Abstract
Myofascial pain syndrome (MPS), a chronic musculoskeletal disorder characterized by myofascial trigger points (MTrPs). The objective is to explore the efficacy and safety of ultrasound-guided myofascial hydrodissection technique (UMHT) compared with trigger point injection of lidocaine (TPI) in the patients with MPS of upper trapezius. This randomized clinical trial was conducted at the rehabilitation medicine center of West China Hospital, Sichuan University, with 41 patients between May and October 2024. Patients were assigned to UMHT group (UMHT) or TPI group (1% lidocaine injection). Outcomes were measured at multiple time points using the Visual Analog Scale (VAS, primary), the short-form McGill pain questionnaire (SF-MPQ), and the neck disability index (NDI). Data were analyzed using SPSS v.29. The VAS scores was significantly decreased after receiving either UMHT (Effect sizes r = 0.716; p < 0.001) or TPI (Effect sizes r = 0.604; p < 0.001) during the 12-week follow-up period, while the SF-MPQ and the NDI showed similar positive results. There was no statistically significant difference between UMHT and TPI in pain reduction and function improvement (p > 0.05). No adverse events had been reported. Both UMHT and TPI demonstrate comparable efficacy and safety profiles in managing MPS of upper trapezius.
Similar content being viewed by others
Introduction
Myofascial pain syndrome (MPS) is a chronic musculoskeletal disorder characterized by the presence of myofascial trigger points (MTrPs), taut bands, and tenderness in skeletal muscles or tendinous insertions1. Its lifetime prevalence reaches up to 85% in the general population2. Prolonged computer use with sustained postures is strongly associated with localized muscle pain, particularly in the neck region3. Such repetitive strain correlates with the formation of MTrPs, with the upper trapezius being the most frequently implicated site in MPS-induced neck pain4,5. In addition, it also induced muscle stiffness, spasms, and restricted joint range of motion6,7. These manifestations can severely impair patients’ quality of life, contributing to insomnia, depression, anxiety, and other emotional disturbances8.
The previous studies demonstrated the association of dysfunctional motor endplates with activation of MTrPs. Dysfunctional motor endplates could induce sustained contractions of skeletal muscle fibers, resulting in localized ischemia, hypoxia, and an energy crisis due to heightened metabolic demands9. Furthermore, the contracted muscle fibers surrounding MTrPs contribute to pathological adhesions, colonic contractions, and scar tissue formation within the myofascial system1. Thus, it is hypothesized that the therapeutic outcomes of MPS depend on the inactivation of MTrPs and the restoration of surrounding myofascial system.
Recently, the treatment of MPS still remains challenging due to the lack of objective evaluation indicators for MTrPs, thus no standard treatment protocol has been established for MPS10,11. The invasive therapies for MPS include dry needling (DN) and trigger point injection (TPI). To inactivate the MTrPs, the DN is performed through rapid in-and-out needle penetration11 whereas the TPI via injection of botulinum toxin type A, lidocaine, 0.9% normal saline, steroid, platelet-rich plasma11,12,13,14,15,16. Of which, TPI with 1% lidocaine has been commonly used to reduce the MTrPs-induced neck and back pain17. However, these two methods failed to completely control the progress of MPS, which stems from the inaccurate localization and incomplete inactivation of MTrPs18.
Based on the mechanisms of activation of MTrPs, ultrasound-guided myofascial hydrodissection technique (UMHT) has been proposed in order to precisely localize and completely inactive the MTrPs. In the operational regimens of UMHT, ultrasound is used to enable real-time visualization and to guide needle placement precisely into MTrPs. Under the ultrasound guidance, an appropriate volume of normal saline is shotted into MTrPs, followed by sequential injection into adjacent myofascial layers for hydrodissection of the perimuscular membrane, perimysium, and epimysium surrounding the MTrPs (Fig. 1). The precise shot and layer hydrodissection could inactivate the MTrPs and restore its surrounding myofascial system.
Ultrasound images showing the ultrasound-guided myofascial hydrodissection technique (UMHT). (A) Long-axper of Upper trapezius (UT); (B) A 22-gauge 10 cm needle was inserted into the upper trapezius (arrow); (C) Normal saline layer after injection (asterisk); (D) Demonstrating the spread of bolus of normal saline 10 mL over the muscle fascias.
