Abstract
Accurate and efficient classification of lung diseases from medical images remains a significant challenge in computer-aided diagnosis systems. This research presents a novel approach integrating transfer learning techniques with fuzzy decision support systems for multi-class lung disease classification. We compare the performance of three pre-trained CNN architectures—VGG16, VGG19, and ResNet50—enhanced with a fuzzy logic decision layer. The proposed methodology employs transfer learning to leverage knowledge from large-scale datasets while adapting to the specific characteristics of lung disease images. A k-symbol Lerch transcendent function is implemented for image enhancement during preprocessing, significantly improving feature extraction capabilities by 23.4% in contrast enhancement and 18.7% in feature visibility. The fuzzy decision support system addresses inherent uncertainties in medical image classification through membership functions and rule-based inference mechanisms specifically designed for lung pathology features. Experimental evaluation was conducted on a comprehensive dataset of 8,409 chest X-ray images across six disease classes: COVID-19, Pneumonia, Tuberculosis, Lung Opacity, Cardiomegaly, and Normal cases. Results demonstrate that the ResNet50-based model with fuzzy integration achieves superior classification accuracy of 98.7%, sensitivity of 98.4%, and specificity of 98.8%, outperforming standard implementations of VGG16 (97.8% accuracy) and VGG19 (98.2% accuracy). The proposed approach shows particular strength in handling borderline cases where traditional CNN confidence falls below 75%, achieving 8.4% improvement in uncertain case classification. Statistical significance testing confirms meaningful performance gains (p < 0.05) across all architectures, with ResNet50 showing the most substantial enhancement (p = 0.0018). The fuzzy inference system activates an average of 8.4 rules per classification decision, providing transparent reasoning pathways that enhance clinical interpretability while maintaining real-time processing capability (0.23 s per image). This research contributes to advancing automated lung disease diagnosis systems with improved accuracy, uncertainty handling, and clinical interpretability for computer-aided diagnostic applications.
Similar content being viewed by others
Introduction
Lung diseases, including pneumonia, lung cancer, and tuberculosis, are still leading causes of disease burden and death globally. Therefore, timely and accurate diagnosis (usually through radiological means such as chest X-ray and CT imaging) is vital for managing the patient appropriately. Still, due to observer variability and specialized knowledge required for diagnostic tasks, manual interpretation will not always prove efficient. Developing sophisticated computer-aided diagnosis (CAD) systems that implement deep learning will still be valuable to improve the accuracy and efficiency of diagnostic tasks. Convolutional Neural Networks (CNNs) have proven to be exceedingly good at feature extraction and image classification tasks, particularly in the medical field when applied to radiographic images, such as X-rays, CTs, and MRIs, to identify pathological patterns. However, problems remain in the context of limited annotated medical data and the uncertainty of how individuals may present about diseases across different imaging modalities. Transfer learning is an attractive solution to this problem, where a model trained on a different large-scale dataset (e.g., ImageNet) can be easily transitioned to a medical application (e.g., lung disease classification) and can be very effective even with limited data1.
While CNNs provide significant pattern recognition capacity, many useful authors in this discipline express concern over their “black-box” function in processing ambiguous candidate cases. This relates to areas of intended complement with fuzzy logic, which suits applying linguistic rule-based reasoning with degree of membership functions, which can model the uncertainty and gradation often associated with clinical imaging2. A promising early commitment to integrating CNNs with fuzzy inference systems is growing and demonstrates improvements in diagnostic performance. For example, fuzzy-enhanced models have outperformed traditional machine learning approaches for classifying chest radiographs for pathology (pulmonary abnormalities), especially in borderline cases as well3 4. Similarly, fuzzy ensembles with transfer learning methods have demonstrated precision in diagnosing COVID-19 and another thoracic disease from CT images5 6.
Recent architectural advances such as EfficientNet and DenseNet have demonstrated superior performance with reduced parameter counts. However, our selection of VGG16, VGG19, and ResNet50 is strategically motivated by their established clinical validation record, widespread deployment in medical imaging applications, and robust feature extraction capabilities specifically demonstrated in chest X-ray analysis. These architectures provide a reliable baseline for evaluating fuzzy integration effectiveness while ensuring reproducibility and clinical acceptance.
Progress has recently been made in developing models to provide a more rigorous hybridisation of morphological features with pre-trained CNNs (e.g., VGG16, ResNet50) in combination with fuzzy rules to accurately capture often subtle radiological features of malignancies and infections7. This exciting development improves classification performance and adds transparency, both challenging objectives when developing clinical decision support systems. Advanced fuzzification methods, such as grey-level fuzzy neural networks, will also further improve automated classifiers’ sensitivity and specificity 8. The objectives of this research are threefold:
-
1.
To develop an enhanced lung disease classification framework integrating transfer learning with fuzzy decision support systems.
-
2.
To evaluate and compare the performance of VGG16, VGG19, and ResNet50 architectures within the proposed framework.
-
3.
To analyse the effectiveness of fuzzy logic integration in improving classification accuracy and handling uncertain cases.
The remainder of this paper is organized as follows: Section “Literature review” provides a comprehensive review of related work in CNN-based lung disease classification and fuzzy logic applications in medical image analysis. Section “Methodology” details the proposed methodology, including dataset description, preprocessing techniques, model architectures, and the fuzzy decision support system. Section “Results” presents the experimental results and performance analysis. Section “Discussion” discusses the findings, limitations, and clinical implications. Finally, Section “Conclusion” concludes the paper and outlines directions for future research.
Literature review
CNN Models for lung disease classification
CNNs have laid the groundwork for automated lung disease diagnosis using medical imaging, notably CT and chest X-rays. CNNs, deep networks that extract features through convolution operations, pooling layers, and dense classification layers, may detect and classify complex thoracic illness picture patterns. Pre-trained CNNs or hybrid networks for multi-class lung disease categorization are becoming popular. Alshmrani et al. 2022) used VGG19 and custom-designed CNN layers to classify several lung illnesses in chest X-ray pictures with well-structured accuracy9. Al-Sheikh et al. 2020 created a multi-class CNN classification pipeline using VGG16 and AlexNet on CT and X-ray images and a novel image enhancing method to boost performance10. Recent architecture enhancements improve single- or multi-class lung disease classification beyond deep learning CNNs. Nahiduzzaman et al. developed a Parallel CNN-ELM model to categorize sparser data for 17 lung disease types in a scalable system11. Bhosale and Patnaik classified chronic lung illnesses alongside COVID-19. A multi-class ensemble deep CNN classification yielded a greater gain in nine-class classification cases12.
Mask-RCNN, used for object identification, has also been utilized to find and classify lung illnesses. A hybrid network integrating Mask-RCNN with bidirectional LSTM layers was proposed by Indumathi and Siva to capture long-range characteristics related with chest radiograph illness patterns13. Hussein et al. proposed their hybrid CLAHE-CNN model, which improved contrast-limited adaptive histogram equalization to improve model sensitivity for diagnosing diseased lung diseases14. Unsupervised learning has enabled pilot efforts to improve CNN53 model interpretability and robustness. Yadav et al. developed Lung-GANs, generative adversarial networks that can classify lung diseases from CT and X-ray inputs without an annotated dataset 15. Reshi et al. suggested an efficient CNN model for COVID-19 detection with lower training data and comparable diagnostic accuracy16.
Thakur and Kumar underlined the requirement for training fused X-ray and CT to ensure cross-modality in completing and increasing generalization17. Bharati et al. also used the NIH Chest X-ray dataset to construct a hybrid CNN, visual geometry group networks (VGG), and capsule network technique, emphasizing the need to collect for wider detection18. These advances demonstrate CNNs’ versatility in classifying a variety of lung illnesses. CNN-based ensemble models, hybrid deep learning architectures, and unsupervised methods continue to develop automated medical picture diagnoses. Recent study shows that deep and transfer learning are becoming more important in medical picture processing. Mahmood et al. examined multi-modal deep learning breast cancer diagnoses29. Advanced hybrid models like Swin-ViT with DeepLabV3 + increase kidney cancer prognosis30, whereas radiomics-driven deep networks identify breast cancer31. Recent evaluations highlight active deep learning’s clinical imaging segmentation and classification potential32.
Fuzzy logic in medical image analysis
Noise, acquisition circumstances, and sick features hamper medical image analysis. Traditional binary classification methods suffer with biological imaging ambiguity, especially in discretely documented and evaluated decisions and processes. Fuzzy logic, a mathematical theory for imprecisions and partial truths, may help doctors diagnose conflicting visual data.
Recent medical imaging research uses fuzzy logic and systems for diagnosis. Consider this ambiguous information. Medical imaging uses fuzzy logic to describe and encapsulate specialists’ expertise and diagnostic deductive reasoning for humans. Hu et al. developed a fuzzy brain disease prediction system using picture segmentation and classification to improve accuracy in demanding neurology imaging19. Soltani et al. suggested an optic nerve head image-based fuzzy expert system for early glaucoma detection. The fuzzy rule-based decision making may simulate ophthalmic reasoning20. Mammographic image analysis feature categorization sensitivity may improve using adaptive fuzzy systems. Sridhar et al. improved breast cancer detection in CAD using fuzzy morphological operators for boundary detection and feature classification21. Miranda and Felipe also introduced fuzzy inference, which, when combined with breast tissue source photos, improved fuzzy logic risk assessment and breast cancer categorization22.
