Abstract
Intranasal dexmedetomidine (IN DEX) is being increasingly used for sedation in children undergoing nonpainful diagnostic procedures. Successful procedural sedation facilitates rapid and accurate clinical diagnosis and treatment, thereby improving clinical management efficiency. As the success rate of sedation is influenced by multiple factors, enhancing sedation success, reducing procedure-related risks, and minimizing adverse events are critical clinical priorities. In this study, children aged 12–72 months (1–6 years) who underwent outpatient examinations requiring sedation with IN DEX were included. Data were extracted from the electronic medical records system of the outpatient sedation suite. The training dataset was analysed using least absolute shrinkage and selection operator (LASSO) and multivariable logistic regression to develop a nomogram-based clinical predictive model. Model performance was evaluated via receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA), with validation performed using a separate validation dataset. The developed nomogram, which incorporates napping before sedation, respiratory diseases, snotty before sedation, neurological diseases, sedation history, sedation duration, and sedation method, exhibited moderate predictive value (AUC = 0.833), favourable calibration, and enhanced clinical benefit. This study establishes a clinical prediction nomogram that can assist anaesthesiologists in the outpatient sedation suite to customize and optimize sedation plans, thereby improving the sedation success rate of IN DEX, enhancing the efficiency of nonpainful diagnostic procedures, and providing substantial clinical benefits.
Similar content being viewed by others
Introduction
Procedural sedation is essential for nonpainful diagnostic examinations in paediatric patients, who have unique demographics because of their young age, incomplete physical and mental development, limited tolerance to unfamiliar environments, and poor emotional regulation. Exposure to unfamiliar health care providers, examination equipment, noise, and dim and confined examination rooms often induces panic, crying, and restlessness, leading to non-cooperation during procedures requiring prolonged immobility1. These behaviours compromise examination efficiency and quality while increasing the risks of adverse reactions, such as catecholamine-mediated tachycardia, increased respiratory secretions, and dyspnoea. Parental separation during procedures further exacerbates challenges1. Thus, moderate-to-deep sedation or anaesthesia is often required to optimize examination outcomes and reduce adverse events in infants and preschoolers2,3.
Intranasal dexmedetomidine (IN DEX), a highly selective α2-adrenergic receptor agonist with minimal respiratory depression, has gained popularity in paediatric sedation4. Its noninvasive administration is well tolerated by children, yielding sedation comparable to intravenous routes and mimicking natural sleep1. A number of sedation studies for nonpainful procedures have reported the efficacy and dosing of dexmedetomidine in a wide range of paediatric age groups4,5,6. While IN DEX has improved sedation success rates in our outpatient suite, sedation failure remains problematic because of multifactorial influences (e.g., patient characteristics, sedation protocols, and environmental factors). Identifying predictors of sedation success is critical for optimizing clinical practice.
Although logistic regression analysis can identify factors affecting the success rate of sedation, relatively few studies have investigated the degree of influence of these factors and how to use them to predict the success rate. A nomogram is a graphical representation of complex mathematical formulas7. We innovatively use clinical variables that affect the success rate, such as patient age and disease name, to construct a statistical prediction model in a graphical manner, thereby generating an individual-specific prediction of the probability of successful sedation. This can help predict the success rate for patients in need of sedation, and targeted optimization of sedation protocols can be carried out accordingly, with the expectation of improving the actual success rate of sedation8.
Materials and methods
Study design and population
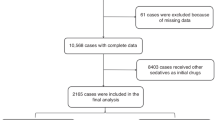
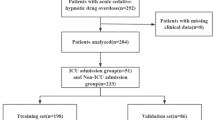
This single-centre retrospective study included 12,095 paediatric patients (12–72 months) who underwent nonpainful diagnostic procedures at Shenzhen Children’s Hospital between January 2022 and June 2023. This study was approved by the Institutional Review Board (IRB no. 202409502) and adhered to the Good Clinical Practice guidelines and the Declaration of Helsinki. Data were extracted from the outpatient sedation suite’s electronic medical records system. The inclusion criteria were as follows: (1) age 12–72 months; (2) ASA I–III; (3) parental consent for intranasal sedation; and (4) complete clinical data. The exclusion criteria were as follows: (1) age < 12 or > 72 months; (2) alternative sedation methods (e.g., chloral hydrate or oral midazolam); (3) incomplete/incorrect records; and (4) changes in the mid-procedure sedation method. A study flowchart is shown in Fig. 1.
Clinical practice
The children were accompanied by their parents or legal guardians to outpatient clinics, during which the attending physician prescribed orders for nonpainful diagnostic examinations on the basis of the child’s condition. These examinations may have included chest X-rays, computed tomography (CT), magnetic resonance imaging (MRI), pulmonary function tests (PFT), auditory brainstem response (ABR) tests, and transthoracic echocardiograms (TTE). Parents or guardians then took the child and the examination orders to the registration desk in the radiology or imaging department to complete the necessary registration procedures.