TPI is currently the most widely used clinical intervention for MTrPs, and thus serves as an appropriate comparator for evaluating the clinical value of UMHT. Both target upper trapezius MTrPs, but their mechanisms differ fundamentally: lidocaine TPI relies on the pharmacological effects of a local anesthetic to temporarily relieve pain and muscle spasms17, whereas UMHT employs ultrasound-guided saline hydrodissection to mechanically release adhesions and restore myofascial integrity, potentially providing longer-lasting benefits19. A direct comparison between these approaches clarifies the efficacy of pharmacological versus non-pharmacological mechanisms and determines whether UMHT can achieve comparable or superior outcomes without drug-related adverse effects. Therefore, this study aimed to evaluate the efficacy and safety of UMHT in patients with upper trapezius MPS and to compare it with TPI using 1% lidocaine.
Methods
Setting and patients
This study was conducted in accordance with the CONSORT guidelines (Fig. 1). This study complied with the Declaration of Helsinki and national ethical regulations, having received formal approval from the Biomedical Research Ethics Committee of West China Hospital, Sichuan University (Approval number: 2023-99).The protocol was registered at the Chinese Clinical Trial Register (ChiCTR2300067984) as a clinical trial on February 2nd 2023. Patients were informed and given written informed consent before being included in the study. They received thorough information regarding the study’s goals, methods, possible risks, and advantages. Joining the study was completely voluntary, and anyone could opt out at any moment without facing any repercussions. We made sure to protect confidentiality and anonymity throughout the research, using the data exclusively for research purposes. This study was designed as a single-blind randomized controlled trial and enrolled 41 patients who received care in the rehabilitation medicine center of West China Hospital, Sichuan University, between May and October 2024.
The patients aged between 20 and 70 with a diagnosis of MPS in the upper trapezius would be recruited. They had to present with active MTrPs within the upper trapezius identified according to the Simon criteria: (1) the presence of a palpable taut band in the upper trapezius; (2) the presence of an excessive MTrPs in the taut band; (3) local twitch response elicited by the snapping palpation of the taut band (where palpation is possible); (4) referred pain due to MTrPs compression20.
The exclusion was performed via physical examination of the experienced consultant based on the exclusion criteria. Patients were excluded if they had: (1) clinical signs of cervical radiculopathy; (2) a history of neck trauma (whiplash, vertebral fractures) or prior cervical surgical interventions; (3) fibromyalgia syndrome; (4) coexisting nausea, vomiting, or vertigo; or (5) received therapeutic nerve blocks or botulinum toxin administration within the preceding 12 months21. The diagnosis of MTrPs was conducted by a clinician with 15 years of experience in MPS-induced pain.
Randomization and blinding
Patients were randomly assigned (1:1) to the UMHT or TPI group via a computer-generated block randomization sequence (block size 6) managed exclusively by the second author using a validated electronic system. To ensure allocation concealment, the randomization sequence had been stored in sequentially numbered opaque envelopes, which would be opened only after the recruited patients completed all the baseline assessments. This study adopted a single-blind design, where patients were blinded to group allocation through the following measures: (1) patients were escorted to a private treatment room physically separated from the medication preparation area, preventing observation of injection solution preparation; (2) Clinicians preloaded syringes with either 0.9% normal saline (UMHT group) or 1% lidocaine (TPI group) in a dedicated preparation room prior to participant entry, ensuring identical appearance and handling of both solutions. Clinicians administering interventions were unblinded due to technical requirements but followed scripted instructions and standardized protocols (e.g., fixed treatment duration, neutral communication). Outcome assessors were fully blinded to group allocation and had no involvement in treatment delivery.
Interventions
Firstly, the recruited patients underwent standardized palpation of MTrPs in the upper trapezius in the seated upright position. Following identification of MTrPs (marked with surgical ink), the surrounding area of MTrPs was prepared with antiseptic manipulation. Secondly, a linear array transducer was positioned along the coronal plane of the upper trapezius to provide continuous real-time sonographic guidance. All injections were performed using an in-plane approach with the needle trajectory aligned parallel to the transducer’s longitudinal axis. A 22-gauge, 10-cm needle was used for both procedures.
For the UMHT group, 5 mL of 0.9% normal saline was injected directly into the MTrPs. This was followed by sequential hydrodissection of the surrounding perimuscular membrane, perimysium, and epimysium. The injection was performed slowly under direct sonographic visualization to ensure controlled fluid dispersion and real-time confirmation of fascial separation. The advancement of the normal saline was observed sonographically as a hypoechoic expansion, effectively cleaving the fibrotic interfaces and restoring fascial mobility. This treatment was administered once per week for two weeks.