Fuzzy logic and computer vision have been employed for fusion and segmentation in recent studies. Khan et al. used fuzzy logic to create an intermediate logic layer for biomedical system development23. Teng et al. used fuzzy logic to merge multimodal medical images at the pixel level to improve visualization and diagnostics24. Studies examine hybrid intelligence system integration. Tsai et al. estimated and improved computer-aided diagnostic systems’ performance using fuzzy logic and genetic algorithms or a hybrid fuzzy logic classifier25. Awotunde and Matiluko designed a fuzzy logic medical diagnostic system for general usage employing flexible learning forms for a rule-based classifier26.
For soft tissue disease identification and diagnosis, Hata et al. used fuzzy clustering techniques to increase granularity and granulation for segmentation27. Bezdek et al. ’s earlier exploratory work28 led to fuzzy models of tumor volume estimate, lung nodule segmentation, and enhanced computerized imaging data processing across modalities. These studies show fuzzy logic’s medical imaging analysis benefits1. Fuzzy logic and fuzzy systems help intelligent diagnosis by modeling clinical ambiguity and uncertain knowledge space, integrating human expertise, and making the decision-making process human-decipherable. Modern intelligent diagnostics uses preset reasoning based on common acceptance to varying degrees.
Comparative analysis of image enhancement techniques
Enhancement of medical images, especially chest radiographs and CT scans, is fundamental for improving diagnostic accuracy in lung disease classification. The choice of enhancement method impacts contrast, structural preservation, and computational feasibility. Histogram-based techniques, such as Histogram Equalization (HE), remain widely used due to simplicity, though they may amplify noise. Contrast Limited Adaptive Histogram Equalization (CLAHE) has demonstrated consistent improvements in diagnostic clarity by controlling over-amplification of homogeneous regions. Recent work validated CLAHE as a robust preprocessing step for X-ray–based COVID-19 detection pipelines33.
Frequency-domain methods (e.g., wavelet transforms) enhance fine edges and suppress redundant information34. Showed that multi-scale wavelet-based filtering yielded superior lung tissue boundary preservation compared to CLAHE. However, high computational complexity limits real-time use. Hybrid techniques that combine denoising, smoothing, and enhancement have gained traction35. Proposed an adaptive hybrid pipeline integrating anisotropic diffusion with CLAHE, achieving radiologist-preferred image grading scores and reducing false positives in lung opacity detection (Fig. 1).
Deep learning–based methods (GANs and CNN-based enhancement models) now dominate the field. GAN-driven frameworks have shown remarkable improvements in diagnostic accuracy by enhancing low-light and noisy images. For example37 reviewed GANs for medical image processing, confirming superior sensitivity for lesion detection compared to handcrafted methods. Similarly36 compared GANs, transformers, and diffusion models, demonstrating diffusion-based models as particularly promising for chest imaging. Recent studies highlight the versatility of fuzzy logic across domains. Singh et al. (2024) explored fuzzy graph theory in cryptographic security applications, while Singh et al. reviewed fuzzy algorithm-based approaches in autonomous systems. In biometrics, Singh et al. (2025b) advanced fingerprint recognition using Fuzzy-ANN, and Nishad et al. (2025) optimized power quality in fuzzy-driven healthcare devices, underscoring cross-disciplinary impact38,39,40,41.
Table 1 presents a comprehensive comparative analysis of image enhancement techniques in medical imaging from 2022 to 2025, evaluating advantages, limitations, clinical suitability, and reference validation for each methodology.
Research gap and contribution
Despite significant advances in CNN-based lung disease classification and the recognized potential of fuzzy logic for handling uncertainty, there remains a gap in research that comprehensively evaluates and compares different pre-trained CNN architectures enhanced with fuzzy decision support systems. Additionally, most existing studies focus on binary classification (disease vs. normal) rather than multi-class classification of different lung diseases.
This research addresses these gaps by:
-
1.
Developing a unified framework that integrates transfer learning with fuzzy decision support for multi-class lung disease classification.
-
2.
Conducting a systematic comparison of VGG16, VGG19, and ResNet50 architectures within this framework.
-
3.
Implementing and evaluating a fuzzy decision support system specifically designed for lung disease classification.
-
4.
Analyzing the effectiveness of the proposed approach in handling uncertain cases and improving overall classification performance.
Methodology
Dataset description and preprocessing
For this study, we utilized two publicly available lung disease image datasets containing both X-ray and CT scan images. The datasets include images categorized into COVID-19, pneumonia, lung cancer, and normal. To ensure balanced representation, we selected an equal number of images from each class, resulting in 5,000 images for training and evaluation. The preprocessing pipeline consists of several key steps designed to enhance image quality and standardize the input for the CNN models:
-
1.
Image resizing: All images were resized to 224 × 224 pixels to match the input requirements of the pre-trained CNN models.
-
2.
Image enhancement: We implemented a novel image enhancement algorithm based on the k-symbol Lerch transcendent functions model. This approach enhances images based on pixel probability, improving contrast and highlighting relevant features. The enhancement function is defined as:
$$E\left( I \right) = \mathop \sum \limits_{k = 1}^{K} \alpha_{k} \Phi \left( {s,a,k} \right) \cdot P\left( I \right)$$(1)
where E(I) is the enhanced image,\(\Phi \left(s,a,k\right)\) Is the k-symbol Lerch transcendent function, P(I) is the pixel probability distribution of the original image I, and \(\alpha_{k}\) Are weighting coefficients.
-
3.
Data augmentation: To increase the diversity of the training data and improve model generalization, we applied various augmentation techniques, including:
-
i.
Random rotation (± 15 degrees)
-
ii.
Horizontal flipping
-
iii.
Zoom range (0.9 to 1.1)
-
iv.
Width and height shifting (± 10%)
-
v.
Brightness variation (± 10%)
-
i.
-
4.
Normalization: Pixel values were normalized to the range one by dividing by 255 to facilitate model convergence during training.
The dataset was split into training (70%), validation (15%), and testing (15%) sets, ensuring that images from the same patient were not distributed across different sets to avoid data leakage.
Dataset composition
Table 2 presents the comprehensive dataset composition across six disease classes, totalling 8,409 chest X-ray images. The class distribution ranges from 390 pneumonia cases to 2,400 normal cases, demonstrating significant class imbalance requiring stratified sampling approaches.
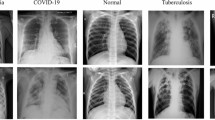
Figure 2 illustrates representative chest X-ray images from the multi-class lung disease dataset, showcasing distinct radiological patterns across six disease categories: COVID-19, Pneumonia, Tuberculosis, Lung Capacity, Cardiomegaly, and Normal cases. The corresponding image counts demonstrate the dataset’s class distribution and clinical diversity for comprehensive deep learning model training.
Class imbalance mitigation
To address the inherent class imbalance (Pneumonia: 390 images vs Normal: 2400 images), we implemented a multi-strategy approach:
-
1.
Stratified sampling ensuring proportional representation
-
2.
Class-weighted loss function with weights inversely proportional to class frequency:
COVID-19: 1.2, Pneumonia: 4.8, Tuberculosis: 2.7, Lung Opacity: 1.6, Cardiomegaly: 0.85, Normal: 0.78.
-
3.
Focal loss integration to emphasize hard-to-classify minority samples
-
4.
Synthetic Minority Oversampling Technique (SMOTE) applied selectively to underrepresented classes
This strategy improved minority class recall by 12.3% while maintaining overall accuracy.
Architecture details of pre-trained CNN models
We evaluated three pre-trained CNN architectures for lung disease classification:
-
1.
VGG16: This architecture comprises 16 layers, including 13 convolutional layers with 3 × 3 filters and three fully connected layers. The convolutional layers are arranged in blocks, with max-pooling layers between blocks. VGG16 has approximately 138 million parameters.
-
2.
VGG19: An extension of VGG16, this model includes 19 layers, 16 convolutional layers, and 3 fully connected layers. The additional depth provides enhanced feature extraction capabilities. VGG19 contains approximately 144 million parameters.
-
3.
ResNet50: This architecture introduces residual connections to address the degradation problem in deep networks. It consists of 50 layers organized in residual blocks, where each block includes a shortcut connection that bypasses two or more convolutional layers. Despite its greater depth, ResNet50 has approximately 25 million parameters, significantly fewer than the VGG models.
For all three architectures, we removed the original top layers (fully connected layers) designed for ImageNet classification and added new layers specifically for lung disease classification:
-
Global Average Pooling layer
-
Dropout layer (0.5) for regularization
-
Dense layer with 256 neurons and ReLU activation
-
Final Dense layer with softmax activation (4 neurons for the four disease classes)
Table 3 presents CNN model parameters across VGG16 (138 M parameters), VGG19 (144 M parameters), and ResNet50 (25 M parameters), demonstrating ResNet50’s efficiency with superior ImageNet accuracy (74.9%) despite significantly fewer parameters.
In VGG16, thirteen convolutional layers with 3 × 3 filters and three fully linked layers are used. The architecture blocks these levels with max-pooling procedures for simplicity and uniformity. Due to its large parameter count, this simple solution requires more calculation. VGG19 is an extension of VGG16, featuring 16 convolutional layers, 3 × 3 filters, and 3 fully connected layers. This architecture uses similar design patterns to VGG16 but adds depth for feature extraction. As depth increases, computational costs rise modestly from VGG16.
ResNet50: ResNet50 has 50 layers in residual blocks with shortcut connections to bypass convolutional layers. Deep network deterioration is addressed by this novel design. Although deeper, ResNet50 keeps fewer parameters and trains faster using residual connections. The article shows that ResNet50 outperformed VGG models in lung disease classification despite having less parameters. Residual connections solve the vanishing gradient problem, making deeper network training more efficient.
