Abstract
Gross hematuria (GH) following COVID-19 mRNA vaccination has been increasingly reported in patients with IgA nephropathy (IgAN). This study assessed the effect of post-vaccination GH on kidney function. A total of 441 Japanese outpatients with biopsy-confirmed IgAN (median age: 51 years; 56% female; baseline eGFR: 57 mL/min/1.73 m²) were classified into three groups: unvaccinated (n = 25), vaccinated without GH (n = 391), and vaccinated with GH (n = 25). Kidney function was evaluated at approximately 1 year (midpoint) and 2 years (endpoint) after baseline. The annual eGFR change (ΔeGFR: mL/min/1.73 m²/year) at midpoint was − 1.16, − 1.03, and − 2.50, respectively. The GH group showed a significantly greater decline compared to the non-GH group (p = 0.015), and GH was significantly associated with greater eGFR decline at midpoint (odds ratio: 2.97, p = 0.038). At the endpoint, the ΔeGFR was − 0.45, − 1.93, and − 1.72, with no significant differences among the groups (p = 0.773). In this cohort, GH after vaccination was observed to be associated with a greater short-term decline in kidney function, although the retrospective design precludes establishing causality.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) pandemic and the global COVID-19 vaccination campaign have impacted patients across a wide range of conditions. Among these, immunoglobulin A nephropathy (IgAN) has attracted particular attention as several reports describe acute kidney injury (AKI) accompanied by gross hematuria (GH) following COVID-19 mRNA vaccination1,2,3,4,5,6,7. Although COVID-19 mRNA vaccination in patients with IgAN may cause renal dysfunction8,9,10 and a slight increase in disease recurrence11,12, the long-term kidney prognosis is relatively favorable9,10,13. However, some patients with IgAN who develop GH following COVID-19 mRNA vaccination subsequently experience AKI, which may require aggressive treatment, such as immunosuppressive therapy and even dialysis, and may result in chronic kidney disease (CKD) as a lasting complication3,4,5,6,7. Previous studies have demonstrated that in patients with IgAN, episodes of GH unrelated to COVID-19 vaccination are often associated with AKI, with approximately 25% progressing to chronic kidney dysfunction14,15. Thus, episodes of GH following COVID-19 vaccination may have lasting adverse effects on kidney function in patients with IgAN. Although some studies16,17 have suggested that GH following COVID-19 mRNA vaccination has minimal impact on kidney function, their findings are limited by methodological issues. For example, one study excluded patients who had received steroid therapy or underwent tonsillectomy during follow-up16, while another did not include a control group for comparison17.
Thus, this retrospective cohort study aimed to identify the factors affecting kidney function after COVID-19 mRNA vaccination in patients with IgAN, with a particular focus on GH following COVID-19 mRNA vaccination.
Results
A flow diagram of participant enrollment is presented in Supplementary Figure S1. Overall, 441 patients with IgAN were included in the study. Baseline clinical characteristics before the first dose of the COVID-19 mRNA vaccine are summarized in Table 1. The median age was 51 years (interquartile range [IQR]: 42–62), and 247 patients (56%) were female. The median duration of IgAN was 12 years (IQR: 5–21), and 182 patients (44%) had hypertension. At baseline, 325 patients (74%) were receiving renin-angiotensin-aldosterone system inhibitors (RAASi), while 240 (54%) had a history of corticosteroid therapy, and 166 (38%) had a history of tonsillectomy. The median estimated glomerular filtration rate (eGFR) was 57 mL/min/1.73 m² (IQR: 43–73), and the median urinary protein-to-creatinine ratio (UPCR) was 0.24 g/g (IQR: 0.08–0.67).
Patients with IgAN were divided into three groups: unvaccinated (n = 25), vaccinated with GH (n = 25), and vaccinated without GH (n = 391). Compared with those in the other two groups, patients in the vaccinated with GH group were significantly younger (p < 0.05), more likely to be female (p < 0.05), had a shorter history of IgAN (p < 0.05), higher eGFR values (p < 0.05), and more frequent episodes of microscopic hematuria (p < 0.001). Additional treatments following vaccination, such as corticosteroid therapy or tonsillectomy, were significantly more common in the vaccinated with GH group than in the other two groups (Table 2).