For the TPI group, following the methodology adapted from Ay et al.22 patients received an injection of 2 mL of 1% lidocaine into the identified MTrPs. Under ultrasound guidance, correct needle positioning was confirmed by real-time visualization of tissue release and, when possible, observation of a local twitch response. After ensuring negative aspiration, the lidocaine was injected slowly under direct sonographic monitoring to visualize fluid distribution. TPI was also performed once weekly for 2 weeks.
All injections were carried out by the corresponding author, an experienced practitioner in ultrasound-guided interventional procedures.
Outcome assessments
Baseline characteristics
Data on demographic characteristics, specifically age, sex, weight, height, employment status, and exercise habits were recorded as baseline information. Each patient reported their medical history, including the side of involvement, the duration of the symptoms, previous treatments (e.g., physical therapy), and current painkillers. The information of all patients was recorded by a study assistant who was blinded to group allocation during personal visits.
All assessments were conducted by two clinicians at baseline and post-treatment of 2, 4, 12 weeks. All assessors underwent standardized training to avoid leading questions and to maintain neutral body language during the administration of assessments. The operational procedures for each scale were clearly specified, including the use of visual aids for the Visual Analog Scale (VAS) and detailed item explanations for the Short-Form McGill Pain Questionnaire (SF-MPQ) and the Neck Disability Index (NDI). To minimize contextual variability, all assessments were conducted in a controlled environment with a consistent room temperature (22 ~ 24 °C) and minimal ambient noise.
Visual Analog Scale
The primary outcome was pain intensity which was assessed post-treatment using a standardized VAS; this tool quantifies self-reported pain perception through a continuous metric measurement system. The VAS is a 10-cm validated scale on which patients are asked to rate their pain intensity from 0 (no pain at all) to 10 (worst possible pain)23. Patients were instructed to mark the point on the line that best represented their pain intensity. The score was determined by measuring the distance from the left end of the line to the point marked by the patient.
Short-form McGill Pain Questionnaire
The SF-MPQ is a multidimensional instrument used to assess pain, comprising 15 descriptors: 11 sensory and 4 affective24. Each item is rated on a 4-point scale (0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain), yielding a total score ranging from 0 to 45. The sum of the scores for all 15 items was calculated and used for analysis.
Neck Disability Index
The NDI is a self-administered questionnaire designed to evaluate the functional status of individuals with neck pain. It consists of 10 items covering pain intensity, personal care, lifting, reading, headaches, concentration, work, driving, sleeping, and recreation. Each item is scored on a scale from 0 to 5, with 0 indicating “no pain” and 5 indicating “the worst pain imaginable,” resulting in a total score ranging from 0 to 50. The total score is obtained by summing the scores of all items.
Sample size
Among the outcome measures, we prioritized the VAS for sample size estimation. Previous studies25 indicate that the minimal clinically important difference for the VAS index is defined as a 10% improvement (Δ = 1.0 cm). Based on preliminary data from our research team and empirical evidence, statistical parameters were set as two-tailed significance level (α = 0.05), pooled standard deviation (σ = 1.5 cm), type II error (β = 0.2; power = 80%). Using PASS 2023 (NCSS LLC, Kaysville, UT), the estimated sample size was 18 patients per group under a two-sample t-test framework (effect size d = 0.67, calculated as Δ/σ). To account for potential attrition, the sample size was adjusted to 21 patients per group, anticipating a 10–20% dropout rate over the study period.
Adverse events
First, to quantify adverse effects associated with UMHT or TPI, a standardized protocol was established to ensure consistent interventions. Second, to comprehensively monitor the adverse events, the patients were provided with a contact number to report both immediate and delayed responses to the intervention, including discomfort, swelling, muscular soreness, bleeding, hematoma, needle pain or any other adverse events.
