the fish in both groups had comparable body lengths (3.2 ± 0.3 cm) before and after the starvation period, aligning with protocols to ensure uniformity in experimental conditions. Both groups were housed separately from the original group tanks in individual preholding tanks (16 L capacity, accommodating 20–25 fish) for six days before the experiments.
Behavioural setup and tracking
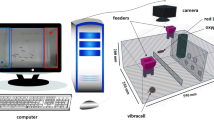
All recording sessions were conducted between 10 a.m. and 4:30 p.m. in a temperature-controlled room maintained at 28 °C. Experiments were conducted in a custom made three chambered glass tank (60 × 15 × 20 cm, LWH). Each chamber of the tank was filled with system water up to two-thirds and the experimental zebrafish (focal fish) was introduced into the middle chamber. In all experimental conditions, two side walls of the middle chamber were covered with visual barriers during the habituation period. Following the habituation phase, the behavioral responses of focal fish exposed to specific stimuli were recorded during a 6-minute period using a Logitech webcam 720p C270 (30fps). A side-view video simultaneously captured the focal fish behavior, with the setup isolated by a black curtain to prevent external disturbances during testing. A sample size of 20 fish was used for each CNTRL and HGRY group, with the tank water replaced after each trial to eliminate residual cues. Behavioral analyses were conducted using Smart V3.0 video-tracking software48,49. The middle chamber of the three-chambered tank was divided virtually into two equal areas: the stimulus zone and the non-stimulus zone. The presentation of stimuli in each experimental condition was counterbalanced50. Once the experiments were completed, the fish were returned to their stock tanks.
Exploratory behavior
The exploratory behavior of zebrafish was assessed to evaluate their natural inclination to explore and interact with an environment lacking external stimuli. The “no stimulus” protocol is a commonly used experimental method to investigate the spatiotemporal exploratory behavior of the focal fish. During the test, the fish were allowed to explore the middle chamber of the tank for a predetermined period, typically between 3 and 10 min. After the test, the fish were carefully returned to the holding tanks until the next recording session. Key behavioral metrics, including total distance traveled, average speed, freezing time, slow time, fast time and number of zone transitions were recorded.
Mirror-induced aggression
We employed the mirror-induced aggression (MIA) paradigm to assess aggression levels in adult zebrafish51. In this setup, the fish was placed in the middle chamber of a three-chambered tank, with a 15 cm x 15 cm mirror positioned at a 22.5º angle in the adjacent chamber to provide a lateral view of the “opponent,” a configuration known to most effectively elicit aggression52. Three minutes after introducing the fish into the middle chamber, aggressive behavior was recorded over a 6-minute period, including cumulative time spent in the mirror zone (MZ), number of zone transitions, number of entries to MZ and mean speed. The valence index (VI) of aggression was calculated using the formula [approaches - avoidances]/[approaches + avoidances]21, where VI = 1 indicates 100% approach and − 1 indicates 100% avoidance, with approach quantified as the sum of entries to and time spent in the MZ, and avoidance as the sum of entries to non-MZ and time spent in the non-MZ. Aggressive behaviors directed toward the mirror image, including biting and tail fin thrashing, were recorded and scored manually by an experienced investigator who was blinded to the experimental groups34.
Social interaction
In social interaction test, zebrafish were exposed to conspecific stimulus consisting of six zebrafish53. The experimental setup for this test featured a three-chambered tank, with chambers arranged horizontally in a line (empty, experimental, and conspecific). Initially, two black panels were placed between the chambers to block the experimental subject’s view of the other chambers. The middle chamber of the tank was divided into two virtual areas: the social ROI (social-region of interest), located closest to the conspecific tank and assumed to be preferred for visual interaction with conspecifics, and the non-social ROI (non-social region of interest). The test consisted of a 3-minute habituation phase in which the experimental fish was placed in the middle chamber, followed by a 6-minute interaction phase. Every test was conducted with the group in a balanced position (either on the left or right of the experimental tank). The parameters analysed were the number of entries to social ROI, mean speed(mm/s), turning index, parallel index, cumulative time spent in the social area (%) and total distance travelled in the social ROI (mm). Reciprocal interaction counts during the social interaction phase were manually scored by an investigator, blind to the experimental groups. This measure represents the number of times the focal fish oriented towards and interacted with an individual conspecific in the adjacent chamber during the first 60 seconds54. The time spent in the social ROI during a 6-minute test period was used as an indicator of social behavior.
Social buffering
The social buffering paradigm utilized test chambers where a focal fish resided centrally, and the adjacent chambers with an inclined mirror (MIA) and a social group were placed side by side but physically separated. Focal fish, both CNTRL and HGRY, were habituated overnight in the middle chamber with no visual access to the adjacent chambers, and behavioral testing occurred the following day. Shoal members were always siblings and tank mates of the focal fish to avoid confounding effects of familiarity, and conspecifics were acclimatized for 60 min before the experiment. Each test lasted 10 min, after which focal fish were transferred to a pre-holding tank. Between treatments, tanks were thoroughly cleaned with 70% ethanol and rinsed multiple times with filtered water to eliminate hormone and odor residues. This paradigm enabled the fish to selectively engage with the social group (social cue) while concurrently being exposed to the mirror to elicit aggression in different metabolic states.
Behavioral data analysis
All tracked data files were imported to python and behavioral parameters were quantified using Smart V3.0 software and custom-made script. A region of interest (ROI), is defined as the area of the test arena closest to the observation glass of the social cue. The focal fish was considered in the ROI when its centroid point was inside that region. The following behavioral parameters were determined and analysed at an individual and group level for each treatment: cumulative of time spent in the ROI, mean speed in the ROI, total distance covered in the arena, zone transition, mean preferred orientation in the arena and directional focus.
The individual mean resultant vector’s angle (α, 0° to 360°) and grouped mean resultant vector length (Rr) were calculated for each fish, with the projection of the vector onto the stimulus tank’s direction (180°) given by Rproj = − Rcosα. Rproj represents the directional focus relative to the stimulus, measured on a linear scale from 1 to − 1, where positive values indicate higher directional focus, negative values indicate reduced directional focus, and zero indicates no directionality55. The Social Preference Index (SPI) was calculated as the difference between the time spent near the conspecific (SC) and the time spent in the equivalent region on the opposite side of the chamber, divided by the total time (360 s) spent in both compartments [SPI = (time spent social ROI – time spent non-social ROI)/Total time]. For the CNTRL and HGRY groups, where no social cue was present, the SPI was computed based on the randomized side of the chamber where the social cue would be added in the subsequent phase of the social interaction1. The Turning Index quantifies the rotational pattern of the animal’s trajectory (Fig. 1I). For each time sample, the angle between the current (p2 to p3) and previous (p1 to p2) direction of movement is calculated. A positive value of + 1 is assigned for rightward rotation, and a negative value of −1 for leftward rotation. The Parallel Index measures the alignment of the current movement direction (Fig. 1g) (p2 to p3) with the previous direction (p1 to p2). If the angle between them is < 20°, the cosine of the angle is used; if < 90°, a value of 0 is assigned; and if > 90°, a value of −1 is assigned. The Parallel Index is the average of all considered samples, with values ranging from − 1 to 1, where 1 indicates straight-line movement and − 1 indicates frequent directional changes. Both indices exclude samples where track displacement is < 0.5 cm. The turning index and parallel index were quantified using Smart software.