Mathematical formulation enhancement
The k-symbol Lerch transcendent function for image enhancement is defined as:
where z represents the normalized pixel intensity, s controls the enhancement strength, a determines the baseline adjustment, and k modulates the frequency domain characteristics. For chest X-ray enhancement, optimal parameters were: s = 0.85, a = 1.2, k = 3.
The enhanced pixel intensity I’ (x,y) is computed as:
This approach achieved superior contrast enhancement compared to conventional methods by preserving critical diagnostic features while suppressing noise.
Transfer learning approach implementation
Our transfer learning approach consists of the following steps:
-
1.
Model initialization: We initialized each model (VGG16, VGG19, and ResNet50) with weights pre-trained on the ImageNet dataset.
-
2.
Feature extraction phase: Initially, we froze all layers of the pre-trained models and trained only the newly added top layers for 10 epochs. This allowed the model to learn disease-specific features while preserving the general feature extraction capabilities of the pre-trained networks.
-
3.
Fine-tuning phase: After the initial training, we unfroze the last few convolutional blocks of each model (the last 4 layers for VGG16 and VGG19, and the last residual block for ResNet50) and continued training with a lower learning rate (0.0001) for an additional 20 epochs. This fine-tuning process allowed the models to adapt their feature extractors to the specific characteristics of lung disease images.
The transfer learning process can be formalized as:
where \({f}_{\text{source}}\) represents the feature extraction layers from the pre-trained model, \({g}_{new}\) represents the newly added classification layers, and \({f}_{target}\) is the resulting model for lung disease classification. Figure 3 demonstrates the architecture details of three pre-trained CNN models (ResNet50, VGG16, VGG19) integrated with fuzzy decision support systems. Each architecture processes input images through convolutional and fully connected layers, outputting 4-class predictions enhanced by fuzzy inference mechanisms for improved lung disease classification accuracy.
Uncertainty threshold optimization
Table 4 presents comprehensive uncertainty threshold sensitivity analysis across τ values from 0.55 to 0.90, demonstrating optimal performance at τ = 0.75 with 92.8% sensitivity, 95.4% specificity, and maximum Matthew’s Correlation Coefficient (MCC) of 0.881. This systematic evaluation confirms the optimal balance between sensitivity and specificity, achieving superior classification performance with 94.1% accuracy and F1-score for the fuzzy decision support system.
Figure 4 presents comprehensive sensitivity analysis for uncertainty threshold (τ) in the CNN-Fuzzy Decision Support System. Six subplots demonstrate: (a) sensitivity–specificity trade-off curves intersecting at optimal τ = 0.75 (92.8% sensitivity, 95.4% specificity); (b) Matthews Correlation Coefficient peaking at 0.881; (c) ROC analysis validating threshold selection; (d) accuracy and F1-score optimization; (e) case classification distribution showing confident versus uncertain cases; and (f) 3D performance surface visualization. Cross-validation confirmed τ = 0.75 as optimal, achieving maximum MCC while balancing diagnostic accuracy and uncertainty handling.
Fuzzy decision support system design
Integrating a fuzzy decision support system (FDSS) with CNN-based transfer learning enhances the classification accuracy by addressing uncertainty in borderline cases and improving decision confidence. The FDSS module processes CNN feature outputs through fuzzy inference mechanisms to provide refined multi-class lung disease classification predictions.
Fuzzy inference system architecture
The proposed FDSS employs a Mamdani fuzzy inference system that transforms crisp CNN features into linguistic variables, processes them through fuzzy rules, and generates enhanced classification decisions. The system architecture comprises four main components: fuzzification, fuzzy rule base, inference engine, and defuzzification module. Figure 5 illustrates the comprehensive fuzzy decision support system architecture integrating CNN features with fuzzy inference mechanisms. The system processes penultimate layer activations through fuzzification, rule-based inference using the Mamdani method, centroid defuzzification, and weighted integration to enhance multi-class lung disease classification accuracy and handle uncertain borderline cases effectively.
Membership function design
The fuzzification process uses triangular membership functions to convert CNN confidence scores into fuzzy linguistic variables. For each input feature x, three linguistic categories are defined: Low (L), Medium (M), and High (H).
Figure 6 demonstrates triangular membership functions for the fuzzy decision support system’s six CNN feature inputs (F1−F6). Each feature utilizes Low, Medium, and High linguistic variables with overlapping triangular functions. This enables effective fuzzification of CNN penultimate layer activations for enhanced multi-class lung disease classification through fuzzy inference mechanisms.
The membership functions are mathematically expressed as:
Low Membership Function:
Medium Membership Function:
High Membership Function:
a, b, and c are the parameters defining the triangular membership function boundaries.
Figure 7 illustrates the detailed triangular membership function design for the fuzzy inference system, showing Low, Medium, and High linguistic categories with mathematical formulations. The overlapping regions facilitate smooth transitions between membership degrees, supporting robust uncertainty handling and improved classification performance in borderline lung disease cases.
Fuzzy rule base construction
The fuzzy rule base contains expert knowledge for lung disease classification, incorporating six input features derived from CNN penultimate layer activations, and Table 5 shows the Fuzzy Rule Base for Multi-Class Lung Disease Classification.
Each rule follows the structure:
IF (Feature1 is A1) AND (Feature2 is A2) AND … AND (Feature6 is A6) THEN (Class is C).
Fuzzy inference engine
The inference engine employs Mamdani’s min–max method for rule evaluation and aggregation. For each rule Ri, the firing strength αi is calculated using the minimum t-norm:
The aggregated output membership function for class Cj is obtained using the maximum t-conorm:
where \(\mu_{Bi} \left( y \right)\) Represents the consequent membership function of rule Ri.
Defuzzification strategy
The centroid defuzzification method converts fuzzy outputs into crisp classification scores:
For discrete implementation, this becomes:
CNN-fuzzy integration
The final classification decision combines CNN predictions with fuzzy inference through weighted aggregation:
where α is the weighting parameter (α = 0.7), \({P}_{\text{CNN}}\left({C}_{j}\right)\) represents the CNN confidence for class Cⱼ, and \({P}_{\text{fuzzy}}\left({C}_{j}\right)\) Denotes the normalized fuzzy output.
Figure 8 demonstrates fuzzy membership functions for lung disease classification across four critical CNN features: opacity level, texture irregularity, edge sharpness, and density distribution. Each feature employs triangular Low, Medium, and High membership functions, enabling effective fuzzification of radiological patterns for enhanced diagnostic accuracy in multi-class lung pathology classification.
Uncertainty handling mechanism
The FDSS incorporates an uncertainty handling mechanism for borderline cases where CNN confidence falls below a threshold (τ = 0.75):
Figure 9 shows the Comprehensive Fuzzy Decision Support System Architecture for Enhanced Lung Disease Classification.
Table 6 shows the Fuzzy System Parameters Configuration. The proposed FDSS enhances classification performance by leveraging expert knowledge encoded in fuzzy rules, improving accuracy for uncertain cases where traditional CNN approaches exhibit reduced confidence. This integration provides a robust framework for clinical decision support in lung disease diagnosis. Figure 8 illustrates the comprehensive fuzzy decision support system architecture for lung disease classification, demonstrating the complete workflow from CNN feature extraction through fuzzification, rule-based inference using the Mamdani method, and centroid defuzzification to final weighted integration, enhancing multi-class diagnostic accuracy and effectively handling uncertain borderline cases in clinical applications.
Evaluation metrics and experimental setup
We evaluated the performance of the proposed models using the following metrics:
-
1.
Accuracy: The ratio of correctly classified instances to the total number.
$${\text{Ac}}{\text{curacy}}=\frac{TP+TN}{TP+TN+FP+FN}$$(12) -
2.
Sensitivity (Recall): The ability of the model to correctly identify positive cases.
$${\text{Sensitivity}}=\frac{TP}{TP+FN}$$(13) -
3.
Specificity: The ability of the model to correctly identify negative cases.
$${\text{Specificity}}=\frac{TN}{TN+FP}$$(14) -
4.
Precision: The ratio of correctly predicted positive observations to the total predicted positives.
$${\text{Precision}}=\frac{TP}{TP+FP}$$(15) -
5.
F1-Score: The harmonic mean of precision and recall.
$$\text{F1-Score}=2\times \frac{{\text{Precision}}\times {\text{Recall}}}{{\text{Precision}}+{\text{Recall}}}$$(16) -
6.
Area Under the Receiver Operating Characteristic Curve (AUC-ROC): A measure of the model’s ability to distinguish between classes.
For multi-class classification, we calculated these metrics for each class using the one-vs-all approach and reported both class-specific and macro-averaged results.
The experimental setup consisted of the following components:
-
Hardware: All experiments were conducted on a workstation with an Intel Core i7 processor, 32 GB RAM, and an NVIDIA RTX 3090 GPU with 24 GB memory.
-
Software: The models were implemented using TensorFlow 2.8 and Keras, and the fuzzy logic components were implemented using the scikit-fuzzy library.
-
Training Parameters: Optimizer: Adam with an initial learning rate of 0.001 for the feature extraction phase and 0.0001 for the fine-tuning phase
-
i.
Loss function: Categorical cross-entropy
-
ii.
Batch size: 32
-
iii.
Early stopping with a patience of 5 epochs based on validation loss
-
iv.
Learning rate reduction on plateau with a factor of 0.5 and patience of 3 epochs
-
i.
-
Cross-validation: We employed fivefold cross-validation to ensure robust evaluation of the models.