Statistical analysis
Statistical analyses were conducted using SPSS version 29.0. Normally distributed quantitative data were presented as mean ± standard deviation (mean ± SD) and analyzed with Student’s t-test for between-group comparisons. Non-normally distributed quantitative data were described using median and interquartile range (IQR) and compared using the Mann-Whitney U test or Wilcoxon signed-rank test. Categorical data were summarized as frequency (n) and percentage (%), with between-group differences assessed via the chi-square test (χ² test). To assess longitudinal differences in pain scores (VAS, SF-MPQ) and functional disability indices (NDI) between both groups, we utilized a linear mixed model (LMM). The model incorporated random intercepts for patients to control for individual variability, with treatment group (UMHT vs. TPI) and time points (categorical variables: baseline, 2-week, 4-week, 12-week) as fixed effects, including the group × time interaction term. An exchangeable correlation matrix was specified to account for within-subject correlations in repeated-measures data. Statistical significance was defined at α = 0.05 (two-tailed). Prior to modeling, Box-Cox transformations were applied to all non-normally distributed continuous variables, and parameters were estimated using Huber-White robust standard errors. Significance of interaction effects was tested via Type III ANOVA with Satterthwaite’s degrees of freedom approximation. When the group × time interaction was nonsignificant (p > 0.05), main effects of group and time were analyzed to evaluate overall treatment efficacy and temporal trends, respectively. Where significant interaction effects existed (p < 0.05), simple effects analyses with Bonferroni correction were performed for multiple comparisons. Effect sizes (ES) for ordinal and non-normally distributed variables were calculated using r score26. The value of the r score (r = Z/√N, where N is the total number of observations) was used to evaluate the effect size. The effect size was considered large for values ≥ 0.5, moderate for values ≥ 0.3 and < 0.5, ≥ 0.1 and < 0.3 small, and < 0.1 trivial.
Results
Characteristics of enrolled subjects
The study started from May 12th, 2024 and follow-up completed on October 15th, 2024. A total of 54 patients had been assessed for eligibility, of which 42 were randomly assigned to either UMHT or TPI treatments. One patient could not complete the follow-up due to undergoing pulmonary nodule resection surgery after treatment conclusion. Ultimately, 41 patients had completed this study (Fig. 2). The demographics and characteristics of enrolled patients were summarized in Table 1. There were no significant differences in the baseline characteristics between these two groups.
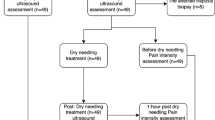
The trial profile of CONSORT. A total of 54 patients have been consecutively enrolled from the rehabilitation medicine center, of which 6 patients were excluded according to exclusion criteria. The recruited 42 patients were randomly assigned at 1:1 to either the UMHT group or the TPI group using the block randomization. Abbreviation: CONSORT, Consolidated Standards of Reporting Trials; TPI, trigger point injection; UMHT, ultrasound-guided myofascial hydrodissection technique.
Results of linear mixed model analysis
Based on the LMM analysis (Table 2), time points had a highly significant effect on VAS, SF-MPQ, and NDI scores (p < 0.001), indicating statistically significant improvement in symptoms over time among all subjects. However, no significant differences were found between the UMHT and TPI treatment groups (p > 0.05), and there was no significant interaction effect between group and time (p > 0.9), suggesting that the efficacy of the two therapies is comparable.
UMHT could effectively alleviate the MTrPs-induced pain
There were no significant differences (r < 0.2; p > 0.05) in the outcome variables (VAS, SF-MPQ) between two treatments, indicating the comparable efficacy between UMHT and TPI for MPS-induced pain (Fig. 3C, F; Table 3A). Either UMHT or TPI treatments demonstrated significant reductions in VAS and SF-MPQ scores during the follow-up comparing to baseline, respectively (Fig. 3A, B, D, E; Table 3B). Pain reduction was sustained through 12 weeks following UMHT and TPI treatments, as evidenced by decreases in VAS (r = 0.716 and r = 0.602; both p < 0.001) and SF-MPQ scores (r = 0.649 and r = 0.644; both p < 0.001).
Comparison of efficacy between UMHT versus TPI on pain and physical function of MPS. (A–C) The VAS scores have been reduced after either UMHT or TPI treatments. (D–F) The SF-MPQ scores have been decreased after either UMHT or TPI treatments. (G–I) The disability index, NDI scores have been improved post-treament of either UMHT or TPI treatments. Abbreviation: VAS, visual analog scale; SF-MPQ, short-form McGill pain questionnaire; NDI, neck disability index; UMHT, ultrasound-guided myofascial hydrodissection technique; TPI, trigger point injection. Data presented as median and interquartile range. *p < 0.001.
UMHT could effectively improve the physical function of MPS
There were no significant differences (r < 0.2; p > 0.05) in the outcome variable of NDI between these two treatments, indicating the similar efficacy between both UMHT and TPI for treating MPS-induced dysfunction (Fig. 3I; Table 3A). Both UMHT and TPI treatments exhibited a significantly sustained improvement in the physical function, as measured by NDI (Fig. 3G, H; Table 3B). The improvements of physical function have been demonstrated in both UMHT and TPI treatments during a follow-up of 12 weeks (r = 0.581 and r = 0.504; both p < 0.001).