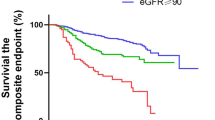
Figure 1a and b illustrate the longitudinal changes in eGFR and UPCR. In the vaccinated without GH group, eGFR declined steadily from baseline to endpoint, whereas in the vaccinated with GH group, a significant decline occurred only from baseline to midpoint. The unvaccinated group showed no significant eGFR change (Fig. 1a). UPCR increased at the midpoint and decreased at the endpoint in the vaccinated without GH group, while no significant changes were observed in the other groups (Fig. 1b). No cases of AKI occurred during the follow-up period.
Changes in kidney functions across groups. (a) Trends in eGFR across the three groups. In the no GH group, eGFR declined progressively at both the midpoint and endpoints compared with that at baseline (*p < 0.001). In the GH group, eGFR decreased at the midpoint compared with that at the baseline (#p < 0.05); however, no significant change was observed between the midpoint and endpoint. (b) Trends in UPCR across the three groups (*p < 0.001, #p < 0.05). (c) The annual change in eGFR (ΔeGFR) at the midpoint among the three groups. The vaccinated with GH group had a significantly greater decline in eGFR than the vaccinated without GH group (*p < 0.05). (d) ΔeGFR at the endpoint among the three groups. No significant differences were observed. (e) The annual percentage change in eGFR (ΔeGFR%) at the midpoint among the three groups. The vaccinated with GH group had a significantly greater decline in eGFR than the vaccinated without GH group (*p < 0.05). (f) ΔeGFR% at the endpoint among the three groups. No significant differences were observed. Abbreviations: eGFR, estimated glomerular filtration rate; GH, gross hematuria; UPCR, urinary protein-to-creatinine ratio.
At the midpoint, the annual change in eGFR (ΔeGFR) was − 1.16, − 1.03, and − 2.50 mL/min/1.73 m²/year in the unvaccinated, vaccinated without GH, and vaccinated with GH groups, respectively, with significantly lower values in the GH group than in the no-GH group (p = 0.015; Fig. 1c). Similarly, the corresponding percentage change in eGFR (ΔeGFR%) was − 2.81, − 1.90, and − 4.66%/year, and was significantly lower in the GH group than in the no-GH group (p = 0.048; Fig. 1e).
Univariable and multivariable logistic regression analyses identified factors associated with a decline in eGFR at the midpoint (ΔeGFR < − 1.11 mL/min/1.73 m²/year) across the cohort (Table 3). In the multivariable model, post–COVID-19 GH (OR 2.97, 95% CI 1.06–8.30, p = 0.038), baseline eGFR ≥ 60 (OR 1.84, 95% CI 1.12–3.05, p = 0.017), and UPCR ≥ 0.3 g/g (OR 2.03, 95% CI 1.26–3.27, p = 0.003) were independently associated with eGFR decline. Additionally, linear regression analysis revealed that GH was significantly associated with ΔeGFR at the midpoint in both univariable and multivariable models (β = − 3.326, p < 0.0001; β = − 2.878, p = 0.003; Table 4).
Supplementary Table S1 shows logistic regression analyses of factors associated with ΔeGFR%. In the multivariable model, post-vaccination GH, UPCR ≥ 0.3 g/g, and SGLT2 inhibitor use were significantly associated with ΔeGFR% (OR 3.22, 95% CI 1.15–9.02, p = 0.026; OR 2.14, 95% CI 1.33–3.44, p = 0.002; OR 2.05, 95% CI 1.13–3.74, p = 0.019). GH was also significantly associated with ΔeGFR% at the midpoint in both crude and adjusted models (β = − 3.724, p = 0.030; β = − 3.792, p = 0.037; Supplementary Table S2).
At the endpoint (median 756 days), the ΔeGFR was − 0.45, − 1.93, and − 1.72 mL/min/1.73 m²/year in the unvaccinated, vaccinated without GH, and vaccinated with GH groups, respectively, with no significant differences (Fig. 1d). Similarly, the ΔeGFR% was − 1.81, − 3.44, and − 2.65%/year, without significant differences (Fig. 1f). Regression analyses (univariable, multivariable, and linear) found no association between post–COVID-19 vaccination GH and ΔeGFR or ΔeGFR% at the endpoint (Tables 3 and 4; Supplementary Tables S1, S2).
