Abstract
Increased expression of programmed cell death protein-1 (PD-1) on T lymphocyte subsets is associated with sepsis immunosuppression and offers potential biomarkers for prognosis. However, few studies have assessed the effect of PD-1 expression on CD3+ T lymphocytes in predicting sepsis. The aim of this study was to investigate the relationship between PD-1 expression on CD3+ T lymphocytes and 30-day mortality in patients with sepsis. A total of 180 patients with sepsis, comprising 117 survivors and 63 non-survivors, were included in the analysis. Lymphocyte counts and PD-1 expression on T lymphocytes were quantified in peripheral blood samples through the application of flow cytometry. Regression models, receiver-operating characteristic curves (ROC) and reclassification analyses were performed to assess the relationship between the biomarkers under investigation and 30-day mortality. Results showed the level of PD-1 expressing CD3+ T cells was obviously increased in the non-survivor group compared with the group that survived (P < 0.05). Based on multiple logistic regression analysis, PD-1+CD3+T cells (OR = 1.02, P < 0.05), SOFA score (OR = 1.20, P < 0.05) and APACHE II score (OR = 1.14, P < 0.05) were independent risk factors for mortality of sepsis. PD-1+CD3+ T cells showed an area under the curve(AUC) of 0.623(95% CI, 0.534–0.713) in predicting sepsis mortality. AUCs for SOFA and APACHE II scores were 0.706 (95% CI, 0.624–0.789) and 0.784 (95% CI, 0.714–0.854), respectively. Adding PD-1+CD3+ T cells to the SOFA and APACHE II scoring systems significantly improved predictive accuracy, with an AUC enhancement and a top Youden’s index of 0.553. The Cox regression analysis revealed significantly lower 30-day survival rates in patients with high PD-1 expressing CD3+ T cells (> 36.56%). PD-1 expression on CD3+T lymphocytes can serve as a promising prognostic biomarker in sepsis patients, enhancing the predictive accuracy of APACHE II and SOFA scores.
Similar content being viewed by others
Introduction
In modern medical research, sepsis is recognised as a serious disease, characterised by an imbalance in the host’s immune response to infection. The elevated rates of morbidity and mortality associated with sepsis pose a considerable challenge to global health1,2.
The pathophysiological mechanisms of sepsis are complex and involve dysregulation of immune cells and pathways. Of these, immunosuppression is key to patient prognosis. Not only does this suppression reduce the body’s ability to clear pathogens, but it also increases the susceptibility to secondary infections, which has a direct impact on the clinical prognosis3.
The suppression of the immune system in sepsis is mainly characterised by the apoptosis and exhaustion of lymphocytes, in particular of T-cells. Dysfunction of T lymphocytes, the central regulators of the adaptive immune response, can severely impair the body’s immune defence mechanisms4. Studies have shown that T cells in patients with sepsis are not only reduced in number, but also functionally lost at the cellular and molecular levels, as evidenced by decreased cytokine production, reduced cytotoxicity, and decreased cell proliferation and differentiation, which has been termed as T cell depletion5,6.
Impaired T-lymphocyte function in sepsis is strongly associated with high expression of the immune checkpoint inhibitory molecule PD-17. PD-1 is mainly expressed at receptors on the surface of activated T cells, and upon binding to its ligands PD-L1 or PD-L2, it can inhibit T-cell activity, leading to impaired T-cell function and affecting their response to pathogens.
The expression of PD-1 in different T cell subsets and its functional impact are hot topics in immunological research. For instance, PD-1 expression on regulatory T cells is likely linked to their suppressive functions, which play an important role in maintaining immune tolerance and preventing autoimmune reactions8,9. On CD8+ T cells, the upregulation of PD-1 in the presence of persistent infections may negatively impact their cytotoxic capabilities, leading to a compromise in immune defense mechanisms10. The expression of PD-1 on memory T cells may have an impact on their functionality and longevity, which is crucial for long-term immune protection and rapid response to re-encountered antigens11,12. Effector T cells, such as Th1 and Tc1, may upregulate PD-1 expression during their activation process, which is intricately linked to their involvement in cell-mediated immune responses13.