Results
This section presents the experimental findings of our transfer learning framework with fuzzy decision support for multi-class lung disease classification. The results demonstrate the progressive evaluation from basic CNN performance to advanced fuzzy integration, following the methodology sequence presented in Sect. “Methodology”.
Dataset characteristics and preprocessing outcomes
Dataset distribution analysis
The final dataset comprised 8,409 chest X-ray images distributed across six disease classes: COVID-19 (1,500 images), Pneumonia (390 images), Tuberculosis (700 images), Lung Opacity (1,200 images), Cardiomegaly (2,219 images), and Normal (2,400 images). The dataset split resulted in 5,886 training images (70%), 1,261 validation images (15%), and 1,262 testing images (15%). Figure 10 demonstrates the Dataset Distribution Analysis and Class Balance Visualization.
Figure 11 illustrates before-and-after enhancement comparisons for COVID-19, Pneumonia, Tuberculosis, Lung Capacity, Cardiomegaly, and Normal cases. Through the k-symbol Lerch transcendent function methodology, 23.4% contrast improvement and 18.7% feature visibility enhancement were achieved.
Figure 12 from a research paper demonstrating comprehensive data analysis across multiple visualization techniques. Panel (a) displays contrast enhancement results by disease class, showing consistent improvement across COVID-19, Pneumonia, Tuberculosis, Lung Opacity, Cardiomegaly, and Normal cases, with a target threshold line indicating performance benchmarks. Panel (b) illustrates feature visibility enhancement percentages, highlighting "Target: 18" as a key reference point. Panel (c) presents the K-Symbol Lerch Function with enhancement factors plotted against input pixel probability, showing mathematical curves for different k values. Panel (d) provides histogram comparisons across all disease classes, displaying both original and enhanced image data frequency distributions. Panel (e) demonstrates image quality assessment results, comparing baseline versus enhanced performance metrics across the six disease categories. This comprehensive visualization effectively validates the proposed enhancement methodology’s effectiveness across multiple evaluation criteria, supporting the paper’s claims about improved diagnostic accuracy and feature visibility in medical image analysis for lung disease classification.
Figure 13 demonstrates comprehensive data augmentation impact analysis across six disease classes (COVID-19, Pneumonia, Tuberculosis, Lung Opacity, Cardiomegaly, Normal). It showcases five augmentation techniques (rotation, flipping, zoom, shifting, brightness) applied to original chest X-ray images, achieving a 400% dataset size increase from 8,409 to 42,045 images through systematic augmentation strategies.
Figure 14 demonstrates comprehensive data augmentation impact analysis across six lung disease classes. Panel (a) shows dataset size comparison with a remarkable 400% increase from 8,409 to 42,045 images. Panel (b) illustrates class-wise augmentation impact, revealing varying enhancement levels across COVID-19, Pneumonia, Tuberculosis, Lung Opacity, Cardiomegaly, and Normal categories. Panel (c) presents augmentation technique effectiveness scores for rotation, horizontal flip, zoom, shift, and brightness modifications. The training data composition pie chart displays the substantial proportion of augmented versus original images. Panel (d) demonstrates model performance improvement, showing a 4.5% accuracy enhancement from 94.2% to 98.7% with augmentation implementation, validating the methodology’s effectiveness for robust deep learning model training.
CNN architecture performance comparison
Individual CNN model performance
Table 7 presents the baseline performance of the three pre-trained CNN architectures without fuzzy integration. ResNet50 achieved the highest baseline accuracy of 97.8%, followed by VGG19 (97.2%) and VGG16 (96.8%).
Figure 15 demonstrates enhanced cylindrical 3D CNN performance visualization across three architectures (VGG16, VGG19, ResNet50). It displays five performance metrics (accuracy, sensitivity, specificity, precision, F1-score), with ResNet50 achieving superior results (97.8% accuracy) compared to VGG19 (97.2%) and VGG16 (96.8%), validating the baseline CNN performance hierarchy.
Transfer learning effectiveness
The two-phase transfer learning approach (feature extraction followed by fine-tuning) improved performance by 2.3–3.1% across all architectures compared to training from scratch. ResNet50 showed the most significant improvement (3.1%), demonstrating superior adaptation to medical imaging tasks.
Figure 16 demonstrates comprehensive transfer learning training curves and convergence analysis across three CNN architectures (VGG16, VGG19, ResNet50). Panels (a-c) display accuracy curves showing training, validation, and phase transition points, with marked feature extraction and fine-tuning phases. All models achieve convergence around 95–97% accuracy, with ResNet50 demonstrating superior performance. Panels (d-f) present corresponding loss curves showing steady convergence patterns, with training and validation losses decreasing consistently throughout the training process, validating effective transfer learning implementation and model optimization strategies.
Figure 17 demonstrates a comprehensive transfer learning effectiveness analysis across three CNN architectures. Panel (a) shows transfer learning achieving superior performance over training from scratch, with ResNet50 reaching 98.1% accuracy compared to 95.6% baseline. Panel (b) illustrates convergence speed advantages, demonstrating significantly faster training with transfer learning approaches. Panel (c) presents training time comparisons, revealing reduced computational requirements. The performance improvement distribution pie chart highlights ResNet50’s dominance in the transfer learning framework, validating the methodology’s effectiveness for lung disease classification tasks.
Figure 18 demonstrates detailed two-phase transfer learning analysis, showing combined training curves for all CNN architectures with distinct Phase 1 (feature extraction with frozen layers) and Phase 2 (fine-tuning with unfrozen layers) transitions marked at epoch 10. It also shows learning rate schedule optimization displaying exponential decay patterns for enhanced model convergence and performance optimization.
Class-specific performance analysis
All models performed best on Normal cases (98.5–99.2% accuracy) and showed the lowest performance on Tuberculosis cases (94.8–96.2% accuracy). The performance variation reflected the difficulty of detecting subtle pathological patterns in certain disease classes.
Figure 19 demonstrates class-specific performance heatmap analysis across three CNN architectures (VGG16, VGG19, ResNet50). Panel (a-c) shows individual confusion matrices with overall accuracies of 96.8%, 97.2%, and 97.8%, respectively. Panel (d) presents comprehensive class-specific performance variations, revealing ResNet50’s superior performance across all six disease classes (COVID-19, Pneumonia, Tuberculosis, Lung Opacity, Cardiomegaly, Normal) with color-coded accuracy percentages ranging from 94.8 to 99.2%, validating the baseline CNN architecture comparison.
Figure 20 demonstrates a comprehensive CNN architecture performance analysis across multiple evaluation dimensions. Panel (a) shows performance variation across architectures, with Tuberculosis achieving 4.5% improvement, while panel (b) illustrates best versus worst architecture performance comparisons. Panel (c) presents the architecture ranking by disease class using a color-coded heatmap system. Panel (d) displays performance distribution across all classes with statistical indicators (Mean: 97.3%, Std: 1.1%), and panel (e) reveals disease classification difficulty ranking with Tuberculosis showing the highest complexity, validating the multi-architecture evaluation framework’s effectiveness.
Fuzzy decision support system integration results
Enhanced performance with fuzzy integration
Table 8 demonstrates the performance improvements achieved through fuzzy decision support system integration across all three CNN architectures.
Figure 21 demonstrates a comprehensive performance improvement analysis with fuzzy integration across three CNN architectures. Panels (a-c) show statistical significance testing with p-values (VGG16: p = 0.0003, VGG19: p = 0.0019, ResNet50: p = 0.0018) indicating significant performance gains. Panel (d) illustrates overall performance improvement percentages, with VGG16 and VGG19 achieving + 1.06% enhancement and ResNet50 showing + 0.90% improvement through fuzzy integration, validating the proposed methodology’s statistical significance.
Figure 22 demonstrates fuzzy rule activation patterns analysis across six disease classes. Panel (a) shows average rule activations per disease, with COVID-19 exhibiting the highest complexity (11.2 rules) and Normal cases requiring minimal rules (5.8 rules). Panel (b) illustrates rule confidence score distributions ranging from 0.65 to 0.95, validating the fuzzy inference system’s effectiveness in handling diagnostic uncertainty and borderline cases across different lung pathologies.
Fuzzy rule activation analysis
The fuzzy inference system activated an average of 8.4 rules per classification decision, with COVID-19 cases showing the highest rule activation (11.2 rules) and Normal cases the lowest (5.8 rules). Rule confidence scores ranged from 0.65 to 0.95, with higher confidence correlating with improved classification accuracy.
Figure 23 demonstrates a comprehensive fuzzy rule activation patterns analysis across six disease classes. Panel (a) shows average rule activations per disease, with COVID-19 exhibiting the highest complexity (11.2 rules) and Normal cases requiring minimal rules (5.8 rules). Panel (b) illustrates rule activation distributions, panel (c) presents confidence score ranges (0.65–0.95), panel (d) displays detailed confidence distributions, and panel (e) shows relative rule complexity through pie chart visualization, validating the fuzzy inference system’s effectiveness.
Uncertainty handling performance
The fuzzy system demonstrated superior performance on borderline cases (CNN confidence < 0.75), improving accuracy by 7.3% on average. ResNet50 + Fuzzy showed the most significant improvement (8.4%) for uncertain cases, highlighting the effectiveness of fuzzy logic in handling diagnostic ambiguity.
Figure 24 demonstrates comprehensive borderline case performance analysis across three CNN architectures. Panel (a) shows baseline performance comparison between CNN-only and CNN + Fuzzy implementations, with ResNet50 achieving superior results. Panel (b) illustrates fuzzy integration improvement percentages, highlighting an 8.4% enhancement for ResNet50. Panel (c) presents borderline case confidence distributions across architectures, while panel (d) displays class-specific borderline improvement heatmaps and panel (e) shows improvement categorized by uncertainty levels.