After reviewing the registration details and inquiring about the child’s fasting status, if the staff in the radiology or imaging department determined that procedural sedation was needed, they recommended that the child fast for more than 1 h, although prolonged fasting was not strictly mandated9,10,11. This information, including the scheduled time for sedation and the examination timeline, was documented on the sedation request form.
Patients were expected to arrive at the sedation room in advance of the scheduled sedation time to undergo an assessment by the anaesthesiologist, during which the patient’s baseline characteristics, such as sex, age, medical condition, and medical history, were evaluated. On the basis of the sedation request form, the estimated duration of the examination, and the level of sedation required during the procedure, the anaesthesiologist performed a comprehensive evaluation to develop a sedation plan tailored to the patient’s needs. The patient’s relevant information was subsequently recorded in the outpatient sedation suite’s electronic medical records system.
After reviewing the relevant Literature and research experience and incorporating practical operational summaries, our outpatient sedation protocol at the sedation suite was refined through repeated adjustments. The sedation protocol involved the administration of IN DEX at a dosage of 2.5–3 mcg/kg via an atomizer or drops or a combination of IN DEX at a dosage of 2.5 mcg/kg plus oral midazolam at a dosage of 0.2–0.3 mg/kg for paediatric patients undergoing nonpainful diagnostic procedures5,11,12,13. Adjustments were made as needed, including the use of intravenous or inhalation sedation when necessary1,14,15. Anaesthesiologists at the sedation suite developed individualized sedation plans tailored to the specific needs of each patient. A dedicated sedation nurse, trained in a structured program to ensure competency in performing sedation according to the sedation protocol, closely monitored and documented the patient’s vital signs (heart rate [HR], respiratory rate [RR], oxygen saturation [SpO2], ECG, and blood pressure [BP]) and level of sedation. Adverse events were also recorded, and the nurse promptly reported any special cases to the anaesthesiologist. Vital signs and sedation levels were recorded at baseline and every 5 min after medication administration16. Sedation levels were assessed using a modified Ramsay score17. A modified Ramsay sedation scale is described in Fig. 2. Patients were considered sedated when they achieved modified Ramsay levels 2–3 (moderate or deep sedation)13,18,19.
Once the patient reached the required sedation level, in conjunction with the child’s baseline vital signs and the anaesthesiologist’s assessment, the sedation nurse transferred the patient to the examination department at the scheduled time to await the diagnostic procedure. If a patient was not adequately sedated after 30 min, an additional 1 mcg/kg adjunct dose of IN DEX was administered, or intravenous or inhalation sedation was utilized. Successful sedation was defined as the ability to complete all examinations after the onset of sedation without any additional sedatives or dose18,20. After completing the examination, patients were transferred to the recovery room for continuous monitoring. Patients were deemed to have recovered once they returned to modified Ramsay level 5 and were only permitted to leave the sedation suite after being evaluated by the anaesthesiologist18.
Data collection and definitions
Fifteen variables were collected: demographics (gender, age, weight), primary diagnosis (neurological/cardiovascular/respiratory diseases), ASA status, sedation duration (> 30 vs. <30 min), snotty before sedation (crying or respiratory infections can lead to increased nasal secretions and oral secretions, which can affect the effectiveness of intranasal sedation), napping before sedation (adequate sleep or sleep deprivation before sedation can also affect the effectiveness of intranasal sedation), fever, baseline vital signs (Pulse/SpO2 before sedation), sedation history and sedation method (IN DEX alone or with oral midazolam). High-risk patients (ASA ≥ III) required intravenous access and rescue equipment.
Statistical analyses
The statistical analyses were conducted using R software (version 4.3.0; The R Foundation for Statistical Computing, Vienna, Austria). The R caret package was employed to randomly partition the data into a training set and a validation set at a theoretical ratio of 7:3. Descriptive statistics were performed to summarize the baseline characteristics, with continuous variables presented as medians and interquartile ranges (IQR), and categorical variables expressed as frequencies with percentages (%). For comparisons of continuous data, the Mann-Whitney U test was applied. Categorical variables were analysed using the chi-square (χ²) test or Fisher’s exact test, depending on the distribution of the data.
For predictor selection and regularization, least absolute shrinkage and selection operator (LASSO) regression analysis was performed21. Lambda_1se (the lambda value corresponding to the most regularized model with the lowest prediction error) was used to identify the optimal model with the fewest independent variables. The features selected by LASSO regression were incorporated into a multivariable logistic regression model to develop a predictive model for sedation success. A nomogram was constructed on the basis of the logistic regression model to facilitate visualization and interpretation of the predictive probabilities22. The characteristics of the predictors are presented as odds ratios (OR) with corresponding 95% confidence intervals (CI). The performance of the nomogram was evaluated in both the training and validation sets using calibration and discrimination analyses23. Calibration curves were constructed to assess the agreement between the predicted and observed probabilities, while Harrell’s concordance index (C-index) and the area under the receiver operating characteristic (ROC) curve (AUC) were calculated to evaluate the discriminative ability of the model24. Additionally, decision curve analysis (DCA) was performed to assess the clinical utility and net benefit of the predictive model25. Statistical significance was defined as a two-sided p value < 0.05.