Analysis of swim kinematics revealed altered exploratory behavior for hungry zebrafish. Total distance travelled (in mm) per fish over a 6 min period, b. Freezing time (in seconds) defined as periods of immobility lasting > 4 s, c. Frequency of zone transition, d. mean speed (in mm/s), e. slow time (≤ 10 mm/s) in seconds, f. fast time (≥ 150 mm/s) in seconds, g. Schematic illustration of Parallel Index. The diagram shows three consecutive points (p1, p2, and p3) in the fish’s movement trajectory. The angle α represents the change in direction between the previous movement vector (p1 to p2) and the current movement vector (p2 to p3), h. Cumulative probability distribution of Parallel Index (PI) values for zebrafish under hungry (black) and control (green) condition, i. Schematic representation of turning index. The movement path is divided into sequential points (p1, p2, p3). The turning angle is determined by comparing the current direction vector (p2 to p3) relative to the previous direction vector (p1 to p2), j. Turning index over 20 sample indexes for control (top panel in green) and hungry (bottom panel in black) condition. Each data point represents the rotational behavior at a given sample index. Dashed lines indicate the general trend and overall directional bias of turning behavior over time, k. Spatial occupancy heat map of control and l. hungry zebrafish for the exploratory behavior. For calculating turning tendency and PI, samples with a displacement of less than 50 mm were excluded from the analysis. If the angle is < 20°, the value of the cosine of this angle is considered for the parallel index calculation. Box and whisker plots are shown with arithmetic median (middle line), and boxes represent the 1 st and 3rd quartiles. Data shown as mean ± s.e.m. P values are based on non-parametric t test comparing response differences. All asterisks indicate significance (*P < 0.05, **P < 0.01, ***P < 0.001 or ****P < 0.0001), ns: not significant; Mann–Whitney U test.
Statistical analysis
All data analysis was conducted using Python 3.10 alongside Graphpad prism 9.5. Python libraries utilized for scientific computing included Numpy56Scipy57 and Scikit-learn. All figures were produced using Matplotlib58. For statistical analysis and comparisons, the nonparametric Mann–Whitney U test was employed, except for result (3.7), which was analyzed using a parametric unpaired t-test. The angles of the group mean resultant vectors were expressed as the mean with 95% confidence intervals (C.I.) when directionality was significant. Correlations for time spent in social interaction were conducted using a non-parametric Spearman rank correlation. A p-value < 0.05 was considered statistically significant.
Results
Influence of feeding regimen on exploratory behavior
We assessed the exploratory behavior of zebrafish under two metabolic states: control (CNTRL) and hungry (HGRY). Hungry zebrafish displayed a significant reduction in exploratory activity compared to control group. Quantitative analysis revealed a marked decrease in overall locomotor activity, as indicated by a reduction in total distance traveled in the HGRY group compared to CNTRL (p = 0.0195; Fig. 1a). The reduced activity in hungry zebrafish was associated with an increase in freezing time (p = 0.0135; Fig. 1b), with a higher proportion of immobility during time spent. The number of zone transitions was similar between groups (p = 0.0951; Fig. 1c), while the mean speed was reduced in the HGRY compared to the CNTRL group (p = 0.0184; Fig. 1d). A detailed analysis of movement dynamics showed an increase in time spent at low speeds (≤ 10 mm/s) in HGRY zebrafish compared to the CNTRL group (p = 0.0001, Fig. 1e), while the duration of time spent in fast movements (≥ 150 mm/s) remained unchanged between groups (p = 0.1533; Fig. 1f). This selective reduction in slow movement, combined with stable fast movement, suggests a distinct behavioral adjustment in response to food deprivation. The consistency of zebrafish alignment with a specific zone was analyzed using a directional alignment metric score, Parallel Index (PI) (Fig. 1g), where values closer to 1 represent straighter, more linear movement patterns, and values closer to −1 indicate frequent directional changes. The cumulative probability function for parallel index for hungry zebrafish (black line) ranged from 0.65 to 0.85 (Fig. 1h) and exhibited a rightward shift for the HGRY group, indicating higher PI values than the control group (green line). The HGRY group exhibited stronger directional alignment, whereas the CNTRL group showed lower PI values and greater variability in movement direction. The turning index of the zebrafish was examined to quantify their rotational behavior over time by measuring the angle between successive movement direction, with positive values representing rightward rotation and negative values representing leftward rotations (Fig. 1i). The control group (Fig. 1j, top panel, green) exhibited no strong directional bias, with turning index values fluctuating around zero. The trend line revealed a slight leftward turning tendency over time, indicating minimal directional preference. In contrast, the hungry group (Fig. 1j, bottom panel, black) exhibited greater turning index variability, with frequent right-left oscillations and a modest rightward bias accompanied by larger fluctuations. Furthermore, spatial exploration patterns revealed distinct differences between control and hungry zebrafish (Fig. 1k) and (Fig. 1l). These findings suggest that metabolic state is closely associated with reduced activity, altered movement patterns, and changes in turning behavior during food restriction. We propose that this metabolic shift induced by different feeding regimens aligns with the observed behavioral change59,60,61.
Differential modulation of aggressive behavior by mirror induced stimuli and intrinsic States
Adult zebrafish consistently demonstrated a pronounced response to their own reflection, characterized by frequent approaches to the mirror62. The Mirror-Induced Aggression (MIA) test demonstrates both mirror stimuli and metabolic state significantly modulate aggression in zebrafish. Specifically, the HGRY + M (hungry + mirror stimulus) group exhibited significantly higher values on several aggression-related endpoints compared to the CNTRL + M group. The HGRY + M group spent significantly more time in the mirror zone (MZ) (p < 0.0001; Fig. 2a), which was associated with a marked decrease in both the number of entries to the MZ (p < 0.0001; Fig. 2b) and zone transition (p < 0.0001; Fig. 2d) compared to CNTRL + M. This reduction coincided with increased time spent in MZ, suggesting the HGRY + M group exhibited prolonged and focused orientation to the mirror stimulus. The HGRY + M group displayed a significant increase in the number of mirror bites, a key aggression indicator (p < 0.0001; Fig. 2e), reflecting a heightened focus on the perceived intruder in the mirror and escalated confrontational behavior. However, no significant differences were observed in mean speed (p = 0.3796; Fig. 2c) between the HGRY + M and CNTRL + M groups.
Metabolic stability and the nature of mirror-induced aggression. Cumulative time spent in the mirror zone (MZ) in seconds, b. Number of entries to MZ, c. mean speed in mm/s, d. Frequency of transitions, e. Mean number of aggressive acts performed in the 6 min agonistic interaction, f. Cumulative probability distribution of Parallel Index (PI) values for zebrafish under HGRY + M (black) and CNTRL + M (green) conditions, g. Turning index over 20 sample indexes for CNTRL + M (bottom panel in green) and HGRY + M (bottom panel in black) conditions, h. Temporal dynamics of aggression in CNTRL + M and HGRY + M groups. The average number of mirror bites over time is plotted for both groups, with 95% confidence intervals (shaded areas) for each (n = 20/group), i. Scatter density plot of mirror bites for CNTRL + M and HGRY + M groups. Black points indicate individual data, with denser regions reflecting higher frequencies of aggression in the HGRY + M relative to CNTRL + M group. Axes values represent the number of mirror bites in each condition, j. Relationship between valence index (VI) for aggression and response probability. The graph shows response probability as a function of Valence Index for Aggression in HGRY + M (black) and CNTRL + M (green), k. Polar scatter plot of the focal fishes’ (in green and black dots) individual mean resultant vector’s angles α (0˚ to 360˚) combined with corresponding vector lengths R (0 to 1), for each group, l. Box plot of the individual resultant vector’s lengths R projected (R proj) onto the stimulus direction (180˚), m. Heatmap of mirror-induced aggression (MIA) in the HGRY + M group; n. Heatmap for the CNTRL + M. The vertical line divides the stimulus (right/mirror zone) and non-stimulus (left) zones. Box and whisker plots are shown with arithmetic median (middle line), and boxes represent the 1 st and 3rd quartiles. Data shown as mean ± s.e.m. P values are based on non-parametric t test comparing response differences. All asterisks indicate significance (*P < 0.05, **P < 0.01, ***P < 0.001 or ****P < 0.0001), ns: not significant; Mann–Whitney U test.
