Abstract
The development of nitric oxide-donating antiphlogistics is intended to improve the tolerability of the parent compounds. The aim of the present study was to explore the potency and tolerability of nitric oxide-donating acetaminophen (NCX 701) in healthy volunteers and to test the hypothesis that NCX 701 could have additional anti-inflammatory efficacy in an experimental model of low-grade human endotoxemia. In this prospective, double-blind, placebo-controlled trial with parallel group design 40 healthy male volunteers were randomized to single oral treatment with NCX 701 (1–2 g), acetaminophen (1 g paracetamol) or placebo before lipopolysaccharide (2ng/kg) infusion. NCX 701 dose-dependently increased plasma and urine nitric oxide concentrations. Pooled analysis of both NCX 701 doses showed a significant, but obviously clinically irrelevant lowering effect on systolic and diastolic blood pressure during the first 5 h. Overall, peak levels of tumor necrosis factor-alpha correlated well with interleukin-6, interleukin-8, monocyte chemoattractant protein-1 and von Willebrand Factor release across all cohorts. There was no major difference between NCX 701 and acetaminophen with respect to their effect on the lipopolysaccharide-induced secretion of inflammation and endothelium activation markers. Overall, a total of 61 adverse events were reported in all treatment arms, mainly related to lipopolysaccharide. Both acetaminophen and NCX 701 effectively reduced the proportion of subjects experiencing headache. Despite substantial nitric oxide release by NCX 701, which reduced arterial blood pressure, nitric oxide did not result in relevant inhibition of lipopolysaccharide-induced inflammation.
ISRCTN registry number: ISTCTN-13,358,268.
Similar content being viewed by others
Introduction
Within the broad spectrum of analgesic pharmacotherapeutics, non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin have traditionally been predominantly used. However, despite the low quality of pain improvement these substances provide compared to placebo1, their high risk for gastrointestinal and cardiovascular side effects also limits their use in the acute setting2,3. Acetaminophen (paracetamol), which has superior gastrointestinal tolerability compared with other analgesics, might alternatively be prescribed in the analgesic setting. However, acetaminophen has no anti-inflammatory effect and is only weakly potent at peripheral sites in comparison to NSAIDs4.
Literature review
Initial animal studies on rats showed that the development of nitric oxide (NO)-donating NSAIDs (NO-NSAIDs) might be a valid strategy to combine an enhanced anti-inflammatory effect with a concurrent NO-mediated favourable cardiovascular and gastrointestinal tolerability and nephroprotective property3,5. For the human setting, the gastrointestinal-sparing effect of similar NO-derivations of aspirin was confirmed in several pharmacodynamic (PD) studies on inflammation and coagulation biomarkers. Enhanced potency and improved safety were attributed to multifactorial benefits of supplementing the NO-moiety to the parent compound, including anti-proliferative and anti-oxidative efficacy, endothelium protection, increased blood flow and cyclooxygenase (COX) sparing besides the anti-inflammatory activity.
Based on these observations it seemed reasonable to test if a beneficial influence on the pharmacological activity might be obtained as well, if a NO-moiety is added to acetaminophen. It was anticipated that developing NO-acetaminophen (“NCX 701”) might open new perspectives in the treatment of pain by improving not only tolerability, but also enhancing the functional regulation of a wide range of immune and inflammatory mediators, including macrophages, T lymphocytes, antigen-presenting cells, mast cells, and natural killer cells3,6.
The aim of the present study was to explore the potency and safety of NCX 701 compared to acetaminophen in humans and to test the hypothesis that NCX 701 may also have anti-inflammatory properties. To this end, we exposed healthy volunteers to a well-standardised model of low-grade human endotoxemia and evaluated both the safety profile and the anti-inflammatory properties of NCX 701 administered in two different oral doses, compared with acetaminophen alone and placebo6,7,8,9. This model was chosen because previous studies have indicated an anti-inflammatory action of different NO donating drugs10.
Research methods
Study design, participants and study drugs
The trial was conducted at the Department of Clinical Pharmacology and approved by the Ethics Committee of the Medical University of Vienna. It was a prospective, randomized, double-blind, placebo-controlled trial with a parallel group design with single oral administration of either NCX 701 (in two different doses of 1 and 2 g), acetaminophen (1 g) or placebo. All methods were performed in accordance with the relevant guidelines and regulations.