Results
Patient baseline data
Among the 12,095 patients (7398 males and 4697 females), 8466 were allocated to the training set, and 3629 were allocated to the validation set, with no significant baseline differences (Table 1).
Screening for predictive factors in the training set
With LASSO regression, seven variables were selected: napping before sedation, respiratory diseases, snotty before sedation, neurological diseases, sedation history, sedation duration, and sedation method (Fig. 3A, B).
Variable selection by logistic LASSO regression. A LASSO coefficient profiles for variables significant in univariate analysis; each coefficient profile plot was produced against the log(lambda) sequence. B seven variables with nonzero coefficients were selected by optimal lambda. By verifying the optimal parameter (lambda) in the LASSO model, the partial likelihood deviance (binomial deviance) curve was plotted versus log(lambda), and dotted vertical lines were set using the minimum criteria and the one standard error of the minimum criteria. LASSO indicates least absolute shrinkage and selection operator.
Predictive model construction
Multivariate logistic regression revealed that all seven variables were significant predictors (Table 2). A nomogram was developed, with risk factors including napping before sedation (OR = 0.584), respiratory/neurological diseases (OR = 0.470/0.333), snotty before sedation (OR = 0.573), sedation history (OR = 0.158), and sedation time (> 30 min) (OR = 0.208), while IN DEX + oral midazolam was protective (OR = 7.259) (Fig. 4A,B).
A predictive model was developed using multivariate logistic regression. (A) Multivariate logistic regression analysis of risk predictors. The ORs, CIs, and p values are displayed, and p < 0.05 indicated a statistically significant difference. (B) Nomogram for predicting the success rate of sedation with IN DEX in children who underwent nonpainful diagnostic procedures.
Predictive model validation (Predictive Performance)
The sensitivity and specificity of the predictive model were measured using the ROC curve. The prediction model had an AUC for the nomogram pooled over the training set of 0.833 (95% CI, 0.815–0.851), a cut-off value of 0.926, precision of 0.845, sensitivity of 0.858, and specificity of 0.654 (Fig. 5A), whereas for the validation set the AUC was 0.853 (95% CI, 0.825–0.881), and the cut-off value was 0.926, with a precision of 0.866, a sensitivity of 0.876, and a specificity of 0.707 (Fig. 5B), indicating fair to good performance.
Validation of the success rate prediction nomogram using a receiver operating characteristic (ROC) curve. The y-axis indicates the true positive rate of the success rate prediction, whereas the x-axis indicates the false-positive rate. The solid line depicts the performance of the nomogram in the training set (A) and validation set (B).
The predictive model was calibrated with the use of a calibration curve and Hosmer-Lemeshow test. The calibration curves exhibited a high level of alignment for both the predictive model and the validation set. Hosmer-Lemeshow analysis demonstrated excellent agreement between the calculated and observed probabilities (Fig. 6A,B).
The calibration curves of the prediction model in the training (A) and validation (B) sets. Calibration curves were used to evaluate how a classifier was calibrated and how the probabilities of predicting each class label differ. The x-axis represents the average predicted probability. The y-axis is the ratio of positives. The ideal calibrated model generates a calibration curve that presents a straight line from (0, 0) and moves linearly.
The DCA nomogram similarly suggested that the prediction model (red) showed greater net benefit than “Intervene All” (thin) or “Intervene None” (thick) management did, indicating that our nomogram has potential for use in clinical decision-making (Fig. 7A,B).
Decision curve analysis for the success rate prediction nomogram from the training set (A) and from the validation set (B). The y-axis represents the net benefit. The thick solid line indicates the assumption that all patients received no intervention. The thin solid line represents the assumption that all patients have intervention, and the red line represents the success rate prediction nomogram. Additionally, the area under the decision curve was used to assess the clinical utility of the prediction model. The net benefit of the prediction model (red) was greater than that of “Intervene All” (thin) or “Intervene None” (thick) management, indicating that our nomogram has potential for use in clinical decision-making.
Discussion
This single-centre study conducted a retrospective analysis by extracting raw data from the sedation suite between January 2022 and June 2023. Our data analysis revealed that age significantly influenced the success rate of sedation with IN DEX. Previous studies have also confirmed that age is a critical factor in sedation failure, with particular attention given to younger and older age groups10,19,26,27. Specifically, Zhou X et al.28. reported that age (0–1 year and > 6 years) was a risk factor for sedation failure, with success rates of 90.2% for the 0–1 year group, 93.1% for the 1–3 year group, 92.7% for the 3–6 year group, and 78.4% for the > 6 years group. These findings underscore that age groups (0–1 year and > 6 years) may not be suitable for establishing a predictive model.