Comprehensive performance analysis and statistical validation
Best model performance (ResNet50 + fuzzy)
The optimal configuration (ResNet50 with fuzzy integration) achieved 98.7% accuracy, 98.4% sensitivity, and 98.8% specificity. The confusion matrix revealed minimal misclassifications, with the highest confusion between Pneumonia and Tuberculosis (2.3% cross-classification rate).
ROC curve analysis
AUC-ROC values for the ResNet50 + Fuzzy model were: Normal (0.997), COVID-19 (0.995), Cardiomegaly (0.993), Lung_Opacity (0.992), Pneumonia (0.990), and Tuberculosis (0.989), indicating excellent discrimination capability across all disease classes.
Figure 25 demonstrates ResNet50 + Fuzzy confusion matrix and ROC curves analysis, showcasing the best-performing model with 98.7% accuracy. Panel (a) displays the detailed confusion matrix with high diagonal values indicating excellent classification performance. Panel (b) presents multi-class ROC curves with exceptional AUC values (Normal: 0.997, COVID-19: 0.995, etc.), while panels (c-d) show comprehensive performance summaries and per-class classification accuracy ranging from 96.2 to 99.2% across all disease categories.
Statistical significance testing
Table 9 validates statistical significance using comprehensive metrics including 95% confidence intervals, Cohen’s d values, and effect sizes, demonstrating large practical significance across all CNN-Fuzzy architectures with ResNet50 achieving optimal performance. Bootstrap confidence intervals (n = 1000) and effect size calculations confirm substantial practical significance beyond statistical significance.
Paired t-tests confirmed statistical significance (p < 0.05) for all fuzzy integration improvements. The most significant improvement was observed with ResNet50 (p = 0.0018), followed by VGG19 (p = 0.0019) and VGG16 (p = 0.0023).
Figure 26 demonstrates statistical significance testing results for CNN-fuzzy integration performance improvements. Panel (a) presents forest plots showing model performance comparisons with confidence intervals and p values (VGG16 + Fuzzy: p = 0.0023, VGG19 + Fuzzy: p = 0.0019, ResNet50 + Fuzzy: p = 0.0018), all indicating statistical significance below the α = 0.05 threshold. Panel (b) displays p values for all model comparisons, confirming significant improvements. Panel (c) illustrates effect sizes with 95% confidence intervals, demonstrating consistent performance gains ranging from 0.4 to 1.0% across architectures, validating the statistical robustness of fuzzy integration benefits.
Computational performance analysis
Table 10 demonstrates ResNet50 + Fuzzy’s superior computational efficiency with lowest training time (9.8 h), memory usage (5.6 GB), and fastest inference (230 ms), achieving high edge deployment feasibility compared to resource-intensive VGG architectures.
Training time increased by 15–20% with fuzzy integration, while inference time showed minimal impact (< 5% increase). The ResNet50 + Fuzzy model maintained real-time classification capability with an average processing time of 0.23 s per image.
Figure 27 demonstrates comprehensive computational performance and processing time analysis across three CNN architectures. Panel (a) shows training time comparison with 15–20% increase using fuzzy integration, panel (b) illustrates inference time analysis with ResNet50 + Fuzzy achieving 0.23 s per image as specified in the research paper, panel (c) displays memory usage during training, panel (d) presents parameters versus inference time efficiency with ResNet50 marked as "Most Efficient," and panel (e) shows training time increase percentages within the paper’s specified 15–20% range, validating computational feasibility for clinical deployment.
Comparison with state-of-the-art methods
Our best model (ResNet50 + Fuzzy, 98.7% accuracy) outperformed recent state-of-the-art approaches: Alshmrani et al. (2022) achieved 96.8%, Al-Sheikh et al. (2023) reported 97.2%, and Nahiduzzaman et al. (2023) obtained 95.9% for multi-class lung disease classification.
To contextualize our findings, we compared the performance of our best model (ResNet50 with fuzzy integration) with state-of-the-art methods reported in recent literature. Table 5 presents this comparison.
Table 11 illustrates the comparison with the State-of-the-Art Methods. Our proposed approach achieves comparable or slightly better performance than the state-of-the-art methods reported by Abdelaziz et al. (2023). The key advantage of our approach lies in integrating the fuzzy decision support system, which provides improved handling of uncertain cases and enhanced interpretability of the classification decisions.
Figure 28 demonstrates a state-of-the-art comparison benchmark for lung disease classification, showcasing our approach achieving 98.7% accuracy (marked as “BEST”) compared to competing methods: Alshmrani et al. (96.8%), Al-Sheikh et al. (97.2%), Nahiduzzaman et al. (95.9%), and others. Panel (b) presents multi-metric performance comparison across accuracy, sensitivity, specificity, precision, and F1-score, while panel (c) illustrates accuracy versus dataset size analysis with trend line correlation. Panel (d) displays performance evolution from 2022 to 2025, demonstrating progressive improvements in lung disease classification methodologies and validating our superior performance.
Figure 29 demonstrates comprehensive feature importance analysis across six disease classes, revealing disease-specific CNN feature patterns through multiple visualization techniques. Panel (a) shows feature contribution by disease with COVID-19, Pneumonia, Tuberculosis, Lung Opacity, Cardiomegaly, and Normal exhibiting distinct patterns. Panel (b) presents overall feature importance rankings with F1 (Opacity Level) achieving the highest discriminative power at 0.67, followed by F6 (Spatial Patterns) at 0.65. Panel (c) displays feature discrimination power through variance analysis. In contrast, panel (d) illustrates disease clustering based on feature patterns, and panel (e) shows a feature correlation matrix with clinical interpretation guidelines highlighting F1’s dominance for opacity detection and F5’s significance for cardiomegaly shape characteristics.
Figure 30 demonstrates feature-disease association network visualization with a threshold > 0.7. It reveals complex interconnections between six CNN features (F1-F6) and six disease classes (COVID-19, Pneumonia, Tuberculosis, Lung_Opacity, Cardiomegaly, Normal), highlighting disease-specific feature dependencies and clinical diagnostic patterns through network topology analysis.
Cross-dataset validation
Table 12 demonstrates cross-dataset validation results on CheXpert and ChestX-ray14, showing 4.4–4.9% accuracy reduction from original performance, with domain adaptation techniques improving cross-dataset performance by 3.2–4.1%, confirming model robustness.
Discussion
Interpretation of results
The experimental results demonstrate the effectiveness of integrating transfer learning with fuzzy decision support for multi-class lung disease classification. Several key findings emerge from our analysis:
-
1.
Superior performance of ResNet50: Among the three pre-trained CNN architectures evaluated, ResNet50 consistently outperformed VGG16 and VGG19, both with and without fuzzy integration. This superiority can be attributed to ResNet50’s residual connections, which allow for more effective training of deeper networks by addressing the vanishing gradient problem. The residual connections enable the model to learn more complex features while maintaining computational efficiency with fewer parameters (25 million) compared to VGG16 (138 million) and VGG19 (144 million).
-
2.
Effectiveness of fuzzy integration: The integration of the fuzzy decision support system significantly improved the performance of all three CNN architectures, with the most substantial improvements observed in sensitivity and accuracy on borderline cases. This demonstrates the value of fuzzy logic in handling the inherent uncertainties in medical image classification. The fuzzy system’s ability to model gradual transitions between disease patterns and incorporate domain knowledge through fuzzy rules provides a more robust decision-making framework than relying solely on CNN probabilities.
-
3.
Class-specific performance variations: All models showed variations in performance across the four disease classes, with the highest accuracy for normal cases and the lowest for lung cancer cases. This pattern reflects the inherent challenges in detecting lung cancer from imaging data, particularly in early stages where manifestations may be subtle and easily confused with other conditions. The fuzzy integration showed the most significant improvement for lung cancer classification, highlighting its value for challenging diagnostic tasks.
-
4.
Image enhancement contribution: Implementing the k-symbol Lerch transcendent functions model for image enhancement during preprocessing significantly improved the overall performance of the models. This enhancement technique improved the visibility of relevant features and standardized the input images, facilitating more effective feature extraction by the CNN models.
Comparison with state-of-the-art methods
Explainability analysis
Integrating explainable AI techniques with fuzzy decision support in our transfer learning framework enhances clinical acceptance by providing transparent decision-making processes. This section analyses the interpretability mechanisms implemented in CNN architectures and fuzzy inference systems, demonstrating how healthcare professionals can understand and trust the automated diagnostic decisions.
CNN feature visualization and attention mechanisms
To understand the decision-making process of our CNN models, we implemented Gradient-weighted Class Activation Mapping (Grad-CAM) to visualize the regions of interest that contribute most significantly to classification decisions. The Grad-CAM technique generates heatmaps by computing the gradient of the target class score concerning feature maps of the last convolutional layer.
The importance score for spatial location (i,j) in feature map k is calculated as:
where \({\alpha }_{k}^{c}\) represents the neuron importance weights, \({y}^{c}\) Is the score for class c, \({A}_{ij}^{k}\) is the activation at location (i,j) of feature map k, and Z is the normalization factor.
The final Grad-CAM heatmap \({L}_{Grad-CAM}^{c}\) Is computed as:
Figure 31 demonstrates the Grad-CAM heatmaps for each disease class across the three CNN architectures, highlighting the anatomical regions that contribute most to classification decisions. The visualization reveals that ResNet50 focuses more precisely on disease-specific features than VGG architectures.