The cumulative probability function plot for the parallel index (PI) revealed a leftward shift in the HGRY + M group (Fig. 2f), with lower PI values compared to the CNTRL + M group. The CNTRL + M fish displayed a higher probability of PI values, with a steep increase in cumulative probability between 0.6 and 0.8, suggesting more consistent, straight-line movements and minimal directional change. In contrast, the HGRY + M group exhibited a broader distribution of PI values between 0.2 and 0.6, reflecting more frequent changes in direction. In the CNTRL + M group (Fig. 2g, top panel), the turning index exhibited a relatively balanced movement pattern with no clear directional bias. While HGRY + M group (Fig. 2g, bottom panel) showed more pronounced fluctuations in turning behavior, with both leftward and rightward rotations occurring at higher magnitudes. The turning index was slightly negative overall, indicating a subtle preference for leftward rotation, though no strong directional bias emerged in response to the mirror stimulus. Figure 2h illustrates the temporal dynamics of aggressive behavior over 300 s, comparing the CNTRL + M and HGRY + M groups. The HGRY + M group displayed more mirror bites during MIA test, with a peak around 120 s, maintaining elevated aggression levels. CNTRL + M group showed a rapid decline in mirror bites over time, reflecting a steady reduction in aggression. The density scatter plot (Fig. 2i) provided a comprehensive visualization of the bivariate relationship between HGRY + M and CNTRL + M groups. Each data point (black dot) represents an individual fish aggressive response under both conditions. The plot revealed a non-uniform distribution of aggressive behavior, with a highest density of observations (indicated by darker red regions) clustered between 600 and 800 mirror bites (HGRY + M) and 50–300 mirror bites (CNTRL + M). This asymmetric distribution is consistent with the hypothesis that hunger is associated with elevated aggressive behavior in zebrafish under mirror exposure.
The Valence Index (VI) for aggression was computed for HGRY + M and CNTRL + M groups (Fig. 2j). The HGRY + M group demonstrated a higher and more linear probability of aggression in response to the mirror stimulus, while CNTRL + M group exhibited a slower escalation, particularly at lower VI values. HGRY + M fish showed a higher threshold for initiating aggression compared to CNTRL + M fish. To assess the directionality of the focal fish, we analyzed individual and group preferred orientations as well as directional focus towards the mirror stimulus55. Circular scatter plots of the mean resultant vector orientations and directional focus revealed distinct distribution patterns for each group (Fig. 2k). Both HGRY + M and CNTRL + M fish showed strong clustering around the stimulus direction, with minimal variation in the CNTRL + M group. The group mean resultant vectors for both treatments were oriented towards the mirror at approximately 180°: αg (CNTRL + M) = 176.57°, Rg = 0.35, n = 20; αg (HGRY + M) = 170.1°, Rg = 0.31, n = 20, with the CNTRL + M group showing a higher directional focus. Additionally, the projection of individual fish vectors (R proj) onto the stimulus direction did not differ significantly between the groups (Fig. 2l). Heatmaps revealed distinct aggression patterns between the two groups (Fig. 2m, n). The HGRY + M (Fig. 2n) showed a focused, intense concentration of mirror bites on the right side (mirror zone) of the heatmap, while the CNTRL + M (Fig. 2m) group exhibited a more diffuse and lower-intensity pattern spread across a larger area.
Prioritization of social interaction independent of intrinsic state
We measured the time spent in proximity to conspecifics (social region-of-interest) as an indicator for social preference63. The cumulative time spent in the social region of interest (ROI) differed significantly between CNTRL + SC (control + social cue) and HGRY + SC (hungry + social cue) groups (Fig. 3a; P < 0.0001). HGRY + SC zebrafish exhibited significantly more time spent in the social ROI compared to CNTRL + SC. In contrast, HGRY + SC fish spent significantly less time in the non-social ROI than CNTRL + SC (Fig. 3b; P < 0.0001). The HGRY + SC group displayed fewer entries into the social ROI (P < 0.0001; Fig. 3c) and reduced mean speed (P = 0.0227; Fig. 3d) compared to the CNTRL + SC group, while total distance moved was similar between groups (Fig. 3e). These observations are consistent with HGRY + SC fish spending extended time within the social ROI, with their location clustered around the social cue under resource-limited conditions.
Metabolic states prioritize social interaction. Cumulative time spent in social ROI; b. Cumulative time spent in nonsocial ROI, c. Number of entries to social ROI, d. mean speed in mm/s, e. total distance travelled, f. Correlation matrix showing the relationship between time spent in social and non-social regions of interest (ROIs) for CNTRL + SC and HGRY + SC, g. Cumulative probability distribution of Parallel Index (PI) values for zebrafish under HGRY + SC (black) and CNTRL + SC (green) conditions, h. Turning tendency over 20 sample indices for control and hungry conditions. The top panel (blue) represents the control, while the bottom panel (red) shows data from the hungry condition, i. Boxplots show the preference for the social area in both CNTRL + SC (n = 19) and HGRY + SC (n = 20) and CNTRL and HGRY in response to stimulus fish, j. Polar scatter plot of the focal fishes’ individual mean resultant vector’s angles α (0˚ to 360˚) combined with corresponding vector lengths R (0 to 1), for each treatment, k. Box plot of the individual resultant vector’s lengths R projected (R proj) onto the stimulus direction (180˚). Positive values indicate directional focus towards the stimulus; zero indicates no directionality; negative values indicate directional focus opposite to the stimulus, l. Temporal dynamics of reciprocal interactions between focal fish and social cue for the first 60-seconds. Shaded areas represent 95% confidence intervals, m. Heatmap of social preference in the CNTRL + SC group; n. Heatmap for the HGRY + SC. The vertical line divides the stimulus (right/without social cue) and social cue (left) zones. Box and whisker plots are shown with arithmetic median (middle line), and boxes represent the 1 st and 3rd quartiles. Data shown as mean ± s.e.m. P values are based on non-parametric t test comparing response differences. All asterisks indicate significance (*P < 0.05, **P < 0.01, ***P < 0.001 or ****P < 0.0001), ns: not significant; Mann–Whitney U test.
Correlation between time spent in social and non-social ROIs revealed key behavioral dynamics influenced by metabolic states (Fig. 3f). Both CNTRL + SC and HGRY + SC showed a moderate positive correlation (rs = 0.29), indicating partially aligned patterns. Negative correlations were observed with their respective non-social ROIs (CNTRL + NSC and HGRY + NSC; rs = −0.29), highlighting a trade-off where increased time in the social ROI infer a decreased non-social ROI presence. Strong negative correlation (rs = −1.00) between social and non-social ROI times further confirmed this inverse relationship, underscoring the influence of hunger on prioritizing social engagement at the expense of non-social exploration (NSC refers to time spent by CNRTL and HGRY in non-social ROI). The cumulative probability of parallel index (Fig. 3g) in HGRY + SC group exhibited PI values below 0.35, reflecting less directed movement towards the social group. While CNTRL + SC group displayed PI values up to 0.6, indicating more linear and purposeful approach to social cue. In the CNTRL + SC group (Fig. 3h top panel green), turning index ranged from − 0.05 to 0.10, showing a slight leftward bias over time. The HGRY + SC (Fig. 3h bottom panel black) group displayed greater variability (−0.10 to 0.15), with an increasing rightward bias and more erratic movement patterns. We assessed the Social Preference Index (SPI) of CNTRL and HGRY (Fig. 3i). In the absence of social cue, both groups displayed low SPI values with no significant difference (ns, p > 0.05). In the presence of conspecifics, SPI increased significantly in both groups, with CNTRL + SC showing an SPI of 0.78 ± 0.06 and HGRY + SC exhibiting a higher SPI of 0.98 ± 0.01 (p < 0.001), indicating a stronger social preference in the HGRY + SC group.