Children younger than 6 years are at elevated risk of adverse events29particularly because of the heightened vulnerability of their respiratory systems to sedating medications. This age group is especially susceptible to the effects of sedation on respiratory drive, airway patency, and protective airway reflexes16. In our sedation suite, children aged 1–6 years constituted more than 70% of the patient population, with a comparable success rate for IN DEX sedation within this range. Therefore, improving sedation success rates and reducing complications in this specific age group are significant advantages. This rationale informed our decision to analyse and develop a predictive model utilizing data from children aged 1–6 years.
We analysed data from 12,095 paediatric patients who met the inclusion criteria and employed LASSO and logistic regression to analyse and select from the 15 recorded variables. Our analysis identified seven statistically significant variables, which were used to construct forest plots and a nomogram. Among these, six variables were categorized as risk factors with ORs < 1: napping before sedation (yes), respiratory diseases (yes), nasal congestion before sedation (yes), neurological diseases (yes), sedation time (> 30 min), and sedation history (yes). Additionally, the use of intranasal and oral sedation was identified as a protective factor, with an OR > 1. These variables are presented in Table 2.
When appropriate, daytime nap durations were considered biologically normal for individuals under the age of 6 years30,31. Missing one nap by preschoolers resulted in more negative emotional responses32and the provision of naps improved toddlers’ performance on a generalization task33 and improved their cognitive performance34. For physiological and health reasons, paediatric patients tend to nap during medical appointments. In the sedation process, children who nap before sedation may have more difficulty falling asleep, potentially affecting the success rate of sedation. In our study, “nap before sedation” had a β value of −0.538 and an OR of 0.584.
Among the various influencing factors, the primary diseases of paediatric patients are crucial19,28. We concluded that “respiratory diseases” had a β value of −0.755 and an OR of 0.470, whereas “neurological diseases” had a β value of −1.100 and an OR of 0.333, highlighting their significant negative influencing factors.
In outpatient examinations, upper respiratory tract infections (URTI) and pneumonia in children constitute the majority of respiratory diseases. Grunwell et al.35 reported that URTI was a significant risk factor for failed sedation. Children aged 1–6 years were at high risk for URTI, with the pathogens invading mainly the child’s upper respiratory tract, including the nose and throat36. Clinical manifestations typically include symptoms such as nasal congestion, runny nose, cough, or fever37. Changes in nasal mucosa conditions could affect the sedative effect of IN DEX27and further investigation is needed to determine whether the administration of antiviral or antibiotic medications for treating respiratory system diseases in children influences the efficacy of IN DEX.
In addition to increased nasal secretions caused by underlying diseases, young age and facing unfamiliar hospital environments and health care workers can lead to fear and resistance among paediatric patients during the IN DEX process. This resistance may manifest as a refusal to cooperate and crying. In such cases, even in patients without respiratory diseases, crying can also result in a rapid increase in nasal secretions and even significant discharge. Dexmedetomidine is absorbed through the nasal mucosa, crosses the blood-brain barrier, and affects the central nervous system. Therefore, any condition that leads to increased nasal mucosa secretions can affect the absorption of dexmedetomidine through the nasal mucosa, impacting the success rate and effectiveness of sedation28. In this study, “snotty before sedation” had a β value of −0.557 and an OR of 0.573.
Another primary disease factor was neurological disease, with a β value of −1.100 and an OR of 0.333. Children with intellectual disabilities or developmental delays in psychomotor development have incomplete neurological development, leading to a low arousal threshold, poor self-control, and self-awareness, which enhance their psychological defence mechanisms. As a result, they may exhibit refusal and avoidance during examinations, making sedation more challenging for these children than for typical children38. Longer procedures that require immobility involving children younger than 6 years or those with developmental delay often require an increased depth of sedation to gain control of their behaviour16. Psychostimulants may increase the dosage requirement of sedatives during anaesthesia39. Long-term use of antiepileptic drugs in children with epilepsy can induce the expression of liver microsomal enzyme P-450, altering the liver metabolism and capacity to clear anaesthetics. This can lead to an increased demand for nondepolarizing muscle relaxants, sedatives, and analgesics in paediatric patients during surgery40,41. Autism spectrum disorders (ASD) are also common neurological disorders in children. When children with autism undergo outpatient examinations, the challenges they face are related to communication, sensory issues, the hospital environment, and health care providers42. Some scholars believe that patients with ASD have reduced sensitivity to pain, whereas others argue that pain sensitivity in patients with ASD is either increased or not abnormal43,44. Medications commonly used by patients with ASD, such as aripiprazole or risperidone, have certain side effects that may include changes in sleep patterns, extrapyramidal symptoms, weight gain, abdominal pain, irritability, headaches, and other effects, additionally inducing hypotension during general anaesthesia and potentially triggering arrhythmias45. Therefore, when paediatric patients with neurological diseases are receiving IN DEX sedation, it is important to consider both the impact of the disease and treatment medications on the sedative effect and to pay attention to the prevention and management of complications.