A total of 40 healthy male volunteers were screened within 3 weeks prior to the day of study medication administration at the clinical site. After giving written informed consent eligible subjects were randomized into 4 evenly sized groups of 10 volunteers each. The randomization list was generated by a contract research organization with a publicly available program (www.randomization.com). Individually sealed opaque envelopes containing the randomisation allocation were used.
Participants were hospitalized at the clinical site on day 0 and fed with 3 meals and water to control dietary nitrate intake until the following morning (day 1). On day 1, after overnight fasting, participants received their randomized treatment (1 g NCX 701, 2 g NCX 701, 1 g acetaminophen or placebo) orally after suspension in at least 180 mL of water. Medication was prepared by an unblinded pharmacist, and the appropriate blinded drug was handed over to the blinded study team for subsequent administration. Intake of study medication was scheduled 60 min before lipopolysaccharide (LPS) infusion under the supervision of the clinical team. Volunteers were allowed to leave the study site eight hours after LPS infusion and were requested to return in the morning of the following day (day 2) for blood sampling for PD analysis and reporting of adverse events. One week after LPS infusion a final follow-up visit was scheduled for the morning of day 8. During this visit PD parameters were analysed again and adverse events were recorded once more.
LPS-induced endotoxemia
Endotoxin infusion in humans is a safe and generally well tolerated standardised model to induce a systemic inflammatory response. Our LPS model has been described in detail previously in earlier publications and thus has been tested in more than 1000 volunteers at the investigational site11. All study subjects received 2 ng/kg LPS (National reference Endotoxin, Escherichia coli, United States Pharmacopeial Convention Inc., Rockville, MD, USA) as an intravenous bolus infusion for 1 to 2 min and a continuous infusion of 200 mL/h NaCl 0.9% was given over 8 h. The LPS-induced inflammatory response is limited in duration and severity and mimics the early phase of human sepsis, producing flu-like symptoms, such as fever, chills, myalgias, arthralgias, nausea and headache. Symptoms are most prominent 2–3 h following LPS injection, and begin to resolve after 6 h. Overall, trial subjects are virtually free of symptoms after 8 h.
Blood analysis of nox and endpoint parameters
All blood samples were collected into citrated (3.8%) or EDTA anticoagulated evacuated tubes (Vacutainer, Becton Dickinson, Vienna, Austria) using an indwelling catheter, the cannula of which was rinsed after each sampling with about 2–5 ml of sterile saline solution. As a measure of NO release NOx plasma and urine concentrations were determined. Blood samples for NO measurement were collected pre-dose and closely monitored over 25 h after a single-dose treatment with 1 g NCX 701, 2 g NCX 701, 1 g acetaminophen or placebo and were calorimetrically determined from a linear standard curve, as described earlier12,13. Acetaminophen plasma levels were determined by a fully validated HPLC-UV analytical method (CIT final report nr.23520ATP, Feb 2003). In addition, the effects of the 4 study treatments on markers of inflammation, endothelium activation, and blood counts were analysed at predetermined time intervals over 25 h after drug administration.The specific parameters measured were: interleukin (IL)-6 and 8, monocyte chemoattractant protein-1 (MCP-1), tumour necrosis factor-alpha (TNF-alpha), matrix metalloproteinases (MMP2 and MMP9), white blood counts (WBC), elastase, von Willebrand Factor (VWF), soluble vascular cell adhesion protein-1 (VCAM-1), and soluble E-selectin14.
Plasma levels of soluble E-selectin, IL-6, IL-8, VCAM-1 and TNF-alpha were measured by high sensitivity enzyme immunoassays15,16,17,18,19, VWF: RCo was assayed by turbidometry using a commercial kit from Behring (Marburg, Germany) consisting of lyophilized platelets and ristocetin14. Plasma MCP-I levels were determined by enzyme-immunoassay7. MMP-2 and MMP-9 serum protein levels were assessed by enzyme immune assays (R&D Systems)16. Blood counts were performed with a cell counter (Sysmex, Milton Keynes, UK). All endpoint parameters were evaluated by means of descriptive and statistical analysis by measuring peak plasma concentration.