CNTRL + SC fish exhibited strong clustering of mean orientation around 180°, directed to the social cue, while HGRY + SC fish showed a similar alignment with minimal variation (Fig. 3j). Group mean resultant vectors consistently pointed toward 180° in both groups, with HGRY + SC fish displaying slightly greater directional focus [αg (CNTRL + SC) = 190.48°, Rg = 0.19, n = 20; αg (HGRY + SC) = 187.02°, Rg = 0.29, n = 20]. Individual vector projection lengths (R proj) showed a non-significant increasing trend in HGRY + SC fish compared to CNTRL + SC fish (Fig. 3k). Reciprocal interaction between focal fish and shoal member differed significantly between HGRY + SC and CNTRL + SC conditions during the initial 60-second test period. HGRY + SC fish consistently exhibited higher interaction counts (5.1-6.0 interactions) compared to CNTRL + SC fish (4.1–4.9 interactions). Interaction patterns also varied over time, with HGRY + SC fish showing a pronounced peak around the 50-second mark, while CNTRL + SC fish displayed a gradual decline, starting at 4.9 interactions at 10 s and dropping to 4.1 interactions by the end of the observation period with lower interaction probability (Fig. 3l). In the CNTRL + SC group (Fig. 3m), heatmap depicts the spatial distribution of zebrafish near the social compartment on the left side of the arena but with moderate intensity and some dispersed activity across other regions, indicates less engagement with the social cue. Conversely, the HGRY + SC group (Fig. 3n) exhibits a marked increase in intensity near the social compartment, with a strong, concentrated presence along the left side of the arena. Minimal exploratory activity in the non-social zone further indicates the enhanced social preference in the HGRY + SC group compared to the CNTRL + SC group.
Social maintenance buffer aggressive behavior
In this study, we developed a behavioral paradigm to investigate the phenomenon of social buffering (SB) in adult zebrafish when exposed to both conspecifics and a mirror stimulus. To test the hypothesis, the presence of conspecific cues mitigates aggressive response in focal fish, individuals were placed in the middle chamber while being exposed to conspecifics and mirror in the adjacent chambers. This experimental setup allowed us to assess the impact of social buffering on aggressive behavior. We measured the cumulative time spent by zebrafish in two distinct zones, the Social ROI and the Mirror Zone (MZ), under the conditions of CNTRL + SC + M (control + social cue + mirror) and HGRY + SC + M (hungry + social cue + mirror) (Fig. 4a). Zebrafish in the HGRY + SC + M group spent significantly more time in the Social ROI than those in the CNTRL + SC + M group (P < 0.0007). Both HGRY + SC + M and CNTRL + SC + M groups spent more time in the Social ROI than in the Mirror Zone (P < 0.0001), but the HGRY + SC + M group exhibit a greater preference for the Social ROI. The HGRY + M group displayed significantly more mirror bites than the CNTRL + M group (P < 0.0001), which is consistent with elevated aggression-related measures in the absence of social cues (Fig. 4b). In the presence of both social cues and the mirror stimulus, both CNTRL + SC + M and HGRY + SC + M groups had lower mirror bite counts. Notably, the HGRY + SC + M group exhibited a larger decrease in mirror bites compared to CNTRL + SC + M (P < 0.001).
Social support counteracts aggressive behavior. a. Cumulative time spent by focal zebrafish in the Social ROI and Mirror Zone (MZ) for CNTRL + SC + M and HGRY + SC + M conditions, b. Mean number of aggressive acts performed in the 6 min agonistic interaction, c. Boxplots show the preference for the social area for CNTRL + SC + M (n = 20) and HGRY + SC + M (n = 20) fish during Social Buffering, d. Cumulative probability distribution of Parallel Index (PI) values for zebrafish under HGRY + SC + M and CNTRL + SC + M, e. Polar scatter plot of the focal fishes’ individual mean resultant vector’s angles α (0˚ to 360˚) combined with corresponding vector lengths R (0 to 1), for each treatment, f. Box plot of the individual resultant vector’s lengths R projected (R proj) onto the stimulus direction (180˚). Positive values indicate directional focus towards the stimulus; zero indicates no directionality; negative values indicate directional focus opposite to the stimulus, g. Heatmap of social preference in the CNTRL + SC + M group; h. Heatmap for the HGRY + SC + M. Box and whisker plots are shown with arithmetic median (middle line), and boxes represent the 1 st and 3rd quartiles. Data shown as mean ± s.e.m. P values are based on non-parametric t test comparing response differences. All asterisks indicate significance (*P < 0.05, **P < 0.01, ***P < 0.001 or ****P < 0.0001), ns: not significant; Mann–Whitney U test.
The CNTRL + SC + M group showed a wide SPI range from 0.1 to 1.0, demonstrating variability in time allocation towards the social cue, while the HGRY + SC + M group had consistently high SPI values between 0.9 and 1.0, reflecting strong and stable social preference regardless of the mirror stimulus (P = 0.0012) (Fig. 4c). The CNTRL + SC + M group exhibited higher PI values, ranging from 0.1 to 0.73 (Fig. 4d), showing a rightward shift compared to the HGRY + SC + M group. The CNTRL + SC + M group demonstrated more consistent directional alignment, while the HGRY + SC + M group displayed lower PI values and greater movement variability. Group mean resultant vectors for both CNTRL + SC + M and HGRY + SC + M were consistently aligned with the stimulus at 180° (Fig. 4e), while HGRY + SC + M fish displayed slightly greater directional focus during SB [αg (CNTRL + SC + M) = 170.03°, Rg = 0.19, n = 20; αg (HGRY + SC + M) = 179.49°, Rg = 0.26, n = 20]. Additionally, the HGRY + SC + M group exhibited significantly greater individual vector projection lengths (R proj), indicating a stronger directional focus compared to the CNTRL + SC + M group (Fig. 4f). Both the CNTRL + SC + M (Fig. 4g) and HGRY + SC + M (Fig. 4h) groups show a clear concentration of activity near the social ROI, indicating that social cues strongly attract the focal fish in both conditions during buffering. Although each heatmap reveals a similar pattern of focused activity toward the social cue, the HGRY + SC + M group appears to exhibit a more pronounced cluster of high-intensity activity. This subtle difference suggests that hunger may enhance the tendency to remain close to conspecifics, but in both groups there is a clear overall preference for social interaction.
Influence of group size on social interaction and aggression modulation during social buffering
To elucidate the role of social dynamics in modulating aggressive behavior, we conducted a series of experiments by varying group sizes. We assess how different levels of social interaction, facilitated by varying group sizes, influence behavioral regulation. Specifically, we manipulated group sizes (3-3SC, 6-6SC, and 9-9SC individuals) and measured aggression levels during social buffering to determine whether increased social interaction mitigates stress-induced behavioral dysregulation. Our analysis focused on the influence of group size on social interaction and its subsequent impact on social buffering. By systematically examining these factors, we aimed to disentangle the mechanisms through which social dynamics counteract aggressive behavior.
During the social interaction test, HGRY groups (HGRY + 3SC, HGRY + 6SC, HGRY + 9SC; [3SC −3 social cues, 6SC- 6 social cue, 9SC- 9 social cue]) showed significantly higher cumulative time in the social ROI compared to the CNTRL groups (CNTRL + 3SC CNTRL + 6SC CNTRL + 9SC − 3SC means 3 social cues) across different social contexts (3SC: P = 0.003, 6SC: P < 0.0001, 9SC: P = 0.004), with the most pronounced difference observed at the 6SC condition (P < 0.0001) (Fig. 5a). The entries into the social ROI were higher in the HGRY + 3SC, HGRY + 6SC, and HGRY + 9SC groups with their respective CNTRL + 3SC, CNTRL + 6SC, and CNTRL + 9SC counterparts (P < 0.003, P < 0.00012, and P < 0.017, respectively) (Fig. 5b). Social Preference Index (SPI) analysis showed significant differences in social affinity across group sizes: the HGRY + 6SC group exhibited a higher SPI (CNTRL + 6SC; P < 0.0001), followed by HGRY + 3SC (CNTRL + 3SC; P = 0.0028) and HGRY + 9SC (CNTRL + 9SC; P = 0.017) (Fig. 5c). The social preference was highest in the medium group size 6SC (HGRY + 6SC + M), while both the hungry conditions of 3SC and 9SC showed lower social preference relative to 6SC (HGRY + 6SC + M).
Group Size Shapes Social Buffering Efficacy. a. Cumulative time spent in social ROI during social interaction [3SC −3 social cues, 6SC- 6 social cue, 9SC- 9 social cue], b. Number of entries to social ROI during social interaction, c. Boxplots show the preference for the social area for varying group size in CNTRL and HGRY groups during social interaction, (d) Cumulative time spent in social ROI during social buffering, (e) Number of entries to social ROI during social buffering, (f) Cumulative time spent in mirror zone during social buffering, (g) Boxplots show the preference for the social area for varying group size in CNTRL and HGRY groups during social buffering, (h) Number of mirror bites during social buffering. Box and whisker plots are shown with arithmetic median (middle line), and boxes represent the 1 st and 3rd quartiles. Data shown as mean ± s.e.m. P values are based on non-parametric t test comparing response differences. All asterisks indicate significance (*P < 0.05, **P < 0.01, ***P < 0.001 or ****P < 0.0001), ns: not significant; Mann–Whitney U test.