After propensity score matching for age, sex, body mass index (BMI), baseline eGFR, UPCR, disease duration, and treatment history, a well-balanced GH and non-GH cohorts were obtained (Supplementary Table S3). In this matched cohort, the GH group showed a significantly greater decline in ΔeGFR and ΔeGFR% at the midpoint compared with the non-GH group (p = 0.021). Due to the limited sample size (N = 50), multivariable analysis was not feasible without the risk of overfitting; therefore, correlations were assessed in a univariable framework, which consistently demonstrated a significant association between GH and both ΔeGFR and ΔeGFR% at the midpoint (Supplementary Tables S4). As the decline events defined by ΔeGFR (< 1.11 mL/min/1.73 m² per year) and ΔeGFR% (< − 2.0% per year) at the midpoint occurred in the same cases, the results of the ΔeGFR% analysis were omitted. Furthermore, in a sensitivity analysis restricted to patients with available renal pathology (n = 41), stratification by the OXFORD MEST-C variables attenuated the association between GH and ΔeGFR at the midpoint, rendering the association no longer statistically significant (Supplementary Table S5).
In the subgroup and interaction analyses, neither post-vaccination steroid therapy nor tonsillectomy significantly modified the association between GH and kidney function decline at the midpoint or endpoint (Supplementary Tables S6 and S7).
Discussion
This study provides novel insights into the clinical significance of GH following COVID-19 mRNA vaccination in patients with IgAN. While GH is a hallmark symptom of IgAN, its implications in the vaccination content have remained unclear. In this study, we investigated the association between post-vaccination GH and kidney function decline. We found that post-vaccination GH was associated with a greater short-term decline in kidney function for approximately one year, even after adjustment for potential confounding factors such as age, sex, duration of IgAN, baseline kidney function, and treatments, including RAASi, corticosteroids, and tonsillectomy. However, during a longer follow-up period of approximately two years, no significant differences in kidney function decline were observed between patients with and without post-vaccination GH.
In patients with IgAN, GH unrelated to COVID-19 mRNA vaccination is a recognized trigger for AKI, with some cases progressing to CKD14,15. The underlying mechanism is hypothesized to involve oxidative stress caused by hemoglobin, heme, and iron released by red blood cell casts, which exert cytotoxic effects on tubular cells15. Several recent reports have described similar episodes of GH and subsequent kidney function decline following COVID-19 mRNA vaccination4,18,19, raising concern that vaccine-associated GH may likewise contribute to long-term deterioration, particularly if recurrent.
Paradoxically, earlier studies have suggested that GH unrelated to vaccination may be associated with better long-term prognosis20,21,22. One possible explanation is lead-time bias, as episodes of GH often prompt earlier clinical evaluation and therapeutic intervention, thereby improving outcomes. In our cohort, 39 patients received additional corticosteroid therapy, and 36 underwent tonsillectomy after vaccination. These interventions, both recognized as effective options for IgAN, may have attenuated subsequent inflammatory activity and preserved kidney function. In the subgroup and interaction analyses, neither post-vaccination steroid therapy nor tonsillectomy significantly modified the association between GH and kidney function decline. The significance of GH observed in the main analysis was attenuated after accounting for these treatments. This attenuation may reflect limited statistical power, as only five additional steroid cases were observed at midpoint and nine at endpoint, while tonsillectomy was performed in two and six cases, respectively). Collectively, our findings support a transient association between post-vaccination GH and short-term decline, without evidence of a sustained difference in long-term trajectories when appropriate clinical management is provided. Prospective studies are warranted to test this hypothesis.