A history of sedation or sedation failure is a risk factor that influences the success rate of sedation. In this study, the β-value for “sedation history” was − 1.847, with an OR of 0.158. Several studies26,27,35 analysing risk factors for failed sedation in children have shown that a history of sedation failure increased the likelihood of sedation failure. Li et al.46 identified a history of sedation failure as the sole risk factor for sedation failure.
Sedation time is another influencing factor and one of the most challenging factors to assess during the sedation process. It is crucial to estimate the sedation time needed for the patient during the examination process before IN DEX. This determines the sedation method and dosage of sedative medication. Estimating the sedation time required for the examination needs to be considered from multiple perspectives and tailored to the individual circumstances. The sedation time is the total duration from the onset of intranasal sedation to the successful completion of nonpainful diagnostic procedures1. This timeframe encompasses waiting times, preexamination preparation times, and, if multiple examinations were involved, the integration of the time required for each procedure, along with the time spent transporting the patient between procedures.
On the basis of the operational experience of the sedation suite, single examinations such as X-rays, CT scans, and transthoracic echocardiography can generally be successfully completed within 30 min under smooth conditions at each stage. However, complex procedures such as MRI scans, auditory monitoring, pulmonary function tests, and multi-item combined examinations require more time and have high quality requirements, usually necessitating sedation times exceeding 30 min. Therefore, we categorized sedation time into > 30 min and < 30 min. Examinations requiring < 30 min have a high success rate, whereas those requiring > 30 min have higher sedation requirements, involving complex examination processes and a higher incidence of unexpected situations, resulting in relatively lower success rates. The β value for the influencing factor of sedation time was − 1.568, with an OR of 0.208.
The decision to administer IN DEX alone or in combination with oral midazolam, as well as to determine the specific dosage, should be based on a comprehensive consideration of various factors, such as the nature of the examination, quality requirements, and the estimated sedation time. Some examinations, such as X-rays, CT scans, and transthoracic echocardiography, are of short duration and are conducted in a relatively quiet environment with minimal external stimuli. For such examinations, IN DEX alone to achieve a moderate sedation depth of modified Ramsay level 3 will be sufficient10. On the other hand, for longer procedures such as MRI scans where the MRI equipment generates considerable noise, for examinations that require the patient to remain still, or in cases of multiple combined procedures, IN DEX alone may not be adequate. In such instances, a combination of IN DEX and oral midazolam is often needed to achieve a sedation depth ranging from moderate to deep, corresponding to modified Ramsay levels 3− 2 1. Therefore, the sedation method and dosage play crucial roles in determining the probability of sedation success. However, it is not advisable to opt solely for a high-dose combination of IN DEX and oral midazolam sedation, uniformly applying deep sedation, as this may lead to excessive and unnecessary complications, resulting in oversedation in medical practice. The optimal sedation target should be achieved by selecting the lowest effective dose of medication with the highest therapeutic index for the procedure1,2.
We analysed the data retrieved from the electronic medical records registry system of the sedation suite, segregating the eligible data into training and validation sets. In the training set, we employed LASSO regression analysis and multivariable logistic regression analysis for variable selection, identifying 7 statistically significant variables from the initial pool of 15. A predictive model was subsequently developed on the basis of these findings, and a ROC curve was generated. The predictive model was subsequently evaluated using the ROC curve. The prediction model had an AUC for the nomogram pooled over the training set of 0.833 (95% CI, 0.815–0.851) (Fig. 3A) and was calibrated with the use of a calibration curve and the Hosmer-Lemeshow test. The calibration curves demonstrated a very good degree of fit for both the predictive model and the validation set. Hosmer-Lemeshow analysis demonstrated excellent agreement between the calculated and observed probabilities (Fig. 4A). The DCA of the nomogram similarly suggested that our nomogram has potential for use in clinical decision-making (Fig. 5A). We also successfully validated the model using a validation set (Figs. 3B, 4B and 5B), which demonstrated that the predictive model could effectively predict the success rate of sedation with IN DEX, offering valuable guidance for clinical practice and making it worthy of emulation in clinical settings.
Conclusions
In conclusion, we constructed a predictive model that incorporates seven variables to develop a nomogram that demonstrates good clinical utility. This nomogram can assist anaesthesiologists in the sedation suite in identifying and screening potential risk factors leading to sedation failure, guiding the formulation of more individualized sedation plans, increasing the success rate of sedation, and ultimately facilitating the smooth completion of outpatient examinations, thereby enhancing the clinical efficiency of care for paediatric patients.