Adverse events
No adverse events were observed or documented during the study period.
Discussion
This single-blind randomized controlled trial aimed to explore the efficacy and safety of UMHT in the patients with MPS of upper trapezius in comparison with TPI. Both groups demonstrated statistically significant improvement in pain intensity and functional impairment. Furthermore, there were no significant differences observed between both UMHT and TPI treatments. No adverse events had been reported.
In this study, the within-group changes with large effect size in pain intensity exceeded the minimal clinically important difference (> 10%) for VAS, indicating clinically meaningful improvements25. These results suggest that UMHT effectively reduce the MTrps-induced pain, with sustained therapeutic effect throughout the 12-week period. The similar findings have been found in the previous studies with regard to the positive role of either myofascial hydrodissection or TPI. A single-blind randomized controlled trial demonstrated that myofascial hydrodissection provided a more clinically significant short and long-term pain reduction compared to dry needling27. Another randomized controlled trial by Hsu et al. showed an effective role of myofascial hydrodissection in decreasing pain in the patients with MPS at 12-weeks follow-up28. Either normal saline (0.9%, 5 ml) or lidocaine (1%, 2 ml) injection have been found to effectively alleviate pain severity in the upper trapezius at intermediate-term follow-ups29. The study conducted by Rezasoltani et al. showed that TPI was safe and effective for managing MPS, with sustained therapeutic benefits for at least 12 weeks30. Lidocaine exhibits a superior analgesic effect and minimal procedural discomfort compared to dry needling31.
This study observed a significant improvement in physical function after either UMHT or TPI treatments. In the previous studies, the effects of myofascial hydrodissection on functional improvement have been rarely documented. The positive effect of TPI on physical function has been reported in NDI scores32,33. However, the magnitude of change in NDI scores (median reduction: 3–4 points) did not reach the established MCID threshold of 5 points34. It is suggested that the effect of UMHT on physical function in patients with MPS requires further investigation .
From the perspective of technique regimen, the main characteristics of UMHT involves three aspects: the inactivation of MTrPs, the sequential hydrodissection of the perimuscular membrane, perimysium, and epimysium surrounding the MTrPs, and the release of soft tissue. It is proposed that the chronic musculoskeletal pain is stemmed from the restricted intermuscular fascial sliding due to the injury of soft tissue35. Thus, it is necessary to restore the normal fascial mobility for the alleviation of pain. Based on this proposal, the manual therapies such as myofascial release technique have been developed and proved to adjust the dysfunctional soft tissues for chronic pain36. An ultrasonography study by Ichikawa et al. demonstrated that myofascial release could improve the fascial mobility. These studies have supported the hypothesis that hydrodissection-mediated fluid distribution within interfascial planes may normalize muscle fascial mobility and further to reduce pain19. Furthermore, myofascial hydrodissection may modulate nociceptive signaling through mechanosensitive receptors in fascial layers, which are densely innervated by nociceptive fibers37. Therefore, the utilization of normal saline could theoretically reduce fascial tension or desensitize these nociceptors.
Our study was grounded in the following hypothesis: the therapeutic effect of UMHT is achieved not only through the inactivation of MTrPs, but more crucially, via the mechanical restoration of the surrounding myofascial structure using hydrodissection technique. We found that UMHT (using normal saline) demonstrated comparable efficacy to TPI (using 1% lidocaine), providing strong indirect support for this mechanistic hypothesis. The fact that a non-pharmacological intervention produced pain relief and functional improvement equivalent to those of a pharmacological agent suggests that the mechanical effect of hydrodissection, rather than the chemical properties of the injectate, may be the primary driver of the therapeutic outcome. This aligns with existing theory that pathological adhesions and restricted fascial sliding are key factors in the chronicity of MPS35. Under ultrasound guidance, fluid delivery in UMHT allows precise separation of adherent fascial layers, potentially restoring normal tissue compliance and reducing nociceptive signaling from mechanosensitive receptors37. Therefore, our findings indicate that beyond pharmacological inactivation of trigger points, mechanical interventions targeting the dysfunctional myofascial environment are equally important, offering a novel therapeutic perspective.
Nonsteroidal anti-inflammatory drugs are commonly prescribed for soft tissue injury management, though their prolonged use has been associated with various adverse effects encompassing both gastrointestinal (mucosal damage, ulceration) and dermatological manifestations (pruritus, erythema)38. As noted by Witt et al., acupuncture interventions may carry risks, with 54% of patients experiencing bleeding or hematoma and 17% reporting needle-associated discomfort39, Notably, neither the UMHT nor TPI groups demonstrated any instances of skin lesions or gastrointestinal complications, during follow-up assessments, thereby highlighting the comparative safety advantages of both treatment approaches.