The cumulative time spent in the social ROI during social buffering (SB) (Fig. 5d) for the smallest group size (3SC − 3 social cue) showed no significant difference between CNTRL + 3SC + M and HGRY + 3SC + M (P = 0.097). However, in the medium group size (6SC- 6 social cue), HGRY + 6SC + M exhibited significantly higher time in the social ROI compared to CNTRL + 6SC + M (P = 0.001). Similarly, for the largest group size (9SC-9 social cue) HGRY + 9SC + M showed greater engagement in the social ROI than CNTRL + 9SC + M (P = 0.018). The number of entries into the social ROI (Fig. 5e) was showed similar pattern for 6SC (HGRY + 6SC + M and CNTRL + 6SC + M) and 9SC groups (HGRY + 9SC + M and CNTRL + 9SC + M) (P = 0.033 and P = 0.024 respectively). In contrast, the 3SC group (CNTRl + 3SC + M and HGRY + 3SC + M) showed no change. The cumulative time spent in the mirror zone during social buffering (SB) (Fig. 5f) showed that CNTRL + 3SC + M exhibited significantly higher time in the mirror zone compared to HGRY + 3SC + M (P = 0.0031), CNTRL + 6SC + M spent more time in the mirror zone than HGRY + 6SC + M (P < 0.0001) and for the largest group size, CNTRL + 9SC + M again showed higher time spent in the mirror zone compared to HGRY + 9SC + M (P = 0.0037). The Social Preference Index (SPI) (Fig. 5g) showed a similar social preference for the 6SC and 9SC groups. Both the hungry (HGRY + 6SC + M) and control (CNTRL + 6SC + M) groups for 6SC, as well as the hungry (HGRY + 9SC + M) and control (CNTRL + 9SC + M) groups for 9SC, exhibited greater sociability (P = 0.006 and P = 0.001, respectively). The 3SC group (CNTRL + 3SC + M and HGRY + 3SC + M) displayed no change in the social preference between them (P = 0.937). The number of mirror bites (Fig. 5h) was analyzed to assess the impact of group size on aggressive behavior across different experimental conditions. For the smallest group size (3SC), the CNTRL + M + 3SC exhibited significantly more mirror bites compared to HGRY + M + 3SC (P = 0.0056). In the medium group size (6SC), the CNTRL + M + 6SC showed a highly significant increase in mirror bites compared to HGRY + M + 6SC (P < 0.0001). Similarly, for the largest group size (9SC), the CNTRL + M + 9SC) demonstrated higher mirror bites than HGRY + M + 9SC (P = 0.0042). These results indicate that the influence of group size on reducing mirror-induced aggression is most pronounced in the medium group size (6SC), where the hungry condition (HGRY + M + 6SC) showed a greater reduction in mirror bites. While the 3SC group (CNTRL + 3SC + M and HGRY + 3SC + M) did not show any difference in social preference, both the 3SC and 9SC hungry group also exhibited reduced mirror-induced aggression, suggesting that social context plays a crucial role in modulating aggressive behavior.
Social preference declines with exposure duration while aggression buffering persists
To investigate the temporal dynamics of social buffering, we conducted experiments aimed at understanding how the timing of social interactions influences their efficacy in mitigating aggressive behaviors. Our analysis began by assessing the temporal impact of social interactions, followed by an evaluation of their subsequent effects on social buffering during exposure to mirror-induced aggression. The cumulative time spent in the social region of interest (ROI) between the CNTRL and HGRY groups during exposure to social cues for two durations: 12 min (SC[12 m]) and 18 min (SC[18 m]) (Fig. 6a). For the 12-minute duration (SC[12 m]), the HGRY + SC[12 m] exhibited significantly higher cumulative time in the social ROI compared to the CNTRL + SC[12 m] (P = 0.0008); however, this difference diminished in magnitude during the 18-minute exposure SC[18 m], where the HGRY + SC[18 m] still showed increased social ROI time relative to CNTRL + SC[18 m] but with reduced statistical significance (P = 0.020). The HGRY + SC[12 m] displayed significantly more entries into the social ROI than the CNTRL + SC[12 m] during the 12-minute duration (P = 0.0026); however, this effect was absent during the 18-minute exposure (SC[18 m]), with no significant difference in entry frequency between HGRY + SC[18 m] and CNTRL + SC[18 m] (P = 0.527) (Fig. 6b). During the 12-minute social cue exposure (SC[12 m]), the HGRY + SC[12 m] demonstrated a markedly higher social preference index (SPI) compared to the control group (CNTRL + SC[12 m]; P < 0.0001). However, this effect weakened with prolonged exposure at 18 min (SC[18 m]): the SPI difference between HGRY + SC[18 m] and CNTRL + SC[18 m] was significantly (P = 0.013) (Fig. 6c). These results highlight that 12-minute social cue exposure strongly enhances social preference, while extended durations attenuate its efficacy. Further supporting this time-dependent trend, SPI increased progressively from 6 min (Fig. 3i) to a maximum at 12 min, followed by a gradual decline in preference for social groups.
Temporal Decay in Social Preference Despite Sustained Buffering. a. Cumulative time spent in social ROI during social interaction [12 m–12 min, 18 m–18 min], b. Number of entries to social ROI during social interaction, c. Preference for conspecifics during social interaction, shown as boxplots for CNTRL (SC [12 m]) and HGRY (SC [12 m]) groups, (d) Cumulative time spent in social ROI during social buffering, (e) Number of entries to social ROI during social buffering, (f) Cumulative time spent in mirror zone during social buffering, (g) Preference for conspecifics during social buffering, shown as boxplots for CNTRL (SC [12 m]) and HGRY (SC [12 m]) groups., (h) Number of mirror bites during social buffering. Box and whisker plots are shown with arithmetic median (middle line), and boxes represent the 1 st and 3rd quartiles. Data shown as mean ± s.e.m. P values are based on non-parametric t test comparing response differences. All asterisks indicate significance (*P < 0.05, **P < 0.01, ***P < 0.001 or ****P < 0.0001), ns: not significant; Mann–Whitney U test.
During social buffering, both 12-minute (SC + M[12]) and 18-minute (SC + M[18]) groups undergone social interaction elicited increased engagement in the social ROI (Fig. 6d). Cumulative time in the social ROI was significantly higher for HGRY + SC + M[12] and CNTRL + SC + M[12] (P < 0.0002), HGRY + SC + M[18] and CNTRL + SC + M[18] (P = 0.0021), though the effect magnitude was stronger at 12 min. The HGRY + SC + M[12] displayed significantly more entries into the social ROI than controls CNTRL + SC + M[12] during social buffering (P = 0.0118), but this difference was absent at 18 min (SC + M[18]), with no significant distinction between HGRY + SC + M[18] and CNTRL + SC + M[18] (P = 0.623) (Fig. 6e). The CNTRL + SC + M[12] spent significantly more cumulative time in the mirror zone than the HGRY + SC + M[12](P = 0.0016), a trend that persisted at 18 min exposure group (SC + M[18]) but with reduced effect strength (CNTRL + SC + M[18] and HGRY + SC + M[18] (P = 0.0184) (Fig. 6f). The hungry group exhibited significantly higher social preference (SPI) than controls during both 12-minute (HGRY + SC + M[12] and CNTRL + SC + M[12], P = 0.0009) and 18-minute (HGRY + SC + M[18] vs. CNTRL + SC + M[18], P = 0.0040) during social buffering, indicating duration-independent enhancement of sociability in hungry individuals, with stronger effects at 12 min of social interaction (Fig. 6g). The hungry group exhibited significantly fewer mirror bites than controls during both 12-minute (Fig. 6h) (HGRY + SC + M[12] and CNTRL + SC + M[12], P < 0.0001) and 18-minute (HGRY + SC + M[18] and CNTRL + SC + M[18], P < 0.001) during social buffering, though the duration of social cues (12 and 18 min) did not further modulate aggression reduction. However, social cue duration differentially influenced non-aggressive social behaviors (e.g., preference, engagement).