Although the interaction of PD-1 with T cells plays an important role in the immunosuppressive mechanisms of sepsis and provides potential biomarkers and targets for prognostic assessment and immunotherapy in sepsis14,15, there is a lack of studies examining the impact of PD-1 expression on CD3+ T lymphocytes concerning sepsis prognosis. CD3+ T cells represent mature T lymphocytes, which are the most important active cells in cellular immunity. This allows a rapid assessment of overall cellular immunity. Therefore, the aim of this research was to investigate PD-1 expression on CD3+ T lymphocytes in sepsis patients and to analyse its association with patients’ clinical conditions and prognosis. By identifying reliable biomarkers, this work may offer a scientific basis for risk assessment and prognosis of sepsis.
Methods
Study population
We consecutively screened septic patients who were already admitted to our ICU for study enrolment from January 2023 to April 2024. A priori power calculation based on data from Zhang et al.16 (assuming α = 0.05 and 80% power) indicated a minimum requirement of 42 patients. Our final enrollment exceeded this number to account for potential attrition and missing data.
The following criteria were employed for recruitment: (1) fulfillment of the Third International Consensus Definitions for Sepsis and Septic Shock17; (2) sepsis diagnosed within 24 h after ICU admission; (3) over 16 years old. Exclusion criteria were based on the following conditions: (1) patients with history of malignancy, autoimmune or hematological diseases; (2) individuals with prolonged use of corticosteroids or other immunosuppressants (e.g., for autoimmune disease or organ transplant); (3) incomplete data; (4) patient or family request to discontinue treatment.
The Affiliated Hospital of Xuzhou Medical University’s Ethics Committee authorised this study in accordance with the Helsinki Declaration and its later revisions (2021-KL173-01). All participants or their legal representatives provided written informed consent for the study. In addition, to protect subjects’ privacy, we removed all identifiable information from personal health data before analysis. All subjects were assessed in the ICU on the first day of admission (within 24 h post admission) and treated in compliance with the Surviving Sepsis Campaign guidelines18.
Collection of clinical data
Data were collected within 24 h of the diagnosis of sepsis on demographic and clinical factors. These include: (1) age and sex; (2) comorbidities: heart failure(HF), chronic heart disease(CHD), renal failure(RF), diabetes mellitus(DM); (3) source of infection; (4) laboratory results: white blood cell(WBC) count, neutrophil(NEU) count; lymphocyte(LYM) count, C-reactive protein(CRP), procalcitonin(PCT).
Acute physiology and chronic health evaluation II (APACHE II), Glasgow Coma Scale (GCS) and sepsis-related organ failure assessment (SOFA) scores were all computed by clinicians in 24 h of patient enrollment based on relevant clinical and demographic information.
To determine 30-day mortality, all patients were followed for 30 days after enrollment in the study. Based on the final outcome, these patients were divided into two groups for analysis: survivors and non-survivors. Within this category, survivors were patients who had a positive response to treatment in the ICU and were subsequently either transferred to other units for further treatment or discharged from hospital. Non-surviving patients included those who died after unsuccessful medical interventions.
Blood collection and processing
Peripheral blood was collected in tubes containing EDTA within 48 h post sepsis diagnosis. The T lymphocyte subsets were delineated using a panel of monoclonal antibodies sourced from BD Biosciences, USA. We used a combination of fluorescently labeled antibodies, including PE-Cy7-conjugated anti-CD45, FITC-conjugated anti-CD3, APC-conjugated anti-CD4, APC-Cy7-conjugated anti-CD8, and PE-conjugated anti-PD-1, alongside isotype-matched controls for background fluorescence normalization.