Limitation
This study has several methodological constraints: First, the single-center design may limit the generalizability of findings to broader populations. Second, the relatively insufficient sample size may compromise the statistical power of the results. Third, pain hypervigilance and psychological factors like somatization and anxiety, which are implicated in post-needling pain, have not been considered in this study and will be evaluated in future studies. Lastly, the study was limited by the lack of relatively objective assessment methods, which should be considered in the future studies.
Conclusion
This study demonstrated the efficacy and safety of UMHT in the patients with MPS of the upper trapezius within 12 weeks post-treatment. The comparison between UMHT and TPI showed no significant difference in treating MPS of upper trapezius. UMHT could be a novel therapeutic option in treating MPS with the superiority of no adverse reaction of medicine. The further study is needed to investigate the long-term effect of UMHT and to optimize the operational regimens.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request via email.
Abbreviations
- CI:
-
Confidence interval
- CONSORT:
-
Consolidated standards of reporting trials
- ES:
-
Effect size
- LMM:
-
Linear mixed models
- IQR:
-
Interquartile range
- MCID:
-
Minimal clinically important difference
- MD:
-
Median difference
- MPS:
-
Myofascial pain syndrome
- MTrPs:
-
Myofascial trigger points
- NDI:
-
Neck disability index
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- SF-MPQ:
-
Short-form McGill pain questionnaire
- TPI:
-
Trigger point injection
- UMHT:
-
Ultrasound-guided myofascial hydrodissection technique
- VAS:
-
Visual analog scale
References
Simons, D. G. & Travell, J. G. Myofascial origins of low back pain. 1. Principles of diagnosis and treatment. Postgrad. Med. 73 (2), 66, 68–70, 73 passim. https://doi.org/10.1080/00325481.1983.11697756 (1983).
Davidson, M. J. et al. Myotonometry reliably measures muscle stiffness in the Thenar and perineal muscles. Physiother Can. 69 (2), 104–112. https://doi.org/10.3138/ptc.2015-85 (2017).
Carter, J. B. & Banister, E. W. Musculoskeletal problems in VDT work: a review. Ergonomics 37 (10), 1623–1648. https://doi.org/10.1080/00140139408964941 (1994).
Treaster, D. et al. Myofascial trigger point development from visual and postural stressor s during computer work. J. Electromyogr. Kinesiology: Official J. Int. Soc. Electrophysiological Kinesiol. 16 (2), 115–124. https://doi.org/10.1016/j.jelekin.2005.06.016 (2006).
Gerdle, B. et al. Bradykinin and Kallidin levels in the trapezius muscle in patients Wit h work-related trapezius myalgia, in patients With whiplash associated pain, and in healthy controls—a Microdialysis study of women. Pain 139 (3), 578–587. https://doi.org/10.1016/j.pain.2008.06.012 (2008).
Lam, C. et al. Myofascial pain—a major player in musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 38 (1), 101944. https://doi.org/10.1016/j.berh.2024.101944 (2024).
Urits, I. et al. Treatment and management of myofascial pain syndrome. Best Pract. Res. Clin. Anaesthesiol. 34 (3), 427–448. https://doi.org/10.1016/j.bpa.2020.08.003 (2020).
Gerber, L. H. et al. A systematic comparison between subjects with no pain and pain associa ted with active myofascial trigger points. PM & R: J. Injury Function Rehabil. 5 (11), 931–938. https://doi.org/10.1016/j.pmrj.2013.06.006 (2013).
Simons, D. G. et al. Endplate potentials are common to midfiber myofacial trigger points. Am. J. Phys. Med. Rehabil. 81 (3), 212–222. https://doi.org/10.1097/00002060-200203000-00010 (2002).
Li, X. et al. Efficacy and safety of low-intensity ultrasound therapy for myofascial pain syndrome: a systematic review and meta-analysis. BMC Musculoskelet. Disord. 25 (1), 1059. https://doi.org/10.1186/s12891-024-08174-7 (2024).
Han, S. C. & Harrison, P. Myofascial pain syndrome and trigger-point management. Reg. Anesth. 22 (1), 89–101. https://doi.org/10.1016/s1098-7339(06)80062-3 (1997).