Pre-Exposure to social buffering suppresses Mirror-Induced aggression
To assess whether the timing of social buffering influences its efficacy in mitigating aggression, we modified our previous protocol by administering social buffering prior to the mirror-induced aggression test. In our earlier findings (Fig. 4), social buffering administered after aggression testing demonstrated behavioral recovery, yet the temporal dynamics of buffering efficacy remained unexplored. Here, we reversed this paradigm, exposing zebrafish to social cues before aggression tests, to evaluate whether proactive social buffering could preemptively reduce aggressive responses. Our findings revealed a significant reduction in aggressive behavior among zebrafish receiving pre-exposure social buffering, with HGRY + pSB-M (hungry + pre social buffering - mirror stimuli) individuals displaying notably less interaction with the mirror stimulus than their CNTRL + pSB-M (control + pre social buffering - mirror stimul). HGRY + pSB-M fish spent less cumulative time in the mirror zone (p < 0.0014; Fig. 7a) and displayed fewer entries into the mirror zone (MZ) relative to CNTRL + pSB-M (p < 0.0030; Fig. 7b), suggesting reduced engagement with the mirror stimulus. Additionally, HGRY + pSB-M individuals showed prolonged latency to approach the mirror (p < 0.0001; Fig. 7c) and reduced mean swimming speed (p < 0.0002; Fig. 7d). In contrast to the earlier MIA test (Fig. 2f), the PI values for CNTRL + pSB-M and HGRY + pSB-M appear more similar, with a rightward shift in the distribution of HGRY + pSB-M fish toward CNTRL + pSB-M levels (Fig. 7e). The narrower gap in the plot reflects a marked increase in PI values and reduced directional change among hungry fish, suggesting that pre-exposure to social buffering enhanced directional stability.
Social Buffering Before Mirror Exposure Mitigates Aggressive Responses. a. Cumulative time spent in the mirror zone for CNTRL + pSB-M and HGRY + pSB-M groups, b. Number of entries to mirror zone, c. Latency to enter mirror zone, d. Mean speed in mm/s, e. Cumulative probability distribution of Parallel Index (PI) values for zebrafish under CNTRL + pSB-M (black) and HGRY + pSB-M (green) conditions, (f) Mean number of aggressive acts performed in the 6 min agonistic interaction, (g) Temporal dynamics of aggression in CNTRL + pSB-M and HGRY + pSB-M. The average number of mirror bites over time is plotted for both groups, with 95% confidence intervals (shaded areas) for each (n = 30/group), (h) Relationship between Valence Index (VI) for aggression and response probability. The graph shows response probability as a function of Valence Index for Aggression in HGRY + pSB-M (black) and CNTRL + pSB-M (green), i Polar scatter plot of the focal fishes’ individual mean resultant vector’s angles α (0˚ to 360˚) combined with corresponding vector lengths R (0 to 1), for each treatment, j. Box plot of the individual resultant vector’s lengths R projected (R proj) onto the stimulus direction (180˚). Positive values indicate directional focus towards the stimulus; zero indicates no directionality; negative values indicate directional focus opposite to the stimulus. Box and whisker plots are shown with arithmetic median (middle line), and boxes represent the 1 st and 3rd quartiles. Data shown as mean ± s.e.m. P values are based on parametric t test (unpaired) comparing response differences. All asterisks indicate significance (*P < 0.05, **P < 0.01, ***P < 0.001 or ****P < 0.0001), ns: not significant; unpaired t test.
The HGRY + pSB-M group showed a significantly lower number of mirror bites than the CNTRL + pSB-M group (P < 0.0001), indicating a marked decrease in aggression (Fig. 7f). The CNTRL + pSB-M group exhibited substantially higher initial aggression levels compared to HGRY + pSB-M (Fig. 7g). This difference was most pronounced during the early observation period. The CNTRL + pSB-M showed a sharp decline in aggressive behavior between 60 and 120 s, with aggression levels decreasing from 15.5 to 1.5 mirror bites. The HGRY + pSB-M group demonstrated relatively stable, low aggression levels throughout the observation period, with a slight declining trend from 2.5 mirror bites at 60 s to near zero at 300 s. The convergence of both conditions suggests that while initial aggressive responses differed significantly between groups, the sustained aggressive behavior remained minimal across both experimental conditions. The HGRY + pSB-M group exhibited a significantly lower Valence Index (VI) compared to the CNTRL + pSB-M group, with reduced aggressive tendencies in individuals subjected to pre-exposure social buffering prior to mirror stimulus presentation (Fig. 7h). Notably, HGRY + pSB-M zebrafish displayed a higher threshold for aggression initiation, with diminished propensity to engage in aggressive behavior, underscoring the capacity of proactive social buffering to suppress aggression even under metabolic stress. Group mean resultant vectors for both CNTRL + pSB-M and HGRY + pSB-M were consistently aligned with the stimulus at 180° (Fig. 7i), while HGRY + SC + M fish displayed greater directional focus during SB [αg (CNTRL + pSB-M) = 188.16°, Rg = 0.15, n = 30; αg (HGRY + pSB-M) = 180.62°, Rg = 0.20, n = 30]. The projection of individual fish movement vectors onto the stimulus direction (R_proj) did not show a significant difference between groups (Fig. 7j), consistent with our earlier observations (Fig. 2l). Our findings demonstrate that pre-exposure to social buffering effectively reduces aggression in zebrafish, with HGRY + pSB-M group exhibiting diminished mirror interaction, prolonged aggression latency and lower Valence Index values. Proactive social buffering suppressed aggression initiation under metabolic stress while stabilizing directional behavior, highlighting its dual role in mitigating aggression across behavioral and temporal domains.
Discussion
In the present study, we showed that social maintenance or social buffering mechanism can attenuate aggression and stress induced by mirror stimuli and food deprivation-induced stress in zebrafish. This underscores the critical role of social interaction in modulating stress-related responses, with evidence suggesting that the presence of interacting conspecifics exerts a stabilizing and calming influence. These interactions seem to shift the focus of stressed individuals, reducing the intensity of aggressive behavior. This highlights the potential adaptive value of social buffering as a strategy for managing both environmental and physiological stressors.
The HGRY zebrafish exhibited a marked reduction in exploratory behavior, highlighting the significant influence of metabolic state on responses to spatial novelty. The increased freezing time observed in the HGRY group is likely indicative of a “startle and freeze” response, characterized by sudden movements followed by brief immobility. This behavioral pattern may serve as an adaptive mechanism to evade threats and enhance camouflage in uncertain environments64. Such hunger-induced behavioral lethargy aligns with energy conservation strategies, reflecting a physiological adaptation to metabolic stress. Notably, the differences in movement patterns, orientation, and interactions with stimuli between the HGRY and CNTRL groups demonstrate the substantial impact of hunger on spatial exploration. These findings emphasize the critical role of metabolic state in shaping behavioral plasticity, offering insights into how internal physiological conditions modulate external behavioral expressions61. Hunger also significantly heightened aggressive responses to mirror stimuli, prolonging territorial interactions and intensifying confrontational behavior. This suggests that aggression may serve as an adaptive strategy for resource acquisition in competitive conditions. The elevated mirror-biting frequency in the HGRY + M group, relative to both CNTRL and HGRY groups, is consistent with a modulatory effect of hunger on aggressive behavior. This observation aligns with previous research showing that nutritional status is closely linked to risk-taking and aggression, with hunger driving animals to adopt more aggressive strategies to secure essential resources47. The altered spatial and directional dynamics in the HGRY + M group, marked by greater movement variability and focused aggression, suggest a heightened focus on perceived rivals. Such aggressive behavior is not unique to zebrafish but is a widespread phenomenon observed across animal taxa, often aimed at protecting group members, families, food, or territory65. Nutritional deficits have been shown to increase aggression across various species, further supporting the link between hunger and escalated confrontational behavior66,67. In the HGRY + SC group, hunger was associated with both reduced individual variability and more uniform social behaviors. This increased social preference may reflect an adaptive strategy for foraging and social learning in resource-scarce and competitive environments, where group living enhances access to food and mitigates predation risks68,69. However, studies in European sea bass have found that food deprivation is associated with reduced social interaction, highlighting the nuanced role of ecological pressures in modulating behavioral adaptations70. The interaction between hunger and social behavior underscores how zebrafish dynamically balance personal and social cues to forage efficiently, often modifying their interaction range in response to group size regardless of food distribution uncertainty3. These findings further highlight the integration of social and feeding circuits, with oxytocin (OXT) playing a key role in prioritizing motivated behaviors, such as seeking social engagement over solitary actions when resources are scarce10.