In our cohort, no patients underwent repeat renal biopsy after vaccination; therefore, direct assessment of histological changes associated with post-vaccination GH was not possible. Previous biopsy studies in patients with IgAN who developed GH following vaccination did not exhibit an increased prevalence of acute lesions, such as endocapillary hypercellularity or crescent formation, nor did they reveal evidence of enhanced endothelial injury2,17. Experimental studies indicate that the SARS-CoV-2 spike protein, particularly its S1 subunit, can impair endothelial function through ACE2 downregulation and induction of oxidative stress23,24. While these mechanisms provide a biological rationale, direct clinical evidence of vaccine-induced endothelial injury in IgAN remains lacking. Thus, these findings suggest that post-vaccination GH in IgAN is unlikely to be driven by overt structural injury but may reflect transient functional disturbances, although further studies are warranted.
To our knowledge, this is the first study to specifically evaluate the association of post-COVID-19 mRNA vaccination GH in IgAN, with a focus on longitudinal eGFR changes. Using a real-world cohort, we provide novel insights into the clinical relevance of post-vaccination GH and its temporal association with kidney function trajectory. However, some limitations should be acknowledged. First, the retrospective nature of the study and relatively small sample size from a single-country (Japanese) cohort may introduce selection bias and limit the generalizability of the findings to broader populations. Second, the classification of post- vaccination GH was based on self-reported data collected during routine outpatient visits, raising the potential for recall bias and underreporting of clinical events. Third, kidney function was evaluated at only three discrete time points (baseline, midpoint, and endpoint), which may not capture transient fluctuations or the full spectrum of kidney function dynamics. More granular longitudinal data would be needed to better characterize the natural course of post- vaccination GH and its clinical consequences. Given the retrospective design, limited sample size, and relatively small number of endpoint events, which preclude causal inference, the findings should be interpreted as associative.
In conclusion, in this cohort, GH after vaccination was observed to be associated with a greater short-term decline in kidney function, without evidence of a sustained difference in long-term trajectories. These observational findings should be considered hypothesis-generating and do not directly inform vaccination or treatment policies. Larger multicenter prospective studies with adjudicated endpoints are required to determine whether any causal relationships exist.
Methods
Study design and patients
This study recruited Japanese outpatients with biopsy-proven IgAN who visited Jikei University Hospital, Jikei Katsushika Medical Center, Jikei Kashiwa Hospital, or Jikei Daisan Hospital between February 1, 2021, and September 20, 2022. The exclusion criteria were the lack of urinalysis and blood tests before the first vaccination, complications of other primary glomerular diseases, kidney or urological cancer, kidney transplant recipients, eGFR of < 15 mL/min/1.73 m2, an age of < 18 years, and pregnancy. This study was approved by the Ethics Review Board of the Jikei University School of Medicine (Approval No. 34–137 [11288]) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was waived by the Ethics Review Board of the Jikei University School of Medicine. This cohort study includes cases that have been previously reported25,26.
Clinical measurements
Clinical characteristics before the first dose of COVID-19 mRNA vaccination, including age, sex, BMI, and medical history, such as the current use of RAASi and prior treatment with corticosteroids or tonsillectomy, were obtained from patients’ medical records. Additional treatments administered after vaccination, including corticosteroids, tonsillectomy, escalation of RAASi, and initiation of sodium-glucose cotransporter 2 inhibitors, which were approved for insurance coverage in Japan for the treatment of CKD after the launch of the COVID-19 vaccination campaign, were also evaluated.
Information on the occurrence of GH was obtained through patient interviews and self-reports during routine outpatient visits. Laboratory data, including serum creatinine (Cr), IgA, complement C3, UPCR, and urinary sediments, were also collected. eGFR was defined using the following formula for Japanese individuals:
eGFR (mL/min/1.73 m2) = 194 × Cr− 1.094 × age− 0.287 (× 0.739 for women) (Eq. 1)27.
The red blood cell (RBC) count in the urinary sediment was graded as follows: 0, < 5 RBC/high-power field (HPF); 1, 5–19 RBC/HPF; 2, 20–49 RBC/HPF; and 3, ≥ 50 RBC/HPF2,25. Laboratory data, including Cr, eGFR, UPCR, and urinary sediments, were collected at three time points: before vaccination (baseline), approximately 1 year after vaccination (midpoint), and approximately 2 years after vaccination (endpoint) (Supplementary Fig. S2). For unvaccinated patients, baseline data were collected at time points comparable to those of vaccinated patients.