Limitations
(1) Clinical data of paediatric patients were manually entered into the electronic medical records system by anaesthesiologists or sedation nurses rather than being automatically collected and stored. Although incomplete data were excluded, some data may not have accurately reflected the original information. (2) Some influencing factors were difficult to assess accurately, such as “sedation time”, and some factors were challenging to classify, perhaps requiring more reasonable classification methods. In practical work, many clinical manifestations of paediatric patients lack specific quantifiable indicators, such as “nap before sedation” or “snotty before sedation”, yet these factors affect the success rate of sedation. (3) The nomogram was well developed with sufficient internal validation but lacked external validation. External validation, ideally across multiple and diverse datasets, is the gold standard and should be performed when feasible. Unfortunately, most nomograms (including those from our institution) typically report only internal validation. While these methods prevent overinterpretation of data, they fail to eliminate all bias from potential overfitting inherent in variable and threshold selection, nor do they assess accuracy across diverse patient populations23. (4) Numerous factors influenced the success rate of procedural sedation, and some influencing factors might not have been included in the statistical analysis and discussion, which could have affected the model’s establishment, such as medication use (e.g., antiepileptics, antipsychotics) and recent upper respiratory tract infections or premedication.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. E-mail address: bestxyt@126.com.
References
Lyu, X., Tao, Y. & Dang, X. Efficacy and safety of intranasal Dexmedetomidine vs. Oral Chloral hydrate for sedation in children undergoing computed tomography/magnetic resonance imaging: A Meta-Analysis. Front. Pediatr. 10, 872900. https://doi.org/10.3389/fped.2022.872900 (2022).
Callahan, M. J. & Cravero, J. P. Should I irradiate with computed tomography or Sedate for magnetic resonance imaging? Pediatr. Radiol. 52 (2), 340–344. https://doi.org/10.1007/s00247-021-04984-2 (2022).
Callahan, M. J. et al. Correction to: Ionizing radiation from computed tomography versus anesthesia for magnetic resonance imaging in infants and children: patient safety considerations. Pediatr. Radiol. 48 (3), 454. https://doi.org/10.1007/s00247-017-4057-9 (2018).
Tervonen, M., Pokka, T., Kallio, M. & Peltoniemi, O. Systematic review and meta-analysis found that intranasal Dexmedetomidine was a safe and effective sedative drug during paediatric procedural sedation. Acta Paediatr. 109 (10), 2008–2016. https://doi.org/10.1111/apa.15348 (2020).
Sulton, C., Kamat, P., Mallory, M. & Reynolds, J. The use of intranasal Dexmedetomidine and Midazolam for sedated magnetic resonance imaging in children: A report from the pediatric sedation research consortium. Pediatr. Emerg. Care. 36 (3), 138–142. https://doi.org/10.1097/PEC.0000000000001199 (2020).
Marra, P. et al. Sedation with intranasal Dexmedetomidine in the pediatric population for auditory brainstem response testing: review of the existing literature. Healthcare 10 (2), 287. https://doi.org/10.3390/healthcare10020287 (2022).
Jiang, J., Chen, X. Y. & Guo, H. Clinical characteristics and nomogram model for predicting the risk of recurrence of complicated urinary tract infection in pediatric patients. Sci. Rep. 14 (1), 25393. https://doi.org/10.1038/s41598-024-76901-0 (2024).
Lin, N. et al. A nomogram for predicting hemorrhagic shock in pediatric patients with multiple trauma. Sci. Rep. 14 (1), 13308. https://doi.org/10.1038/s41598-024-62376-6 (2024).
Coulthard, P. et al. Current UK dental sedation practice and the National Institute for health and care excellence (NICE) guideline 112: sedation in children and young people. Br. Dent. J. 218 (8), E14–E14. https://doi.org/10.1038/sj.bdj.2015.338 (2015).
Yu, Q. et al. Median effective dose of intranasal Dexmedetomidine sedation for transthoracic echocardiography in pediatric patients with noncyanotic congenital heart disease: an up-and‐down sequential allocation trial. Pediatr. Anesth. 27 (11), 1108–1114. https://doi.org/10.1111/pan.13235 (2017).
Inserra, E. et al. Safety and effectiveness of intranasal dexmedetomidine together with midazolam for sedation in neonatal MRI. Pediatr. Anesth. 32(1):79–81 (2022) .https://doi.org/10.1111/pan.14307
Freriksen, J. J. M., Van Der Zanden, T. M., Holsappel, I. G. A., Molenbuur, B. & De Wildt, S. N. Best Evidence-Based dosing recommendations for Dexmedetomidine for premedication and procedural sedation in pediatrics: outcome of a Risk-Benefit analysis by the Dutch pediatric formulary. Pediatr. Drugs. 24 (3), 247–257. https://doi.org/10.1007/s40272-022-00498-y (2022).
Hongbin, G. Combined use of intranasal Dexmedetomidine and an oral novel formulation of Midazolam for sedation of young children during brain MRI examination: a prospective, single-center, randomized controlled trial. Published online 2022.