After the LPS bolus infusion, the following safety parameters were continuously monitored every 15 min during the first 6 h: ECG, heart rate, oxygen saturation and lying blood pressure. A final routine blood analysis was performed after one week. During the entire study period the occurrence of adverse events was monitored. When an adverse event typical of endotoxin (e.g. headache, feeling cold) occurred in temporal relationship with the LPS challenge, its relationship with the study drugs was rated as unknown or not related.
Statistical methods
The effects of 1 g NCX 701, 2 g NCX 701, and 1 g acetaminophen on LPS-induced secretion of inflammation and endothelium activation markers were investigated and compared with each other and with placebo. The LPS-induced concentration changes of these markers, observed in the placebo group represented the net LPS effects, against which the activity of NCX 701 and acetaminophen were compared.
Data were grouped for treatment and summarised by appropriate statistics consisting of arithmetic mean, standard deviation and standard error of the mean for continuous variables and, absolute and relative frequencies for categorical variables.
After data robustness was tested using repeated measures analysis of variance, non-parametric tests were employed for comparisons of continuous variables. Specifically, the Kruskal Wallis ANOVA and the Mann-Whitney U test were used to test differences between groups. One-way ANOVA was used for pooled analysis of NCX treatment on systemic blood pressure. Linear correlation between TNF-alpha values and endpoint parameters were expressed by the Spearman correlation coefficient. The Fisher exact test was used for post hoc comparison of adverse event frequencies between groups.
Descriptive statistics were computed using SAS version 8.2 procedures (Proc Univariate, Proc Means, Proc Freq and Proc Tabulate). Additional statistical calculations were performed using commercially available statistical software (Statistica Vers. 5.0, Tulsa, OK. USA). Statistical significance was defined as p-values less than 0.05.
Results
The mean age of the 40 screened participants was 26 ± 0.6 years and the mean body mass index was 23 ± 0.3 kg/m². All participants finished the study without withdrawal. No relevant changes from the screening period and between treatments were seen for electrocardiograms, safety laboratory markers and physical examinations on day 1.
Overall, a total of 61 adverse events were reported in all treatment arms. There were no serious or severe adverse events in the active treatment groups and the majority of participants completely recovered from adverse events until the end of the observation period. Adverse events among the 4 study arms are summarized in more detail with respect to their severity, outcome and relation with the study drugs in Table 1: 23 adverse events were reported in the placebo group, 19 in the acetaminophen group, 12 in the 1 g NCX 701 group and 7 in the 2 g NCX 701 group. The most frequently reported adverse events in all 4 groups were headache and coldness, both known to be potential side effects of LPS infusion. All subjects in the placebo group experienced headache, whereas 40–50% experienced headache in the active treatment groups (Fisher exact test: p = 0.02 and p = 0.04).
Systolic blood pressure in supine position was significantly lower at 1 and 3 h after intake of the 1 g NCX 701 dose, and at 3 h after intake of the 2 g NCX 701 dose, compared with placebo. Additionally, at 3 h after either NCX 701 dose, the systolic blood pressure was significantly lowered compared with acetaminophen (p < 0.01 for all comparisons). Diastolic blood pressure in supine position was significantly lower at 1 h after intake of either dose compared with placebo or acetaminophen (p < 0.01 for both comparisons).
Pooled analysis of both NCX 701 doses versus acetaminophen and versus placebo showed a lowering effect of NCX 701 on supine systolic blood pressure at 1–3 h (p < 0.01; p = 0.07 and p < 0.01) and lying diastolic blood pressure at 1 and 2 h (p < 0.01 and p = 0.04).
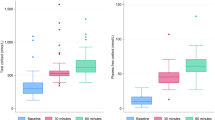
Plasma and urine nitrate concentrations are illustrated in Fig. 1: Peak plasma nitrate values were higher after 1–2 g of NCX 701 than after 1 g acetaminophen or placebo and showed a rapid release after intake of NCX 701. Plasma nitrate AUC also increased dose-dependently.
Illustrates plasma and urine concentrations of nitrate (in µM/l) over the first 25 h after single oral administration of either NCX 701 (in two different doses of 1 g (triangle upwards) and 2 g (triangle downwards), acetaminophen, 1 g (circles) or placebo (squares). Data are presented as mean +/- standard error of the mean (SEM).