The results of this study provide compelling evidence for the efficacy of social buffering (SB) in mitigating aggressive behavior in zebrafish. Social buffering’s capacity to mitigate aggression in zebrafish is modulated by contextual, temporal, and proactive factors: group size critically shapes efficacy, with medium-sized groups (6SC) optimizing aggression suppression through enhanced social preference, while smaller (3SC) and larger (9SC) groups retain partial effectiveness, suggesting scalability across social contexts. The pronounced preference for the social ROI in the HGRY + SC + M group, alongside reduced mirror-biting in both HGRY + SC + M and CNTRL + SC + M groups, underscores social cues critical role in attenuating aggression, even under metabolic stress. For HGRY + SC + M individuals, hunger enhances their reliance on social cues, with elevated SPI values indicating a prioritization of affiliative behaviors and a strong directional focus toward conspecifics. These findings align with studies on zebrafish exposed to alarm substances, where combined olfactory and visual social cues dampen fear responses more effectively than isolation, highlighting conserved neural pathways for social buffering21,35. Prolonged social exposure weakens social attraction (declining SPI) yet sustains aggression reduction, dissociating sociability from stress regulation, while pre-exposure to social cues preemptively suppresses aggression under metabolic stress, prolonging latency and stabilizing behavior. Together, these results demonstrate that social buffering operates through distinct yet complementary mechanisms like social context, temporal dynamics, and proactive timing to regulate aggression, with metabolic state acting as a key modulator. This multifaceted framework not only elucidates behavioral resilience in stress-inducing environments but also offers insights into the evolutionary benefits of social living and potential applications for managing aggression-related disorders in animal models. In conclusion, we found that exposure to conspecific cues reduces aggressive responses in zebrafish. These findings establish zebrafish as an ideal, genetically tractable vertebrate model for investigating the neural mechanisms underlying social buffering, particularly in the context of aggression mitigation. Given the evolutionary conservation of social decision-making networks across vertebrates71our work provides a foundation to explore the deep-rooted neural and behavioral mechanisms underlying sociality.
Supplementary materials
Supplementary figures and the accompanying video can be accessed via the link below: https://pdf.ac/t7OyM-8nxb.
Data availability
Video recordings of all four behavioral paradigms (Exploration, Aggression [MIA], Social Interaction [SI], and Social Buffering [SB]) are available at Mendeley Data [https://data.mendeley.com/datasets/ktfr2cwz95/2]71. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request; please contact binuramachandran@uoc.ac.in.
Code availability
The software used to analyze the data generated in this study was developed in Python (v3.10) and the analysis code is available on Github.
References
Dreosti, E., Lopes, G., Kampff, A. R. & Wilson, S. W. Development of social behavior in young zebrafish. Front Neural Circuits 9, (2015).
Marras, S. et al. Fish swimming in schools save energy regardless of their Spatial position. Behav. Ecol. Sociobiol. 69, 219–226 (2015).
Harpaz, R. & Schneidman, E. Social interactions drive efficient foraging and income equality in groups of fish. eLife 9, e56196 (2020).
Kappel, J. M. et al. Visual recognition of social signals by a tectothalamic neural circuit. Nature 608, 146–152 (2022).
Tulogdi, Á. et al. Effects of resocialization on post-weaning social isolation‐induced abnormal aggression and social deficits in rats. Dev. Psychobiol. 56, 49–57 (2014).
Groneberg, A. H. et al. Early-Life social experience shapes social avoidance reactions in larval zebrafish. Curr. Biol. 30, 4009–4021e4 (2020).
Anneser, L. et al. The neuropeptide Pth2 modulates social behavior and anxiety in zebrafish. iScience 25, 103868 (2022).
Bergan, J. F. Neural computation and neuromodulation underlying social behavior. Integr. Comp. Biol. 55, 268–280 (2015).
Anderson, D. J. Circuit modules linking internal States and social behaviour in flies and mice. Nat. Rev. Neurosci. 17, 692–704 (2016).
Wee, C. L. et al. Social isolation modulates appetite and avoidance behavior via a common oxytocinergic circuit in larval zebrafish. Nat. Commun. 13, 2573 (2022).
Burnett, C. J. et al. Hunger-Driven Motivational State Competition Neuron 92, 187–201 (2016).
Lischinsky, J. E. & Lin, D. Neural mechanisms of aggression across species. Nat. Neurosci. 23, 1317–1328 (2020).
Rohles, F. H. & Wilson, L. M. Hunger as a catalyst in aggression. Behav 48, 123–129 (1974).
Janson, C. & Vogel, E. Hunger and aggression in capuchin monkeys. in Feeding ecology in apes and other primates 285–312Cambridge University Press, (2006).
Fokidis, H. B., Prior, N. H. & Soma, K. K. Fasting increases aggression and differentially modulates local and systemic steroid levels in male zebra finches. Endocrinology 154, 4328–4339 (2013).
Smith, N. K. & Grueter, B. A. Hunger-driven adaptive prioritization of behavior. FEBS J. 289, 922–936 (2022).
Schwartz, M. W., Woods, S. C., Porte, D., Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Wang, T., Hung, C. C. Y. & Randall, D. J. THE COMPARATIVE PHYSIOLOGY OF FOOD DEPRIVATION: from feast to famine. Annu. Rev. Physiol. 68, 223–251 (2006).
Carlini, E. A., Hamaoui, A. & Märtz, R. M. W. Factors influencing the aggressiveness elicited by Marihuana in food-deprived rats. Br. J. Pharmacol. 44, 794–804 (1972).
Padilla, S. L. et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 19, 734–741 (2016).
Filosa, A., Barker, A. J., Dal Maschio, M. & Baier, H. Feeding state modulates behavioral choice and processing of prey stimuli in the zebrafish tectum. Neuron 90, 596–608 (2016).
Chathooth, N. et al. Hunger Induced Perceptional Shift Influence Decisive Behavior In Zebrafish. Preprint at (2024). https://doi.org/10.21203/rs.3.rs-4925537/v1
Zaupa, M. et al. The Calmodulin-interacting peptide Pcp4a regulates feeding state-dependent behavioral choice in zebrafish. Neuron 112, 1150–1164e6 (2024).
Wee, C. L. et al. A bidirectional network for appetite control in larval zebrafish. eLife 8, e43775 (2019).
Jennings, J. H. et al. Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 565, 645–649 (2019).
Larson, E. T., O’Malley, D. M. & Melloni, R. H. Aggression and vasotocin are associated with dominant–subordinate relationships in zebrafish. Behav. Brain. Res. 167, 94–102 (2006).
Oliveira, R. F., Silva, J. F. & Simões, J. M. Fighting zebrafish: characterization of aggressive behavior and Winner–Loser effects. Zebrafish 8, 73–81 (2011).
Teles, M. C. & Oliveira, R. F. Quantifying aggressive behavior in zebrafish. In Zebrafish Vol. 1451 (eds Kawakami, K. et al.) 293–305 (Springer New York, 2016).
Chou, M. Y. et al. Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. Science 352, 87–90 (2016).
Zabegalov, K. N. et al. Understanding zebrafish aggressive behavior. Behav. Process. 158, 200–210 (2019).
Ariyomo, T. O. & Watt, P. J. Aggression and sex differences in lateralization in the zebrafish. Anim. Behav. 86, 617–622 (2013).