Olgun, G. & Ali, M. H. Use of intranasal Dexmedetomidine as a solo sedative for MRI of infants. Hosp. Pediatr. 8 (2), 68–71. https://doi.org/10.1542/hpeds.2017-0120 (2018).
Cui, Y. et al. Predictors of pediatric sedation failure with initial dose of intranasal Dexmedetomidine and oral Midazolam. Pediatr. Res. 94 (6), 2054–2061. https://doi.org/10.1038/s41390-023-02758-0 (2023).
Coté, C. J., Wilson, S., AMERICAN ACADEMY OF PEDIATRICS & AMERICAN ACADEMY OF PEDIATRIC DENTISTRY. Guidelines for monitoring and management of pediatric patients before, during, and after sedation for diagnostic and therapeutic procedures. Pediatrics 143 (6), e20191000. https://doi.org/10.1542/peds.2019-1000 (2019).
Ramsay, M. A. E., Savege, T. M., Simpson, B. R. J. & Goodwin, R. Controlled sedation with Alphaxalone-Alphadolone. BMJ 2 (5920), 656–659. https://doi.org/10.1136/bmj.2.5920.656 (1974).
Saudek, D. E. et al. Intranasal dexmedetomidine: the ideal drug for sedation in the pediatric echo lab? Cardiol. Young. 32 (4), 545–549. https://doi.org/10.1017/S1047951121002493 (2022).
Jung, S. M. Drug selection for sedation and general anesthesia in children undergoing ambulatory magnetic resonance imaging. Yeungnam Univ. J. Med. 37 (3), 159–168. https://doi.org/10.12701/yujm.2020.00171 (2020).
Van Hoorn, C. E. et al. Off-label use of Dexmedetomidine in paediatric anaesthesiology: an international survey of 791 (paediatric) anaesthesiologists. Eur. J. Clin. Pharmacol. 77 (4), 625–635. https://doi.org/10.1007/s00228-020-03028-2 (2021).
Tibshirani, R., Friedman, J. A. & Pliable Lasso J. Comput. Graph Stat. ;29(1):215–225. doi:https://doi.org/10.1080/10618600.2019.1648271 (2020).
Tong, C., Miao, Q., Zheng, J. & Wu, J. A novel nomogram for predicting the decision to delayed extubation after thoracoscopic lung cancer surgery. Ann. Med. 55 (1), 800–807. https://doi.org/10.1080/07853890.2022.2160490 (2023).
Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: more than Meets the eye. Lancet Oncol. 16 (4), e173–e180. https://doi.org/10.1016/S1470-2045(14)71116-7 (2015).
Sadatsafavi, M., Saha-Chaudhuri, P., Petkau, J., Model-Based, R. O. C. & Curve Examining the effect of case mix and model calibration on the ROC plot. Med. Decis. Mak. 42 (4), 487–499. https://doi.org/10.1177/0272989X211050909 (2022).
Vickers, A. J. & Holland, F. Decision curve analysis to evaluate the clinical benefit of prediction models. Spine J. 21 (10), 1643–1648. https://doi.org/10.1016/j.spinee.2021.02.024 (2021).
Cui, Y. et al. Analysis of risk factors for Chloral hydrate sedative failure with initial dose in pediatric patients: a retrospective analysis. Pediatr. Drugs. 24 (4), 403–412. https://doi.org/10.1007/s40272-022-00511-4 (2022).
Liu, J., Du, M., Liu, L., Cao, F. & Xu, Y. Sedation effects of intranasal Dexmedetomidine combined with ketamine and risk factors for sedation failure in young children during transthoracic echocardiography. Pediatr. Anesth. 29 (1), 77–84. https://doi.org/10.1111/pan.13529 (2019).
Zhou, X. et al. The effect of age on outpatient pediatric procedural sedation with intranasal dexmedetomidine and oral midazolam. Eur J. Pediatr. https://doi.org/10.1007/s00431-023-05240-5 (2023).
Xie, H., Zhao, J., Tu, H., Wang, W. & Hu, Y. Combined sedation in pediatric magnetic resonance imaging: determination of median effective dose of intranasal Dexmedetomidine combined with oral Midazolam. BMC Anesthesiol. 24 (1), 112. https://doi.org/10.1186/s12871-024-02493-x (2024).
Paruthi, S. et al. Consensus statement of the American academy of sleep medicine on the recommended amount of sleep for healthy children: methodology and discussion. J. Clin. Sleep. Med. 12 (11), 1549–1561. https://doi.org/10.5664/jcsm.6288 (2016).
Mesas, A. E. et al. The role of daytime napping on salivary cortisol in children aged 0–5 years: a systematic review and meta-analysis. Eur. J. Pediatr. 181 (4), 1437–1448. https://doi.org/10.1007/s00431-021-04371-x (2022).
Berger, R. H., Miller, A. L., Seifer, R. & Cares, S. R. Acute sleep restriction effects on emotion responses in 30- to 36-Month-Old children. Published online 2013.