The annual change in eGFR (ΔeGFR) at the midpoint or endpoint was calculated by dividing the difference from the baseline value by the number of days between visits and multiplying by 365 to standardize the value to an annual rate. The percent change in eGFR (ΔeGFR%) was calculated from the percentage of ΔeGFR in eGFR at baseline.
Renal histopathology
We evaluated renal biopsy specimens of the 50 patients in the propensity score–matched cohort according to the Oxford classification (MEST-C)28. Pathological data were obtained from electronic medical records, pathology reports, and direct review of biopsy slides by nephrologists experienced in renal pathology.
Statistical analysis
Continuous variables are presented as medians with interquartile ranges (IQR), and categorical variables as percentages. Nonparametric continuous variables were compared using the Friedman test, followed by pairwise comparisons using the Wilcoxon signed-rank test with Bonferroni correction for repeated measures among the three groups, and the Kruskal–Wallis test, followed by the Steel–Dwass post hoc test for independent group comparisons. Categorical variables were compared using Pearson’s chi-square test. Clinically relevant factors identified through subgroup comparisons, along with potential confounders, such as prior treatments for IgAN (including RAASi, corticosteroids, and tonsillectomy), were included in multivariable logistic and linear regression analyses.
Propensity score matching was performed to minimize potential selection bias. The propensity score was calculated using a logistic regression model including age, sex, baseline eGFR, proteinuria, BMI, and treatment history (RAASi, corticosteroids, and tonsillectomy). Patients with and without GH were matched 1:1 using the nearest neighbor method with a caliper of 0.2 of the standard deviation of the logit of the propensity score. Balance between groups after matching was assessed using standardized mean differences.
Sensitivity analyses were additionally performed by incorporating each component of the MEST-C score (M0/1, E0/1, S0/1, T0/1/2, C0/1/2) as covariates or by stratification.
To explore whether the association between GH and kidney function decline was modified by subsequent interventions, subgroup analyses were performed according to post-vaccination corticosteroid treatment and post-vaccination tonsillectomy. Interaction terms (GH × post-vaccination steroid treatment and GH × post-vaccination tonsillectomy) were introduced into logistic regression models to formally test for effect modification.
Statistical analyses were performed using SPSS (version 29.0; IBM Corp., Armonk, NY, USA), GraphPad Prism (version 8.4.3; GraphPad Software, La Jolla, CA, USA), and EZR software package (ver. 1.61; Saitama Medical Center, Jichi Medical University, Japan). A two-sided p-value < 0.05 was considered statistically significant.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy concerns, but are available from the corresponding author upon reasonable request.
References
Bomback, A. S., Kudose, S. & D’Agati, V. D. De Novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am. J. Kidney Dis. 78, 477–480 (2021).
Yokote, S. et al. First diagnosis of Immunoglobulin A nephropathy following SARS-CoV-2 mRNA vaccination in Japan. Kidney Int. Rep. 8, 179–182 (2023).
Ritter, A. et al. Clinical spectrum of gross haematuria following SARS-CoV-2 vaccination with mRNA vaccines. Clin. Kidney J. 15, 961–973 (2022).
Ma, Y. & Xu, G. New-onset IgA nephropathy following COVID-19 vaccination. Q. J. M. 116, 26–39 (2023).
Park, K. et al. Letter regarding: A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. Rep. 6, 2246–2247 (2021).
Klomjit, N. et al. COVID-19 vaccination and glomerulonephritis. Kidney Int. Rep. 6, 2969–2978 (2021).
Lim, C. C., Choo, J. & Tan, C. S. COVID-19 vaccination in Immunoglobulin A nephropathy. Am. J. Kidney Dis. 78, 617 (2021).
Lim, R. S., Goh, S. M. & Yeo, S. C. Renal outcomes in Immunoglobulin A nephropathy following COVID-19 vaccination: a retrospective cohort study. Clin. Kidney J. 15, 1789–1791 (2022).
Sun, K., Shang, D., Hao, C. & Lai, L. Renal outcomes in IgA nephropathy following inactivated SARS-CoV-2 vaccination. Clin. Exp. Nephrol. 28, 23–30 (2024).