Lymphocyte populations were electronically gated based on forward and side scatter characteristics. T cells were first identified within the CD45+ leukocyte population by gating off the CD3+ cells. Subsequently, the lymphocyte populations were then characterized, distinguishing T-helper (CD4+) from T-cytotoxic (CD8+) cells through their respective CD4 and CD8 expressions. Additionally, the proportion of PD-1 expression was measured in these T cell subsets. The FACS CantoTM II flow cytometer (BD Bioscience) and FACS Diva software (BD FACSDiva™ Software, Version 8.0.1, BD Biosciences, USA; https://www.bdbiosciences.com/en-us/products/software/instrument-software/bd-facsdiva-software) were employed for comprehensive data analysis, with a minimum of 50,000 lymphocytes evaluated per sample to ensure statistical robustness. Flow cytometry was performed within 2 h of blood collection by technicians blinded to clinical outcome. Sample IDs were anonymized, and mortality data were linked only after completion of all testing and 30-day follow-up. Clinicians responsible for patient care were not provided with flow cytometry results during the treatment period.
Statistical analysis
All data collected were statistically analysed and presented using SPSS (version 26.0) and Graph Pad Prism (version 9.5). Categorical variables, expressed as frequency and percentage, were compared by the chi-square test. Continuous data that adhered to a normal distribution were expressed as mean ± standard deviation and group comparisons were performed using the Students T-test. In cases where data did not comply with normal distribution, median and interquartile ranges were reported, with group comparisons made using the Mann-Whitney U-test.
The Spearman correlation test was employed to examine relationships between variables. Multivariate regression was used to determine factors that influence the prognosis of sepsis patients. Trend tests were used to observe a possible dose-response relationship. The predictive accuracy of various patient prognosis indicators was assessed through ROC analysis. Internal validation was performed with 1000 bootstrap resamples. For each resample, we re-fitted the logistic model and calculated AUC/ORs. The 2.5th-97.5th percentiles of the bootstrap distributions provided 95% confidence intervals. Using cut-off values acquired from ROC analysis, Kaplan-Meier survival curves and Cox regression were calculated after stratification. In this study, P < 0.05 was used to define statistical significance.
Results
Basic characteristics of people with sepsis
In this study, 266 individuals were initially considered for inclusion (Fig. 1). Subsequently, 32 patients were excluded for not meeting the inclusion criteria and 54 patients withdrew from the study. Consequently, a total of 180 patients completed the study. Pulmonary infections were the primary cause of sepsis, affecting 131 patients (72.78%), followed by abdominal infections in 23 patients (12.78%), and urinary tract infections in 9 patients (5%).
There were a number of notable distinctions between the two groups. Nonsurvivors exhibited higher APACHE scores (22 vs.14, P < 0.001) and SOFA scores (7.00 vs. 5.00, P < 0.001) compared to survivors. Furthermore, statistically significant variances were identified between the two groups in terms of age (74 vs. 69, P < 0.001), lymphocyte levels (0.50 vs. 0.70, P = 0.003), CHD (25.40% vs. 11.97%, P = 0.021), and GCS scores (8 vs. 12, P = 0.003).
No notable distinctions were found concerning gender, white blood cell, neutrophil, C-reactive protein, procalcitonin, heart failure, renal failure, diabetes mellitus, and the source of infection between the survivors and non-survivors. A comprehensive overview of the patient demographics and respective measurements is encapsulated in Table 1.
Analysis of T lymphocyte subset and PD-1 expression on these subsets
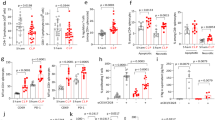
The analysis of lymphocyte subpopulations showed that in nonsurvivors group, the percentages of CD3+ and CD4+ lymphocytes significantly lower compared to survivors group (P < 0.05), but no difference in CD8+ lymphocytes was observed. Meanwhile, the analysis of PD-1 expression on lymphocyte subpopulations revealed that percentage of PD-1 expression on CD3+, CD4+ and CD8+ lymphocytes were significantly higher in nonsurvivors than in survivors (P < 0.05) (Table 2).