Lew, J. et al. Comparison of dry needling and trigger point manual therapy in patient s with neck and upper back myofascial pain syndrome: a systematic revi Ew and meta-analysis. J. Man. Manip. Ther. 29 (3), 136–146. https://doi.org/10.1080/10669817.2020.1822618 (2021).
Benecke, R. et al. Botulinum type A toxin complex for the relief of upper back myofascial pain syndrome: how do fixed-location injections compare with trigger point-focused injections? Pain medicine (Malden, Mass) 12 (11), 1607–1614. https://doi.org/10.1111/j.1526-4637.2011.01163.x (2011).
Kim, J. Y. et al. Ultrasound-guided 5-in-1 trigger point injection for treating tension-type headache: A case report. Med. (Baltim). 101 (31), e29987. https://doi.org/10.1097/md.0000000000029987 (2022).
Yilmaz, O. et al. Comparison of the efficacy of botulinum toxin, local anesthesia, and P latelet-Rich plasma injections in patients with myofascial trigger poi Nts in the masseter muscle. J. Oral Maxillofacial Surgery: Official J. Am. Erican Association Oral Maxillofacial Surg. 79 (1), 88.e81–88.e89. https://doi.org/10.1016/j.joms.2020.09.013 (2021).
Alnahhas, M. F. et al. Outcomes of Ultrasound-Guided trigger point injection for abdominal wall pain. Dig. Dis. Sci. 61 (2), 572–577. https://doi.org/10.1007/s10620-015-3857-8 (2016).
Yanuck, J. et al. Pragmatic randomized controlled pilot trial on trigger point injections with 1% Lidocaine versus conventional approaches for myofascial pain in the emergency department. J. Emerg. Med. 59 (3), 364–370. https://doi.org/10.1016/j.jemermed.2020.06.015 (2020).
Ferrante, F. M. et al. Evidence against trigger point injection technique for the treatment of cervicothoracic myofascial pain with botulinum toxin type A. Anesthesiology 103 (2), 377–383. https://doi.org/10.1097/00000542-200508000-00021 (2005).
Ichikawa, K. et al. Comparative analysis of ultrasound changes in the Vastus lateralis Mus Cle following myofascial release and thermotherapy: a pilot study. J. Bodyw. Mov. Ther. 19 (2), 327–336. https://doi.org/10.1016/j.jbmt.2014.11.018 (2015).
Muñoz-Muñoz, S. et al. Myofascial trigger points, pain, disability, and sleep quality in Indi viduals with mechanical neck pain. J. Manipulative Physiol. Ther. 35 (8), 608–613. https://doi.org/10.1016/j.jmpt.2012.09.003 (2012).
Rodríguez-Jiménez, J. et al. Immediate effects of dry needing or manual pressure release of upper T Rapezius trigger points on muscle activity during the craniocervical flexion test in people with chronic neck pain: a randomized clinical tr Ial. Pain Med. 23 (10), 1717–1725. https://doi.org/10.1093/pm/pnac034 (2022).
Ay, S. et al. Comparison of injection methods in myofascial pain syndrome: a randomized controlled trial. Clin. Rheumatol. 29 (1), 19–23. https://doi.org/10.1007/s10067-009-1307-8 (2010).
Almog, S. et al. The pharmacokinetics, efficacy, and safety of a novel selective-dose cannabis inhaler in patients with chronic pain: a randomized, double-blinded, placebo-controlled trial. Eur. J. Pain. 24 (8), 1505–1516. https://doi.org/10.1002/ejp.1605 (2020).
Melzack, R. The short-form McGill pain questionnaire. Pain 30 (2), 191–197. https://doi.org/10.1016/0304-3959(87)91074-8 (1987).
Bourdel, N. et al. Systematic review of endometriosis pain assessment: how to choose a scale? Hum. Reprod. Update 21 (1), 136–152. https://doi.org/10.1093/humupd/dmu046 (2015).
Cohen, J. A power primer. Psychol. Bull. 112 (1), 155–159. https://doi.org/10.1037/0033-2909.112.1.155 (1992).
Suarez-Ramos, C. et al. Effectiveness of ultrasound guided interfascial hydrodissection with the use of saline anesthetic solution for myofascial pain syndrome of the upper trapezius: a single blind randomized controlled trial. Front. Rehabil Sci. 4. https://doi.org/10.3389/fresc.2023.1281813 (2023).
Hsu, C. Y. et al. Additional effect of interfascial hydrodissection with dextrose on Sho ulder and neck function in patients with myofascial pain syndrome: A R andomized control trial. Am. J. Phys. Med. Rehabil. 103 (9), 827–834. https://doi.org/10.1097/PHM.0000000000002442 (2024).