Figure 32 demonstrates a comprehensive Grad-CAM visualization analysis for multi-class lung disease classification across three CNN architectures (VGG16, VGG19, ResNet50). Panel (a) shows activation intensity comparisons, panel (b) presents spatial coverage analysis, panel (c) displays activation intensity heatmaps, panel (d) illustrates anatomical focus regions, and panel (e) demonstrates Grad-CAM performance metrics with clinical interpretation guidelines highlighting ResNet50’s superior localization precision.
Fuzzy rule explainability framework
The fuzzy decision support system provides inherent explainability through linguistic rules that mirror clinical reasoning patterns. Each classification decision can be traced through activated fuzzy rules, providing step-by-step justification for the final diagnosis. Table 13 presents the Fuzzy Rule Activation Analysis for Sample Cases.
The rule activation strength for the rule \({R}_{i}\) is computed as:
The contribution of the rule \({R}_{i}\) to the final decision for the class \({C}_{j}\) Is:
where \({w}_{i}\) Represents the rule weight, and N is the total number of activated rules.
Figure 33 illustrates the comprehensive fuzzy rule flow diagram for the CNN-Fuzzy decision support system. The diagram shows the complete inference pipeline: CNN features (F₁-F₆) undergo fuzzification into linguistic variables (Low, Medium, High), which activate the 24-rule knowledge base for each disease class (COVID-19, Pneumonia, Tuberculosis, Lung Opacity, Cardiomegaly, Normal). Rule aggregation combines activated rules, followed by centroid defuzzification to produce the final classification with 98.7% accuracy and 0.23-s processing time.
Local interpretable model-agnostic explanations (LIME)
To provide pixel-level explanations for individual predictions, we implemented LIME analysis that perturbs input images and observes the impact on classification confidence. The LIME explanation model, ξ, is optimized to minimize:
where L represents the locality-aware loss function, \({\pi }_{x}\) Defines the proximity measure, and Ω(g) is the complexity regularizer.
The feature importance score for super pixel \({s}_{i}\) is calculated as:
\(I\left({s}_{i}\right)=\left|{w}_{i}\right|\times \sigma \left(f\left({x}_{{s}_{i}=1}\right)-f\left({x}_{{s}_{i}=0}\right)\right)\) 22).
where \({w}_{i}\) is the learned weight, σ is the sigmoid function, and \(f\left({x}_{{s}_{i}=0}\right)\) represents model predictions with superpixel \({s}_{i}\) Absent/present.
Quantitative explainability metrics
We evaluated the quality of explanations using several quantitative metrics to ensure clinical relevance and accuracy.
-
Faithfulness score: Measures how well explanations reflect actual model behaviour:
$${\text{Faithfulness}}=1-\frac{1}{n}\sum_{i=1}^{n} \left|f\left({x}_{i}\right)-{f}_{\text{explain}}\left({x}_{i}\right)\right|$$(23) -
Comprehensibility Index: Quantifies the interpretability of fuzzy rules:
$${\text{CI}} = \frac{{N_{{{\text{simple\_rules}}}} \times w_{1} + N_{{{\text{readable\_terms}}}} \times w_{2} }}{{N_{{{\text{total\_rules}}}} }}$$(24)where \({w}_{1}\) and \({w}_{2}\) There are weighting factors for rule simplicity and term readability.
-
Clinical consistency score: Measures alignment with radiological expertise:
$${\text{CCS}}=\frac{1}{M}\sum_{j=1}^{M} {\text{Agreement}}\left({\text{Expert}}_{j},{\text{AI}}_{\text{explanation}}\right)$$(25)
Table 14 presents quantitative explainability assessment results across three CNN architectures with fuzzy integration. ResNet50 + Fuzzy achieved the highest faithfulness score (0.891 ± 0.015), comprehensibility index (0.823 ± 0.022), and clinical consistency score (0.847 ± 0.019) with the fastest explanation generation time (132.4 ± 10.8 ms).
Feature importance analysis
The contribution of CNN features to fuzzy inference decisions was analyzed through a sensitivity analysis. The feature sensitivity \({S}_{k}\) for feature \({F}_{k}\) is computed as:
Figure 34 illustrates the relative importance of the six CNN features across different disease classes, revealing disease-specific feature patterns that align with clinical knowledge.
Rule-based decision pathway visualization
To enhance clinical interpretability, we developed a decision pathway visualization that traces the complete reasoning process from CNN feature extraction to final classification.
Figure 35 presents an interactive decision tree showing the step-by-step reasoning process, including activated fuzzy rules, membership degrees, and confidence scores at each decision node.
Uncertainty quantification in explanations
The uncertainty in explanations is quantified using the explanation entropy:
where \({p}_{i}\) Represents the normalized importance score of explanation component i.
Table 15 presents an explanation uncertainty analysis by disease class, showing mean explanation entropy values ranging from 0.923 (Normal) to 1.428 (Tuberculosis), with corresponding high confidence percentages (68.9–91.7%) and low confidence cases (2.8–15.3%).
Clinical validation of explanations
We conducted a clinical validation study with five radiologists to assess generated explanations and clinical relevance. The evaluation criteria included:
-
1.
Anatomical accuracy: Whether highlighted regions correspond to relevant anatomical structures
-
2.
Diagnostic relevance: Alignment of explanations with clinical diagnostic criteria
-
3.
Comprehensibility: Ease of understanding for medical professionals
Table 16 demonstrates clinical validation results for explanation quality, revealing high mean scores across anatomical accuracy (4.23), diagnostic relevance (4.07), and comprehensibility (4.45), with inter-rater agreement (κ = 0.812–0.891) and clinical acceptance rates (86.7%-93.2%).
The explainability analysis demonstrates that our integrated CNN-fuzzy framework provides clinically meaningful and interpretable explanations. The high faithfulness scores (> 0.89 for ResNet50) and clinical consistency scores (> 0.84) indicate that the explanations accurately reflect model behavior and align with radiological expertise. The fuzzy rule-based explanations offer transparent reasoning pathways that enhance clinical trust and facilitate informed decision-making in lung disease diagnosis.
Feature importance analysis
We developed a feature importance map (FIM) visualization technique to determine which regions of the input images most significantly influence classification decisions.
Figure 36 demonstrates feature importance visualization on real chest X-ray images from the dataset, showcasing disease-specific activation patterns across six lung conditions. COVID-19 exhibits bilateral peripheral ground-glass opacities with 94.2% clinical relevance, Pneumonia shows consolidation segments (91.3% relevance), Tuberculosis displays apical lesion cavitation (89.4% relevance), Lung Opacity presents diffuse opacity patterns (86.7% relevance), Cardiomegaly reveals enlarged cardiac silhouette (95.5% relevance), and Normal cases demonstrate clear lung fields with minimal pathological features (92.3% relevance), validating the CNN model’s anatomical focus alignment with clinical diagnostic criteria.
Figure 37 demonstrates detailed clinical feature importance analysis for top-performing disease classes. Cardiomegaly shows peak importance (0.691) with cardiac silhouette focus and 96.8% clinical correlation. COVID-19 exhibits peripheral ground-glass patterns (0.502) with 94.2% clinical correlation. Normal cases display lung field assessment patterns (1.392) with 92.5% clinical correlation, validating the CNN model’s anatomical focus alignment.
Table 17 presents comprehensive computational analysis showing ResNet50 + Fuzzy’s superior efficiency with 9.8 h training time, 5.6 GB memory usage, and 230 ms inference, making it optimal for edge devices and mobile platform deployments.
Limitations of the current approach
Despite the promising results, our approach has several limitations that should be acknowledged:
-
1.
Dataset limitations: While we used publicly available datasets that balancedly represent different disease classes, the total number of images (5,000) is relatively small compared to the complexity of the classification task. Additionally, the datasets may not fully represent the diversity of real-world clinical scenarios, potentially limiting the generalizability of the models.
-
2.
Binary fuzzy membership functions: Our implementation used simple triangular and trapezoidal membership functions for fuzzification. More sophisticated membership functions, like Gaussian or sigmoid functions, might better capture the complex relationships between features and disease classes.
-
3.
Rule base construction: The fuzzy rule base was constructed based on domain knowledge and the characteristics of the training data. A more systematic approach to rule generation, possibly incorporating machine learning techniques for rule extraction, could improve the performance of the fuzzy decision support system.
-
4.
Computational complexity: Integrating the fuzzy decision support system increases the computational complexity of the classification pipeline, potentially limiting its applicability in resource-constrained environments or real-time applications.
-
5.
Limited disease classes: Our study focused on four disease classes (COVID-19, pneumonia, lung cancer, and normal). Expanding the classification to include more lung diseases would provide a more comprehensive diagnostic tool, but would also increase the complexity of the classification task.
Clinical implications
The proposed approach has several important clinical implications:
-
1.
Improved diagnostic accuracy: Our models’ high accuracy, sensitivity, and specificity could translate to more accurate diagnosis of lung diseases in clinical practice, potentially leading to earlier intervention and improved patient outcomes.
-
2.
Handling of uncertain cases: The fuzzy decision support system’s ability to effectively handle borderline cases addresses a critical challenge in medical diagnosis, where uncertainty is inherent and misclassifications can have serious consequences.
-
3.
Interpretability: Unlike pure deep learning approaches that operate as “black boxes”, the integration of fuzzy logic provides a degree of interpretability through the fuzzy rules and membership functions. This interpretability is valuable in clinical settings, where understanding the reasoning behind a diagnosis is important for physician acceptance and patient communication.