Table 2; Fig. 2A and B compare changes in inflammation parameters in response to LPS infusion between the four study groups during study day 1. There was no significant difference in peak plasma concentrations and AUC of IL-6 between the active treatment groups and the placebo group. Elevation of WBC counts in response to LPS infusion were observed in all study volunteers, whereby these leukocytoses did not show relevant variations among the four study groups. Lower AUC of WBC counts in the acetaminophen group compared with placebo, although statistically significant, were not considered of clinical relevance. Peak plasma concentrations of LPS-induced TNF-alpha were not different between the treatment groups (Table 2).
(a) Illustrates the effect of single oral administration of NCX 701 (in two different doses of 1 g (triangle upwards) and 2 g (triangle downwards)), acetaminophen, 1 g (circles) or placebo (squares) on plasma concentrations of tumor necrosis factor-alpha (TNF-alpha; in pg/ml), Interleukin-6 (IL-6; in pg/ml) and Inerleukin-8 (IL-8; in pg/ml) after infusion of 2ng/kg endotoxin LPS. Data are presented as mean +/- standard error of the mean (SEM). (b) Illustrates the effect of single oral administration of NCX 701 (in two different doses of 1 g (triangle upwards) and 2 g (triangle downwards)), acetaminophen, 1 g (circles) or placebo (squares) on plasma concentrations of monocyte chemoattractant protein-1 (MCP-1; in pg/ml), matrix metalloproteinase 2 (MMP2; in ng/ml) and matrix metalloproteinase 9 (MMP9; in ng/ml) after infusion of 2ng/kg endotoxin LPS. Data are presented as mean +/- standard error of the mean (SEM).
Analysing all volunteers together, there was a strong correlation between circulating TNF-alpha levels during day 1 and values of IL-6 (r = 0.79; p < 0.01), IL-8 (r = 0.57; p < 0.01), VWF (r = 0.64; p < 0.01), MCP-1 (r = 0.72; p < 0.01), but not for the values of MMP2, MMP9, Elastase and WBC.
Table 3; Fig. 3 illustrate changes in endothelium activation markers in response to LPS infusion between the four study groups during study day 1. The LPS-dependent increase in VWF appears reduced in the three active treatment groups in comparison to placebo, in particular in the 1 g NCX 701 treated group, in which peak values were significantly lower (p = 0.02) compared with the placebo group.
Illustrates the effect of single oral administration of NCX 701 (in two different doses of 1 g (triangle upwards) and 2 g (triangle downwards)), acetaminophen, 1 g (circles) or placebo (squares) over the course of 25 h on plasma concentration curves of von Willebrand Factor (VWF; in U/dl), soluble E-selectin (in ng/ml) and soluble vascular cell adhesion protein-1 (VCAM-1; in ng/ml) after infusion of 2ng/kg endotoxin LPS. Data are presented as mean +/- standard error of the mean (SEM).
No relevant LPS-induced modification of platelet counts was observed in any of the active treatment groups, compared with placebo.
Discussion
Oral treatment with 1–2 g of NCX 701 induced a dose-dependent NO release into plasma and increased NOx excretion in urine of human volunteers.
The main finding was that the NO moiety at neither dose exhibited significant anti-inflammatory properties, despite an obvious haemodynamic effect, as indicated by lower systolic blood pressure in NCX 701 treated participants compared to those treated with acetaminophen or placebo. In addition, NCX 701 and acetaminophen consistently reduced the proportion of subjects reporting headache.
A bolus infusion of the bacterial endotoxin LPS is a well-accepted model to elicit a systemic inflammatory response in healthy volunteers, characterized by a mild and transient clinical syndrome of low-grade fever with concomitant biochemical evidence of activation of the proinflammatory cytokine network7,8,20.
Also, endotoxin dose-dependently induces the coagulation cascade and an activation of the endothelium20. Therefore, infusion of small doses of LPS into humans has emerged as a valuable tool to explore the PD of new anti-inflammatory and anticoagulant treatments5.