Tantanatip, A. et al. Comparison of the effects of physiologic saline interfascial and Lidocaine trigger point injections in treatment of myofascial pain syndrome: a double-blind randomized controlled trial. Arch. Rehabil Res. Clin. Transl. 3 (2), 100119. https://doi.org/10.1016/j.arrct.2021.100119 (2021).
Rezasoltani, Z. et al. Granisetron vs. lidocaine injection to trigger points in the management of myofascial pain syndrome: a double-blind randomized clinical trial. Scandinavian J. Pain 21 (4), 707–715. https://doi.org/10.1515/sjpain-2020-0154 (2021).
Kamanli, A. et al. Comparison of Lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol. Int. 25 (8), 604–611. https://doi.org/10.1007/s00296-004-0485-6 (2005).
Korkmaz, N. et al. Comparison of the efficacy of oxygen-ozone and Lidocaine injections in the treatment of myofascial pain syndrome: a randomized clinical Tria l. Turkish J. Phys. Med. Rehabilitation 69(3), 294–302. https://doi.org/10.5606/tftrd.2023.11516 (2023).
Raeissadat, S. A. et al. Comparison of Ozone and Lidocaine injection efficacy vs dry needling in myofascial pain syndrome patients. J. Pain Res. 11, 1273–1279. https://doi.org/10.2147/JPR.S164629 (2018).
Smith, A. et al. Cervical facet joint platelet-rich plasma in people with chronic Whipl ash-associated disorders: a prospective case series of longer term 6- and 12- month outcomes. Interventional Pain Med. 2(1), 100237. https://doi.org/10.1016/j.inpm.2023.100237 (2023).
Laimi, K. et al. Effectiveness of myofascial release in treatment of chronic musculoske letal pain: a systematic review. Clin. Rehabil. 32(4), 440–450. https://doi.org/10.1177/0269215517732820 (2018).
Trigger Point Dry Needling. J. Orthop. Sports Phys. Ther. 47(3), 150. https://doi.org/10.2519/jospt.2017.0502 (2017).
Domingo, T. et al. Is interfascial block with ultrasound-guided puncture useful in treatment of myofascial pain of the trapezius muscle? Clin. J. Pain. 27(4), 297–303. https://doi.org/10.1097/AJP.0b013e3182021612 (2011).
Laine, L. The Gastrointestinal effects of nonselective NSAIDs and COX-2–selectiv e inhibitors. Semin. Arthritis Rheum. 32(3), 25–32. https://doi.org/10.1053/sarh.2002.37217 (2002).
Witt, C. M. et al. Pragmatic randomized trial evaluating the clinical and economic effect Iveness of acupuncture for chronic low back pain. Am. J. Epidemiol. 164(5), 487–496. https://doi.org/10.1093/aje/kwj224 (2006).
Acknowledgements
We are grateful for the technical assistance provided by the Rehabilitation Medicine Center and Institute of Rehabilitation Medicine, West China Hospital.
Funding
This research was supported by the 1·3·5 project for disciplines of excellence–Clinical Research Fund, West China Hospital, Sichuan University (024HXFH013).
Author information
Authors and Affiliations
Contributions
Y.J.Chen was responsible for the conception and design of the study. Q.Wang was responsible for funding acquisition, supervisor and implemented the intervention. S.J.Liu, and Y.N.Sun were responsible for the acquisition of data. Y.J.Chen, S.J.Liu, and Y.N.Sun were responsible for participant recruitment and data analysis. Y.J.Chen, prepared the initial draft of the manuscript. Q.Wang gave critical feedback during the study or during the submission of the manuscript. All authors had given final approval of the version to be submitted and agreed on the journal to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study design was approved by the Ethics Committee for Biomedical Research of West China Hospital of Sichuan University (Approval number: 2023-99). All procedures performed in this study were in accordance with the ethical standards of the 1964 Helsinki Declaration.
Consent for publication
Not applicable. The consent was obtained from all of the patients prior to the study.
Statement for consort
This study was conducted in accordance with the CONSORT guidelines. A completed CONSORT checklist was provided as supplementary materials to ensure full transparency of reporting.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Liu, S., Sun, Y. et al. Efficacy of ultrasound-guided myofascial hydrodissection technique in myofascial pain syndrome of upper trapezius: a randomized controlled trial. Sci Rep 15, 33444 (2025). https://doi.org/10.1038/s41598-025-19107-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19107-2