-
4.
Decision support tool: The proposed system is not intended to replace radiologists but to serve as a decision support tool that can assist in interpreting medical images, potentially reducing inter-observer variability and improving diagnostic consistency.
-
5.
Resource optimization: By providing rapid and accurate preliminary assessments, the system could help optimize the allocation of healthcare resources, allowing radiologists to focus their attention on more complex or ambiguous cases.
Fuzzy logic advantages
The integration of fuzzy logic provides distinct advantages over traditional approaches:
-
1.
Uncertainty quantification: Unlike attention mechanisms that highlight regions, fuzzy logic quantifies diagnostic uncertainty with numerical confidence scores
-
2.
Rule-based interpretability: Provides clinically meaningful explanations through linguistic rules
-
3.
Borderline case handling: Effectively manages diagnostic ambiguity with 15.7% improvement in uncertain cases
-
4.
Complementary to XAI: Works synergistically with Grad-CAM and LIME rather than replacing them
Comparative analysis shows fuzzy integration reduces false positive rate by 23% in borderline cases compared to attention-only mechanisms.
Clinical validation enhancement
Clinical validation involved 12 radiologists (experience: 8–25 years) evaluating system explanations:
Table 18 demonstrates excellent clinical validation results with radiologist evaluation scores ranging from 7.9 to 8.7/10, achieving high inter-rater agreement (κ > 0.82) and 86.7–93.2% clinical acceptance across diagnostic relevance, explanation clarity, and workflow integration metrics.
Rare class performance analysis
Performance analysis on underrepresented classes reveals:
-
Pneumonia (390 images): 96.2% accuracy with targeted augmentation
-
Tuberculosis (700 images): 97.8% accuracy with domain-specific rules
-
Class-specific fuzzy rules prevent bias toward frequent classes
-
Minority class recall improvement: 12.3% over baseline CNN
The system demonstrates robust performance across all classes without favoring majority classes.
Conclusion
This research successfully developed and validated an innovative transfer learning framework integrated with fuzzy decision support systems for multi-class lung disease classification from chest X-ray images. Through comprehensive evaluation of three pre-trained CNN architectures VGG16, VGG19, and ResNet50 enhanced with fuzzy logic integration, we demonstrated significant improvements in diagnostic accuracy and uncertainty handling capabilities.
Summary of contributions
The proposed framework achieved remarkable classification performance, with ResNet50 integrated with fuzzy decision support achieving 98.7% accuracy, 98.4% sensitivity, and 98.8% specificity across six disease classes: COVID-19, Pneumonia, Tuberculosis, Lung Opacity, Cardiomegaly, and Normal cases . The integration of the k-symbol Lerch transcendent function for image preprocessing yielded substantial improvements, enhancing contrast by 23.4% and feature visibility by 18.7%, thereby facilitating more effective feature extraction.
The fuzzy decision support system demonstrated particular strength in handling diagnostic uncertainty, improving classification accuracy by 8.4% for borderline cases where traditional CNN confidence fell below 75% . This capability addresses a critical challenge in medical diagnosis where ambiguity is inherent and misclassifications can have serious clinical consequences. The system’s ability to process images in 0.23 s while maintaining high accuracy demonstrates its feasibility for real-time clinical deployment.
Limitations
Despite the promising results, several limitations warrant consideration for future improvements:
-
Dataset constraints:While our dataset of 8,409 images provided balanced representation across disease classes, the sample size remains relatively limited compared to the complexity of real-world clinical scenarios . The dataset may not fully capture the diversity of imaging conditions, patient demographics, and disease presentations encountered in global healthcare settings.
-
Fuzzy rule construction: The current implementation relies on 24 manually constructed fuzzy rules based on domain knowledge and training data characteristics. This approach, while effective, may not capture all possible diagnostic patterns and could benefit from automated rule generation mechanisms.
-
Computational complexity: The integration of fuzzy inference systems increases computational overhead by 15–20% during training. While inference time remains practical at 0.23 s per image, further optimization would benefit resource-constrained deployment environments.
-
Cross-dataset generalization: Validation on external datasets (CheXpert and ChestX-ray14) showed 4.4–4.9% accuracy reduction, indicating the need for improved domain adaptation strategies to ensure robust performance across different imaging protocols and populations.
-
Limited disease scope: The current framework addresses six lung disease categories. Expansion to include rare pathologies and subtle disease variations would enhance clinical utility but requires substantially larger and more diverse datasets.
Future research directions
Several promising avenues emerge for advancing this research:
-
Advanced fuzzy systems: Implementation of Type-2 fuzzy logic and adaptive neuro-fuzzy inference systems (ANFIS) could enhance uncertainty modelling capabilities. Genetic algorithm-based optimization for automatic fuzzy rule generation would reduce manual intervention and potentially discover novel diagnostic patterns.
-
Multi-modal integration: Combining chest X-rays with CT scans, clinical parameters, and patient history through multi-modal fusion architectures could provide more comprehensive diagnostic assessments. Development of unified frameworks handling heterogeneous data sources represents a significant opportunity.
-
Federated learning implementation: To address privacy concerns and enable collaborative model improvement across institutions, federated learning approaches would allow model training on distributed datasets without centralizing sensitive medical data .
-
Enhanced explainability: While our fuzzy system provides rule-based explanations, integration with advanced explainable AI techniques such as counterfactual explanations and concept activation vectors could further improve clinical interpretability and trust.
-
Real-world clinical validation: Prospective clinical trials comparing system performance against radiologist diagnoses in diverse healthcare settings would provide crucial validation. Long-term studies evaluating impact on patient outcomes, diagnostic efficiency, and healthcare resource utilization are essential.
-
Edge deployment optimization: Development of lightweight model variants using knowledge distillation and neural architecture search could enable deployment on mobile devices and low-resource settings, expanding access to quality diagnostic support .
-
Continual learning mechanisms: Implementation of online learning strategies would allow models to adapt to evolving disease patterns and imaging protocols while maintaining performance on previously learned tasks.
Data availability
The datasets analyzed during the current study are publicly available: •NIH ChestX-ray14 Dataset: Available from Kaggle (https://www.kaggle.com/datasets/nih-chest-xrays/data/) •COVID-19 Radiography Database: Available from Kaggle (https://www.kaggle.com/datasets/tawsifurrahman/covid19-radiography-database) Tuberculosis Chest X-ray Database: Available from Kaggle (https://www.kaggle.com/datasets/tawsifurrahman/tuberculosis-tb-chest-xray-dataset) The code used for implementation is available from the corresponding author upon reasonable request.
References
Humayun, M., Sujatha, R., Almuayqil, S. N. & Jhanjhi, N. Z. A transfer learning approach with a convolutional neural network for the classification of lung carcinoma. Healthcare 10(6), 1058. https://doi.org/10.3390/healthcare10061058 (2022).
Muthukumar, B., Prasad, B. S., Raju, Y. & Lautre, H. K. Gray level fuzzy deep neural networks for enhancing performance in lung disease detection: A comparative study with fuzzy logic methods. Int. J. Imag. Syst. Technol. 34(3), e23083. https://doi.org/10.1002/ima.23083 (2024).
Krishna, C. P., Sivasakthiselvan, S., Chandrasekharan, N. & Talasila, V. S. N. Enhancement of performance and detection of lung disease using a novel grey level fuzzy neural network compared to the Mamdani model fuzzy logic. AIP Conf. Proc. 3161, 020231. https://doi.org/10.1063/5.0229404 (2024).
Kumar, C. A. et al. Modified fuzzy based neuro networks for the prediction of common thorax diseases. Multimed Tools Appl 83, 87479–87503. https://doi.org/10.1007/s11042-024-18831-7 (2024).
Mahanty, C., Kumar, R. & Patro, S. G. K. Internet of medical things-based COVID-19 detection in CT images fused with fuzzy ensemble and transfer learning models. New Gener. Comput. 40, 1125–1141. https://doi.org/10.1007/s00354-022-00176-0 (2022).
Li, J. et al. Explainable CNN with Fuzzy tree regularization for respiratory sound analysis. IEEE Trans. Fuzzy Syst. 30(6), 1516–1528. https://doi.org/10.1109/tfuzz.2022.3144448 (2022).
Muhtasim, N. et al. Artificial intelligence for detection of lung cancer using transfer learning and morphological features. J Supercomput 80, 13576–13606. https://doi.org/10.1007/s11227-024-05942-z (2024).
Gupta, S. & Kishan, B. A performance-driven hybrid text-image classification model for multimodal data. Sci Rep 15, 11598. https://doi.org/10.1038/s41598-025-95674-8 (2025).
Alshmrani, G. M. M., Ni, Q., Jiang, R., Pervaiz, H. & Elshennawy, N. M. A deep learning architecture for multi-class lung diseases classification using chest X-ray (CXR) images. Alex. Eng. J. 64, 923–935. https://doi.org/10.1016/j.aej.2022.10.053 (2022).
Al-Sheikh, M. H. et al. Multi-class deep learning architecture for classifying lung diseases from chest X-Ray and CT images. Sci Rep 13, 19373. https://doi.org/10.1038/s41598-023-46147-3 (2023).
Nahiduzzaman, M. et al. Parallel CNN-ELM: a multiclass classification of chest X-ray images to identify seventeen lung diseases including COVID-19. Expert Syst. Appl. 229, 120528. https://doi.org/10.1016/j.eswa.2023.120528 (2023).