While the inhibition of COX by ibuprofen or indomethacin increases cytokine release, probably by blocking negative feedback loops, aspirin and acetaminophen including its derivative NCX 701 do not induce such effects21,22. In this context, our model corroborates earlier findings showing an antipyretic effect of acetaminophen without altering LPS-induced changes of established inflammation parameters (e.g. TNF-alpha, IL-6, IL-8, MCP-1 and WBC)23. In line with our findings, inhaled NO, given as a low dose combination therapy with glucocorticoids, neither modified the inflammatory cascade with respect to clinical signs and cytokine response in a human experimental inflammatory model24. The authors discussed the lack of an advantageous effect was related to an inappropriate dose and/or timing of the treatment administration, and suggested that there might nevertheless be some anti-inflammatory effect in the lungs25. On the basis of the present results, we were not able to clarify potential mechanisms, by which NCX 701 might mediate a reduction of headache.
In an earlier study Ricciotti et al. showed that NCX-4040 reduced LPS-induced COX-2 and cytokine production by stabilizing IkB-alpha and not via a NO-dependent pathway. Therefore, the authors suggested that NCX does not directly reduce cytokine activity, but needs to be transformed to an active metabolite first in order to exert its inhibitory effect towards the expression of cytokine-enzymes or their subunits, such as proteasomes26.
Further experiments would be required not only to confirm a beneficial clinical effect, but also to allocate and elucidate the exact mechanism of NCX-701.
Other types of adverse events, besides headache and coldness occurred at a similar frequency in the active treatment groups and the placebo group, suggesting these events being caused by the LPS infusion rather than the study treatments.
For acetaminophen, its effect on blood pressure appears to be unimportant, because changes in blood pressure do not occur at therapeutic doses of acetaminophen. In the same way, repeated blood pressure measurements did not indicate that NCX 701 causes symptoms of clinically relevant orthostatic hypotension.
A LPS-induced TNF-alpha release is a driver and determinant of the response in other inflammatory reactions in experimental endotoxemia27. This appears to be also true in the current investigation, because TNF-alpha concentrations for the entire study population strongly correlated with concentrations of certain interleukins, MCP-1 and VWF. However, reductions in peak TNF-alpha concentrations and also in IL-6 were not statistically significant between the active treatment groups and the placebo group.
The LPS-dependent increase in VWF seemed reduced in all three active treatment groups in comparison to placebo, in particular in the group treated with 1 g NCX 701. These results are in line with earlier publications, reporting a dampening effect of endogenous NO production on increased VWF concentrations, no matter if the enhanced VWF release was stimulated by exercise, histamine or other secretagogues28,29,30. A similar blunting effect on increased VWF antigen has been shown for L-NMMA, however, this effect was not interpreted as intrinsic, but merely to occur in combination with a further stimulus, only28.
As the present study used NCX 701 doses considerably lower than those used in earlier animal trials31, the lack of some in vivo effects in our experiment could be due to insufficient concentrations of NO in humans. However, further dose escalation of NCX 701 in humans is limited by the maximum recommended single doses of acetaminophen and could also be limited by the dose-dependent NO-mediated hypotension.
Limitations of the study
Our setting is only an acute inflammation model and findings are therefore explorative. We cannot exclude that NO may have more pronounced anti-inflammatory effects in lower grade inflammation, but we did not use an appropriate disease model to transfer our findings. Furthermore, it is unlikely that NO has an effect on chronic inflammation32. Due to the small sample size, it cannot be ruled out that small effects may have been missed. Finally, NCX 701 may show a different profile in patients with compromised endothelial function where it may compensate for impaired NO release32.
Strengths of the study
Previous studies have shown that NO levels are difficult to interpret due to high background levels33. As our volunteers were set on a very strict nitrate-free diet, we were able to rule out alimentary confounders, and saw an unequivocal signal over noise ratio. The inflammation response observed in this study was similar to previous endotoxemia trials across multiple countries and regions34,35, indicating external validity of the LPS model.
In summary NO was rapidly released from NCX 701 which was reflected in an early transient decrease in blood pressure in our LPS model. Similar to acetaminophen, NCX 701 prevented headache, but increased NO availability and release had limited anti-inflammatory effects in our LPS model of human endotoxemia.
Data availability
Data availabilityThe data presented in this study are available on request from the corresponding author. The data are not publicly available due to data privacy reasons.