Horváth, K., Liu, S. & Plunkett, K. A daytime nap facilitates generalization of word meanings in young toddlers. Sleep 39 (1), 203–207. https://doi.org/10.5665/sleep.5348 (2016).
Dutheil, F. et al. Effects of a short daytime nap on the cognitive performance: A systematic review and Meta-Analysis. Int. J. Environ. Res. Public. Health. 18 (19), 10212. https://doi.org/10.3390/ijerph181910212 (2021).
Grunwell, J. R., McCracken, C., Fortenberry, J., Stockwell, J. & Kamat, P. Risk factors leading to failed procedural sedation in children outside the operating room. Pediatr. Emerg. Care. 30 (6), 381–387. https://doi.org/10.1097/PEC.0000000000000143 (2014).
Davidson, L. E., Gentry, E. M., Priem, J. S., Kowalkowski, M. & Spencer, M. D. A multimodal intervention to decrease inappropriate outpatient antibiotic prescribing for upper respiratory tract infections in a large integrated healthcare system. Infect. Control Hosp. Epidemiol. 44 (3), 392–399. https://doi.org/10.1017/ice.2022.83 (2023).
Amin, M. T. et al. Over prescription of antibiotics in children with acute upper respiratory tract infections: A study on the knowledge, attitude and practices of non-specialized physicians in Egypt. Qasim M, ed. PLOS ONE. ;17(11):e0277308. (2022). https://doi.org/10.1371/journal.pone.0277308
Salabura, C. et al. [Pain assessment for children and adolescents with autism spectrum disorders (ASD): A systematic review]. L’Encephale. Published online July 5, 2024:S0013-7006(24)00116-7. https://doi.org/10.1016/j.encep.2024.04.007
Courtman, S. P. & Mumby, D. Children with learning disabilities. Pediatr. Anesth. 18 (3), 198–207. https://doi.org/10.1111/j.1460-9592.2007.02323.x (2008).
Larkin, C. M., O’Brien, D. F. & Maheshwari, D. Anaesthesia for epilepsy surgery. BJA Educ. 19 (12), 383–389. https://doi.org/10.1016/j.bjae.2019.08.001 (2019).
Penna, H. D. M. et al. Comparison between oral Midazolam versus oral ketamine plus Midazolam as preanesthetic medication in autism spectrum disorder: double-blind randomized clinical trial. Braz J. Anesthesiol Engl. Ed. 73 (3), 283–290. https://doi.org/10.1016/j.bjane.2022.09.003 (2023).
Sahyoun, C. et al. Safety and efficacy associated with a Family-Centered procedural sedation protocol for children with autism spectrum disorder or developmental delay. JAMA Netw. Open. 6 (5), e2315974. https://doi.org/10.1001/jamanetworkopen.2023.15974 (2023).
Failla, M. D. et al. Initially intact neural responses to pain in autism are diminished during sustained pain. Autism 22 (6), 669–683. https://doi.org/10.1177/1362361317696043 (2018).
Gu, X. et al. Heightened brain response to pain anticipation in high-functioning adults with autism spectrum disorder. Eur. J. Neurosci. 47 (6), 592–601. https://doi.org/10.1111/ejn.13598 (2018).
Maneeton, N. et al. Risperidone for children and adolescents with autism spectrum disorder: a systematic review. Neuropsychiatr Dis. Treat. 14, 1811–1820. https://doi.org/10.2147/NDT.S151802 (2018).
Li, B. L. et al. Using intranasal Dexmedetomidine with buccal Midazolam for magnetic resonance imaging sedation in children: A single-arm prospective interventional study. Front. Pediatr. 10, 889369. https://doi.org/10.3389/fped.2022.889369 (2022).
Acknowledgements
We thank all involved in assessing our participants, and all the participants for taking the time needed for this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Contributions: Conception and design: Yuetao Xie, Huatian Lin, Lihua GaoQuery of literature and information: Guangwu Liao, Ningning CuiData collection: Lihua Gao, Xueqing Wang, Taohua PengData analysis and interpretation: Yuetao Xie, Huatian LinManuscript revision and final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study was reviewed and approved by the Clinical Trial and Biomedical Ethics Committee of Shenzhen Children’s Hospital, Guangdong, China, with the approval number IRB no. 202409502. All patients (or their proxies/legal guardians) agreed to intranasal sedation and signed informed consent. Because this study analysed only anonymized data without involving privacy leakage or harm and because the potential risks to patients’ rights and welfare were no greater than those associated with routine medical care or minimal risk, the ethics committee waived the requirement for informed consent for this retrospective design.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, H., Gao, L., Cui, N. et al. Nomogram for predicting the success rate of sedation with intranasal dexmedetomidine in paediatric nonpainful diagnostic procedures: a retrospective study. Sci Rep 15, 33430 (2025). https://doi.org/10.1038/s41598-025-18931-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-18931-w