Wang, C. S. et al. Association of COVID-19 versus COVID-19 vaccination with kidney function and disease activity in primary glomerular disease: A report of the cure glomerulonephropathy study. Am. J. Kidney Dis. 83, 37–46 (2024).
Canney, M. et al. A population-based analysis of the risk of glomerular disease relapse after COVID-19 vaccination. J. Am. Soc. Nephrol. 33, 2247–2257 (2022).
Kronbichler, A., Anders, H. J. & mRNA COVID-19 vaccines and their risk to induce a relapse of glomerular diseases. J. Am. Soc. Nephrol. 33, 2128–2131 (2022).
Chen, C. H., Wu, M. J. & Tsai, S. F. Safety and effectiveness of COVID-19 vaccines in patients with IgA nephropathy: a retrospective cohort study from the TriNetX global collaborative networks. EClinicalmedicine 65, 102306 (2023).
Praga, M. et al. Acute worsening of renal function during episodes of macroscopic hematuria in IgA nephropathy. Kidney Int. 28, 69–74 (1985).
Moreno, J. A. et al. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin. J. Am. Soc. Nephrol. 7, 175–184 (2012).
Nagatsuji, K. et al. Adverse reactions and effects on renal function of COVID-19 vaccines in patients with IgA nephropathy. Clin. Exp. Nephrol. 28, 1168–1177 (2024).
Aoki, R. et al. Gross hematuria after the COVID-19 mRNA vaccination: nationwide multicenter prospective cohort study in Japan. Kidney360 5, 1322–1332 (2024).
Ota, Y. et al. Association between COVID-19 vaccination and relapse of glomerulonephritis. Clin. Exp. Nephrol. 27, 236–242 (2023).
Chen, C. C. et al. Acute kidney disease following COVID-19 vaccination: a single-center retrospective study. Front. Med. (Lausanne). 10, 1189243 (2023).
D’Amico, G. et al. Idiopathic IgA mesangial nephropathy. Clinical and histological study of 374 patients. Med. (Baltim). 64, 49–60 (1985).
D’Amico, G. et al. Prognostic indicators in idiopathic IgA mesangial nephropathy. Q. J. Med. 59, 363–378 (1986).
Yoshikawa, N. et al. Macroscopic hematuria in childhood IgA nephropathy. Clin. Nephrol. 28, 217–221 (1987).
Lei, Y. et al. SARS-CoV-2 Spike protein impairs endothelial function via downregulation of ACE 2. Circ. Res. 128, 1323–1326 (2021).
Terentes-Printzios, D. et al. The effect of an mRNA vaccine against COVID-19 on endothelial function and arterial stiffness. Hypertens. Res. 45, 846–855 (2022).
Yokote, S. et al. Predictors of gross hematuria after SARS-CoV-2 mRNA vaccination in patients with IgA nephropathy. Kidney360 4, 943–950 (2023).
Okabe, M. et al. Does COVID-19 vaccination trigger gross hematuria in patients with IgA nephropathy? Clin. Kidney J. 17, sfae160 (2024).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992 (2009).
Trimarchi, H. et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int. 91, 1014–1021 (2017).
Acknowledgements
We would like to thank Editage (www.editage.jp) for providing English language editing services.
Funding
This research has not received specific funding from any public, commercial, or charitable source. Institutions had no role in the study design, data collection, analysis, interpretation of data, writing of the report, or decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
SY designed the study, drafted the manuscript, and prepared the figure. SY, MO, AS, SF, KH, TF, HU, and NT collected the data. MO, AS, SF, KH, TS, HU, NT, and TY revised the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was approved by the Ethics Review Board of the Jikei University School of Medicine (Approval No. 34–137 [11288]) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was waived by the Ethics Review Board of the Jikei University School of Medicine.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yokote, S., Okabe, M., Shimizu, A. et al. Gross hematuria after COVID-19 mRNA vaccination and kidney function trajectory in IgA nephropathy. Sci Rep 15, 35169 (2025). https://doi.org/10.1038/s41598-025-19068-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19068-6