References
Enthoven, W. T., Roelofs, P. D., Deyo, R. A., van Tulder, M. W. & Koes, B. W. Non-steroidal anti-inflammatory drugs for chronic low back pain. Cochrane Database Syst. Rev., 2(2), Cd012087 (2016).
Stubanus, M., Riegger, G. A., Kammerl, M. C., Fischereder, M. & Krämer, B. K. Renal side-effects of cyclo-oxygenase-type-2 inhibitor use. Lancet 355 (9205), 753 (2000).
Wallace, J. L., Soldato, P. D., Cirino, G. & Muscará, M. N. Nitric oxide-releasing nsaids: GI-safe antithrombotics. IDrugs 2 (4), 321–326 (1999).
Prescott, L. F. Paracetamol: past, present, and future. Am. J. Ther. 7 (2), 143–147 (2000).
del Soldato, P., Sorrentino, R. & Pinto, A. NO-aspirins: a class of new anti-inflammatory and antithrombotic agents. Trends Pharmacol. Sci. 20 (8), 319–323 (1999).
Levi, M., van der Poll, T., ten Cate, H. & van Deventer, S. J. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur. J. Clin. Invest. 27 (1), 3–9 (1997).
Mayr, F. B. et al. Duffy antigen modifies the chemokine response in human endotoxemia. Crit. Care Med. 36 (1), 159–165 (2008).
Suffredini, A. F., Hochstein, H. D. & McMahon, F. G. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J. Infect. Dis. 179 (5), 1278–1282 (1999).
van Lier, D., Geven, C., Leijte, G. P. & Pickkers, P. Experimental Hum. Endotoxemia as Model. Systemic Inflamm. Biochimie, 159: 99–106. (2019).
Fiorucci, S., Antonelli, E., Burgaud, J. L. & Morelli, A. Nitric Oxide—Releasing NSAIDs Drug Saf., 24(11): 801–811. (2001).
Derhaschnig, U. et al. Effects of aspirin and NO-aspirin (NCX 4016) on platelet function and coagulation in human endotoxemia. Platelets 21 (5), 320–328 (2010).
Jilma, B. et al. Sex differences in concentrations of exhaled nitric oxide and plasma nitrate. Life Sci. 58 (6), 469–476 (1996).
Roth, E. et al. L-Arginine deficiency after liver transplantation as an effect of arginase efflux from the graft. Influence on nitric oxide metabolism. Transplantation 57 (5), 665–669 (1994).
Kovacevic, K. D. et al. The aptamer BT200 effectively inhibits von Willebrand factor (VWF) dependent platelet function after stimulated VWF release by Desmopressin or endotoxin. Sci. Rep. 10 (1), 11180 (2020).
Endler, G. et al. The interleukin-6 G(-174)C promoter polymorphism does not determine plasma interleukin-6 concentrations in experimental endotoxemia in humans. Clin. Chem. 50 (1), 195–200 (2004).
Jilma, B. et al. Pharmacodynamics of active site-inhibited factor VIIa in endotoxin-induced coagulation in humans. Clin. Pharmacol. Ther. 72 (4), 403–410 (2002).
Leitner, J. M. et al. Recombinant human antithrombin inhibits thrombin formation and Interleukin 6 release in human endotoxemia. Clin. Pharmacol. Ther. 79 (1), 23–34 (2006).
Schoergenhofer, C. et al. Defibrotide enhances fibrinolysis in human endotoxemia - a randomized, double blind, crossover trial in healthy volunteers. Sci. Rep. 9 (1), 11136 (2019).
Thaler, B. et al. Differential in vivo activation of monocyte subsets during low-grade inflammation through experimental endotoxemia in humans. Sci. Rep. 6, 30162 (2016).
Kuhns, D. B., Alvord, W. G. & Gallin, J. I. Increased Circulating cytokines, cytokine antagonists, and E-selectin after intravenous administration of endotoxin in humans. J. Infect. Dis. 171 (1), 145–152 (1995).
Spinas, G. A., Bloesch, D., Keller, U., Zimmerli, W. & Cammisuli, S. Pretreatment with ibuprofen augments Circulating tumor necrosis Factor-α, Interleukin-6, and elastase during acute endotoxinemia. J. Infect. Dis. 163 (1), 89–95 (1991).