Teles, M. C., Dahlbom, S. J., Winberg, S. & Oliveira, R. F. Social modulation of brain monoamine levels in zebrafish. Behav. Brain. Res. 253, 17–24 (2013).
De Abreu, M. S. et al. Neuropharmacology, pharmacogenetics and pharmacogenomics of aggression: the zebrafish model. Pharmacol. Res. 141, 602–608 (2019).
Reichmann, F., Pilic, J., Trajanoski, S. & Norton, W. H. J. Transcriptomic underpinnings of high and low mirror aggression zebrafish behaviours. BMC Biol. 20, 97 (2022).
Mathuru, A. S. et al. Familiarity with companions aids recovery from fear in zebrafish. Preprint At. https://doi.org/10.1101/098509 (2017).
Faustino, A. I., Tacão-Monteiro, A. & Oliveira, R. F. Mechanisms of social buffering of fear in zebrafish. Sci. Rep. 7, 44329 (2017).
Gilmour, K. M. & Bard, B. Social buffering of the stress response: insights from fishes. Biol. Lett. 18, 20220332 (2022).
Kikusui, T., Winslow, J. T. & Mori, Y. Social buffering: relief from stress and anxiety. Phil Trans. R Soc. B. 361, 2215–2228 (2006).
Mikami, K., Kiyokawa, Y., Takeuchi, Y. & Mori, Y. Social buffering enhances extinction of conditioned fear responses in male rats. Physiol. Behav. 163, 123–128 (2016).
Culbert, B. M., Gilmour, K. M. & Balshine, S. Social buffering of stress in a group-living fish. Proc. R Soc. B. 286, 20191626 (2019).
Davitz, J. R. & Mason, D. J. Socially facilitated reduction of a fear response in rats. J. Comp. Physiological Psychol. 48, 149–151 (1955).
Kiyokawa, Y., Takeuchi, Y. & Mori, Y. Two types of social buffering differentially mitigate conditioned fear responses. Eur. J. Neurosci. 26, 3606–3613 (2007).
Smith, A. S. & Wang, Z. Hypothalamic Oxytocin mediates social buffering of the stress response. Biol. Psychiatry. 76, 281–288 (2014).
Teles, M. C., Almeida, O., Lopes, J. S. & Oliveira, R. F. Social interactions elicit rapid shifts in functional connectivity in the social decision-making network of zebrafish. Proc. R. Soc. B. 282, 20151099 (2015).
Cook, A., Beckmann, H., Azap, R. & Ryu, S. Acute stress modulates social approach and social maintenance in adult zebrafish. eNeuro 10, ENEURO0491–222023 (2023).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacotherapeutics. 1, 94–99 (2010).
Nakajo, H. et al. Hunger potentiates the habenular winner pathway for social conflict by Orexin-Promoted biased alternative splicing of the AMPA receptor gene. Cell. Rep. 31, 107790 (2020).
Won, S., Park, K., Lim, H. & Lee, S. J. Sexual dimorphism in cognitive disorders in a murine model of neuropathic pain. Behav. Brain Funct. 16, 1 (2020).
Sivarajan, D. & Ramachandran, B. Antibiotics modulate frequency and early generation of epileptic seizures in zebrafish. Exp. Brain Res. 241, 571–583 (2023).
Ladu, F. et al. Live predators, robots, and Computer-Animated images elicit differential avoidance responses in zebrafish. Zebrafish 12, 205–214 (2015).
Norton, W. H. J. et al. Modulation of Fgfr1a signaling in zebrafish reveals a genetic basis for the Aggression–Boldness syndrome. J. Neurosci. 31, 13796–13807 (2011).
Gerlai, R., Lahav, M., Guo, S. & Rosenthal, A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol. Biochem. Behav. 67, 773–782 (2000).
Angiulli, E. et al. Increase in environmental temperature affects exploratory behaviour, anxiety and social preference in Danio rerio. Sci. Rep. 10, 5385 (2020).
Stednitz, S. J. et al. Forebrain control of behaviorally driven social orienting in zebrafish. Curr. Biol. 28, 2445–2451e3 (2018).
Abril-de-Abreu, R., Cruz, J. & Oliveira, R. F. Social eavesdropping in zebrafish: tuning of attention to social interactions. Sci. Rep. 5, 12678 (2015).
Harris, C. R. et al. Array programming with numpy. Nature 585, 357–362 (2020).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in python. Nat. Methods. 17, 261–272 (2020).
Hunter, J. D. & Matplotlib A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Speakman, J. R. & Selman, C. Physical activity and resting metabolic rate. Proc. Nutr. Soc. 62, 621–634 (2003).
Dametto, F. S. et al. Feeding regimen modulates zebrafish behavior. PeerJ 6, e5343 (2018).
Carreño Gutiérrez, H., Vacca, I., Pons, A. I. & Norton, W. H. J. Automatic quantification of juvenile zebrafish aggression. J. Neurosci. Methods. 296, 23–31 (2018).
Gemmer, A. et al. Oxytocin receptors influence the development and maintenance of social behavior in zebrafish (Danio rerio). Sci. Rep. 12, 4322 (2022).
Lopez-Luna, J., Al-Jubouri, Q., Al-Nuaimy, W. & Sneddon, L. U. Reduction in activity by noxious chemical stimulation is ameliorated by immersion in analgesic drugs in zebrafish. J. Exp. Biol. 220, 1451–1458 (2017).
Helmy, M., Zhang, J. & Wang, H. Neurobiology and neural circuits of aggression. In Neural Circuits of Innate Behaviors Vol. 1284 (ed. Wang, H.) 9–22 (Springer Singapore, 2020).
Arber, B., Huntingford, F. A. & Crompton, D. W. T. The effect of hunger and cestode parasitism on the shoaling decisions of small freshwater fish. J. Fish Biol. 47, 524–536 (1995).
Chapman, B. B., Morrell, L. J. & Krause, J. Unpredictability in food supply during early life influences boldness in fish. Behav. Ecol. 21, 501–506 (2010).
Bhattacharya, K. & Vicsek, T. Collective foraging in heterogeneous landscapes. J. R Soc. Interface. 11, 20140674 (2014).
Bhattacharya, K. & Vicsek, T. To join or not to join: collective foraging strategies. J. Phys. : Conf. Ser. 638, 012015 (2015).
Aimon, C., Le Bayon, N., Le Floch, S. & Claireaux, G. Food deprivation reduces social interest in the European sea bass Dicentrarchus labrax. Journal Experimental Biology Jeb. 190553 https://doi.org/10.1242/jeb.190553 (2019).
O’Connell, L. A. & Hofmann, H. A. Evolution of a vertebrate social Decision-Making network. Science 336, 1154–1157 (2012).
Ramachandran, Binu et al., “Social maintenance masks induced aggression in zebrafish”, Mendeley Data, V3, doi:https://doi.org/10.17632/ktfr2cwz95.31 (2025).
Acknowledgements
The Department of Zoology, University of Calicut, is greatly acknowledged for the infrastructure support.
Funding
The Department of Science and Technology, Science Engineering and Research Board (DST-SERB), Government of India is gratefully acknowledged for awarding research funding under the Early Career Research Scheme (ECR/2018/002479) and Seed grant from (U.O. No.16249/2022/Admn) University of Calicut to Dr Binu Ramachandran.
Author information
Authors and Affiliations
Contributions
BR: conceptualization, writing, supervision, project administration, funding acquisition., MSM: statistical analysis., MSM, BR: interpretation of the data., AAK, MSM, RKV, GKTM, DS, FPF, NC: methodology, formal analysis, writing, investigation. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The University of Calicut’s Ethics Committee and the Committee for the Control and Supervision of Experiments on Animals (CCSEA) regulation 2021 were followed for all animal-related research projects. Every effort was made to reduce the number of animals utilised and their suffering.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kuniyil, A.A., Malik, M.S., Vellattu, R.K. et al. Social maintenance masks induced aggression in zebrafish. Sci Rep 15, 34396 (2025). https://doi.org/10.1038/s41598-025-17318-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17318-1