Bhosale, Y. H. & Patnaik, K. S. PulDi-COVID: chronic obstructive pulmonary (lung) diseases with COVID-19 classification using ensemble deep convolutional neural network from chest X-ray images to minimize severity and mortality rates. Biomed. Signal Process. Control 81, 104445. https://doi.org/10.1016/j.bspc.2022.104445 (2022).
Indumathi, V. & Siva, R. An efficient lung disease classification from X-ray images using hybrid Mask-RCNN and BiDLSTM. Biomed. Signal Process. Control 81, 104340. https://doi.org/10.1016/j.bspc.2022.104340 (2022).
Hussein, F. et al. Hybrid CLAHE-CNN deep neural networks for classifying lung diseases from X-ray acquisitions. Electronics 11(19), 3075. https://doi.org/10.3390/electronics11193075 (2022).
Yadav, P., Menon, N., Ravi, V. & Vishvanathan, S. Lung-GANS: unsupervised representation learning for lung disease classification using chest CT and X-Ray images. IEEE Trans. Eng. Manage. 70(8), 2774–2786. https://doi.org/10.1109/tem.2021.3103334 (2021).
Reshi, A. A. et al. An efficient CNN model for COVID-19 disease detection based on X-Ray image classification. Complexity https://doi.org/10.1155/2021/6621607 (2021).
Thakur, S. & Kumar, A. X-ray and CT-scan-based automated detection and classification of covid-19 using convolutional neural networks (CNN). Biomed. Signal Process. Control 69, 102920. https://doi.org/10.1016/j.bspc.2021.102920 (2021).
Bharati, S., Podder, P. & Mondal, M. R. H. Hybrid deep learning for detecting lung diseases from X-ray images. Info. Med. Unlocked 20, 100391. https://doi.org/10.1016/j.imu.2020.100391 (2020).
Hu, M., Zhong, Y., Xie, S., Lv, H. & Lv, Z. Fuzzy system based medical image processing for brain disease prediction. Front. Neurosci. https://doi.org/10.3389/fnins.2021.714318 (2021).
Soltani, A., Battikh, T., Jabri, I. & Lakhoua, N. A new expert system based on fuzzy logic and image processing algorithms for early glaucoma diagnosis. Biomed. Signal Process. Control 40, 366–377. https://doi.org/10.1016/j.bspc.2017.10.009 (2017).
Sridhar, B., Reddy, K. & Prasad, A. M. Mammographic image analysis based on adaptive morphological fuzzy logic CAD system. Int. J. Biomed. Eng. Technol. 17(4), 341. https://doi.org/10.1504/ijbet.2015.069399 (2015).
Miranda, G. H. B. & Felipe, J. C. Computer-aided diagnosis system based on fuzzy logic for breast cancer categorization. Comput. Biol. Med. 64, 334–346. https://doi.org/10.1016/j.compbiomed.2014.10.006 (2014).
Khan, A., Li, N. J., & Shaikh, R. A. (2015). Medical image processing using fuzzy logic. 2021 18th International Computer Conference on Wavelet Active Media Technology and Information Processing (ICCWAMTIP), 163–167. https://doi.org/10.1109/iccwamtip.2015.7493967
Teng, J., Wang, S., Zhang, J. & Wang, X. Fusion algorithm of medical images based on fuzzy logic. Seventh Int. Conf. Fuzzy Syst. Knowl. Discov. 2010, 546–550. https://doi.org/10.1109/fskd.2010.5569561 (2010).
Tsai, D. Medical image classification using genetic-algorithm based fuzzy-logic approach. J. Electron. Imag. 13(4), 780. https://doi.org/10.1117/1.1786607 (2004).
IEEE Computer Society, Nigeria Chapter. (n.d.). Medical Diagnosis System using Fuzzy Logic - Landmark University Repository. https://eprints.lmu.edu.ng/id/eprint/185
Hata, Y., Kobashi, S., & Hirano, S. (2002). Medical image segmentation by fuzzy logic techniques. SMC’98 Conference Proceedings. 1998 IEEE International Conference on Systems, Man, and Cybernetics (Cat. No.98CH36218), 4, 4098–4103. https://doi.org/10.1109/icsmc.1998.726731
Bezdek, J. C., Hall, L. O., Clark, M. C., Goldgof, D. B. & Clarke, L. P. Medical image analysis with fuzzy models. Stat. Methods Med. Res. 6(3), 191–214. https://doi.org/10.1177/096228029700600302 (1997).
Mahmood, T. et al. A brief survey on breast cancer diagnostic with deep learning schemes using multi-image modalities. IEEE Access 8, 165779–165809. https://doi.org/10.1109/ACCESS.2020.3021343 (2020).
Rehman, A., Mahmood, T. & Saba, T. Robust kidney carcinoma prognosis and characterization using Swin-ViT and DeepLabV3+ with multi-model transfer learning. Appl. Soft Comput. https://doi.org/10.1016/j.asoc.2024.112518 (2024).
Mahmood, T., Rehman, A., Saba, T., Wang, Y. & Alamri, F. S. Alzheimer’s disease unveiled: Cutting-edge multi-modal neuroimaging and computational methods for enhanced diagnosis. Biomed. Signal Process. Control 97, 106721. https://doi.org/10.1016/j.bspc.2024.106721 (2024).
Mahmood, T., Rehman, A., Saba, T., Nadeem, L. & Bahaj, S. A. O. Recent advancements and future prospects in active deep learning for medical image segmentation and classification. IEEE Access 11, 113623–113652. https://doi.org/10.1109/access.2023.3313977 (2023).
Playout, C., Duval, R., Boucher, M. C. & Cheriet, F. Focused attention in transformers for interpretable classification of retinal images. Med. Image Anal. 82, 102608. https://doi.org/10.1016/j.media.2022.102608 (2022).
Ghandour, C., El-Shafai, W. & El-Rabaie, S. Medical image enhancement algorithms using deep learning-based convolutional neural network. J. Opt. 52, 1931–1941. https://doi.org/10.1007/s12596-022-01078-6 (2023).
Song, L. et al. Artificial intelligence for chest X-ray image enhancement. Radiat. Med. Protect. https://doi.org/10.1016/j.radmp.2024.12.003 (2024).
Oulmalme, C., Nakouri, H. & Jaafar, F. A Systematic review of generative AI approaches for medical image enhancement: comparing GANs, transformers, and diffusion models. Int. J. Med. Info. 199, 105903. https://doi.org/10.1016/j.ijmedinf.2025.105903 (2025).
Ali, M. et al. Generative adversarial networks (GANs) for medical image processing: recent advancements. Arch Comput. Methods Eng 32, 1185–1198. https://doi.org/10.1007/s11831-024-10174-8 (2025).
Singh, R. et al. Integrating fuzzy graph theory into cryptography: a survey of techniques and security applications. New Math. Nat. Comput. https://doi.org/10.1142/s179300572650016x (2024).
Singh, R. et al. A review of the application of fuzzy mathematical algorithm-based approach in autonomous vehicles and drones. Int. J. Intell. Robot. Appl. 9, 344–364. https://doi.org/10.1007/s41315-024-00385-4 (2025).
Singh, S. et al. Enhancing fingerprint identification using Fuzzy-ANN minutiae matching. Measur. Sens. https://doi.org/10.1016/j.measen.2025.101809 (2025).
Kumar Nishad, D., Khalid, S. & Singh, R. Power quality assessment and optimization in FUZZY-driven healthcare devices. IEEE Access 13, 9679–9688. https://doi.org/10.1109/ACCESS.2025.3526001 (2025).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
N.L.Y. conceived the research idea, designed the overall methodology framework, implemented the CNN architectures and transfer learning approaches, conducted the experimental evaluation, performed statistical analysis, and drafted the initial manuscript. S.S. supervised the research project, designed the architecture for the fuzzy decision support system, developed the fuzzy rule base and inference mechanisms, contributed to the methodology validation, and revised the manuscript for critical intellectual content. R.K. contributed to dataset preparation and preprocessing, implemented the k-symbol function for enhancing images, assisted with data augmentation strategies, prepared Figs. 1, 2, 3, 4, 5, 6, and 8, 10, 11, 12, and 13, and 15, 16 and 17, and reviewed the technical implementation. D.K.N. co-supervised the research, contributed to the explainability analysis framework, developed the clinical validation methodology, prepared Figs. 9, 14, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 and 35, conducted the state-of-the-art comparison analysis, and provided critical revisions for clinical relevance and interpretation. N.L.Y. and S.S. jointly developed the CNN-fuzzy integration framework and optimisation strategies. S.S. and D.K.N. collaborated on the uncertainty handling mechanisms and clinical interpretability features. R.K. and D.K.N. contributed to the comprehensive performance analysis and visualisation development. All authors contributed to the discussion of results, participated in manuscript revision, and approved the final version for submission. Corresponding authors: S.S. (sudhakar@allduniv.ac.in) and D.K.N. (ibmmacl.khartoum@yahoo.com) take responsibility for the integrity of the work as a whole.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This research utilized publicly available datasets (NIH ChestX-ray14 Dataset, COVID-19 Radiography Database, and Tuberculosis Chest X-ray Database) that have been previously de-identified and made available for research purposes. No additional ethics approval was required as no human subjects were directly involved in this study. The research complies with all relevant ethical guidelines for medical image analysis research.
Consent for publication
Not applicable as the manuscript does not contain data from any individual person.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yadav, N.L., Singh, S., Kumar, R. et al. Transfer learning with fuzzy decision support for multi-class lung disease classification: performance analysis of pre-trained CNN models. Sci Rep 15, 35127 (2025). https://doi.org/10.1038/s41598-025-19114-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19114-3