Summ, O., Andreou, A. P., Akerman, S. & Goadsby, P. J. A potential nitrergic mechanism of action for indomethacin, but not of other COX inhibitors: relevance to indomethacin-sensitive headaches. J. Headache Pain. 11 (6), 477–483 (2010).
Pernerstorfer, T. et al. Acetaminophen has greater antipyretic efficacy than aspirin in endotoxemia: a randomized, double-blind, placebo-controlled trial. Clin. Pharmacol. Ther. 66 (1), 51–57 (1999).
Hållström, L., Berghäll, E., Frostell, C., Sollevi, A. & Soop, A. L. Nitric oxide inhalation and glucocorticoids as combined treatment in human experimental endotoxemia. Crit. Care Med. 36 (11), 3043–3047 (2008).
Pompe, J. C., Kox, M., Hoedemaekers, C. W., van der Hoeven, J. G. & Pickkers, P. Nitric oxide inhalation and glucocorticoids as combined treatment in human experimental endotoxemia: it takes not always two to Tango. Crit. Care Med., 37(9), 2676–2677 (2009).
Ricciotti, E. et al. NCX 4040, a nitric oxide-donating aspirin, exerts anti-inflammatory effects through Inhibition of I kappa B-alpha degradation in human monocytes. J. Immunol. 184 (4), 2140–2147 (2010).
Suffredini, A. F. et al. Effects of Recombinant dimeric TNF receptor on human inflammatory responses following intravenous endotoxin administration. J. Immunol. 155 (10), 5038–5045 (1995).
Jilma, B. et al. Partial Blockade of nitric oxide synthase blunts the exercise-induced increase of von Willebrand factor antigen and of factor VIII in man. Thromb. Haemost. 78 (4), 1268–1271 (1997).
Jilma, B. et al. Effects of Histamine and nitric oxide synthase Inhibition on plasma levels of von Willebrand factor antigen. J. Lab. Clin. Med. 131 (2), 151–156 (1998).
Pernerstorfer, T., Stohlawetz, P., Kapiotis, S., Eichler, H. G. & Jilma, B. Partial Inhibition of nitric oxide synthase primes the stimulated pathway of vWF-secretion in man. Atherosclerosis 148 (1), 43–47 (2000).
Curros-Criado, M. M. & Herrero, J. F. Antinociceptive effects of NCX-701 (nitro-paracetamol) in neuropathic rats: enhancement of antinociception by co-administration with Gabapentin. Br. J. Pharmacol. 158 (2), 601–609 (2009).
Roy, R., Wilcox, J. & Webb, A. J. and K. O’Gallagher, Dysfunctional and dysregulated nitric oxide synthases in cardiovascular disease: mechanisms and therapeutic potential. Int. J. Mol. Sci. 24(20), 15200–15221 (2023).
Turnbull, C. M. et al. Mechanism of action of novel NO-releasing Furoxan derivatives of aspirin in human platelets. Br. J. Pharmacol. 148 (4), 517–526 (2006).
Dorresteijn, M. J. et al. Atazanavir-induced unconjugated hyperbilirubinemia prevents vascular hyporeactivity during experimental human endotoxemia. Front. Immunol. 14, 1176775 (2023).
Suffredini, A. F. & Noveck, R. J. Human endotoxin administration as an experimental model in drug development. Clin. Pharmacol. Ther. 96 (4), 418–422 (2014).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.B.-Z., M.S., B.J. ; methodology, S.B.-Z., M.S., B.J. ; validation, C.S, B.J. ; formal analysis, S.B.-Z., M.S. ; investigation, U.D., C.S., M.F.-B. ; resources, B.J. ; data curation, S.B.-Z., M.S. ; writing—original draft preparation, S.B.-Z., M.S. ; writing—review and editing, S.B.-Z., M.S., S.S., B.J. ; visualization, S.B.-Z., M.S. ; supervision, B.J. ; project administration, B.J. ; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Brunner-Ziegler, S., Staudacher, M., Schörgenhofer, C. et al. A randomized placebo-controlled trial in healthy volunteers examining the effects of acetaminophen and NO-acetaminophen NCX 701 in human endotoxemia. Sci Rep 15, 33612 (2025). https://doi.org/10.1038/s41598-025-19046-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19046-y