Spearman correlation analysis was applied to assess the relationships of CD3+ lymphocytes with CD4+ and CD8+ lymphocytes, respectively, and to explore the correlations between PD-1 expression on CD3+ lymphocytes and its expression on CD4+ and CD8+ lymphocytes. The proportion of CD3+ lymphocytes cells demonstrated a significant positive correlation with both CD4+ lymphocytes(r = 0.669, P < 0.001) and CD8+ lymphocytes(r = 0.498, P < 0.001) (Fig. 2A, B). The proportion of PD-1 expression on CD3+ lymphocytes exhibited a significantly positive association with the proportion of PD-1 on both CD4+ lymphocytes(r = 0.895, P < 0.001) and CD8+ lymphocytes(r = 0.801, P < 0.001) (Fig. 2C, D).
Correlation of CD3+T cell with CD4+T cell and CD8+T cell (A, B). Correlation of the expression of PD-1+CD3+T cell with PD-1+CD4+T cell and PD-1+CD8+T cell (C, D). (A) The percentage of CD3+T cell was positively correlated with that of CD4+T cell. (B) The percentage of CD3+T cell was positively correlated with that of CD8+T cell. (C) The Expression of PD-1+CD3+T cell was positively correlated with that of PD-1+CD4+T cell. (D) The expression of PD-1+CD3+T cell was positively correlated with that of PD-1+CD8+T cell.
Correlation analysis of CD3+ and PD1+CD3+ T cells with disease severity
Spearman correlations were performed among CD3+ T cells, PD-1+CD3+ T cells, APACHE II, PCT and SOFA score. Analysis of correlation between factors depicted as a heatmap(Fig. 3). The results of the correlation analyses showed that CD3+ T cells had a negative correlation with PCT(r=-0.211, P < 0.05), APACHEII score(r=-0.232, P < 0.05), and SOFA score(r=-0.204, P < 0.05). However, no significant correlation was observed between the expression of PD-1 on CD3+ T cells and APACHE II score, SOFA score, or PCT. Nor were significant correlations found between CD3+ T cells and PD-1+CD3+ T cells.
Prognostic risk factors in sepsis patients
Multivariate logistic regression analysis was used to find an independent factor for variables that showed significance in the univariate study. Before multivariate analysis, linear relationships between potential risk factors were analyzed.
Since the percentage of CD3+ T cells was highly related to the percentage of CD4+ and CD8+ T cells, and that the proportion of PD-1+CD3+ T cells was highly correlated with the proportion of PD-1+CD4+ and PD-1+CD8+ T cells, to avoid the multicollinearity issue in the multivariable multilevel analysis, the percentage of CD3+ T cells and PD-1+ CD3+ T cells were assessed. The colinear indicators (percentage of CD4+, PD-1+CD4+, CD8+, and PD-1+CD8+ T cells) were excluded.
The variables ultimately included in the multifactorial analysis were Age, CHD, CD3+, CD3+ PD-1+, SOFA score, GCS score, and APACHE II score. The results of multivariate regression analysis indicated that PD-1+ CD3+ T cells (OR = 1.02, P < 0.05), APACHE II score (OR = 1.14, P < 0.05), and SOFA score (OR = 1.20, P < 0.05) were independent risk factors affecting the prognosis of sepsis(Table 3).
The dose-response relationship between PD1+CD3+T cell and sepsis prognosis
To observe the possible dose-response relationship between PD1+CD3+ T cell and sepsis prognosis, we performed a test for trend using the median value of each tertile of PD1+CD3+ T cell count as a continuous variable to test for trend.
The results showed that in univariate analyses, higher PD1+CD3+ T cell counts were associated with a higher risk of poor sepsis prognosis(P trend < 0.05). After multivariate adjustment, the correlation remained significant(P trend < 0.05), with a 3.07-fold increased risk of sepsis death when the highest tertile of PD1+CD3+ T cell counts was compared with the lowest tertile in fully adjusted Model 3 (OR = 3.07, 95% CI = 1.18–7.94)(Table 4).
Predictive value of mortality risk factors in sepsis patients
The prediction accuracy for sepsis death rate was measured using AUC values for PD-1+CD3+ T cell percentage (AUC = 0.623, 95% CI = 0.534–0.713), SOFA score (AUC = 0.706, 95% CI = 0.624–0.789), and APACHE II score (AUC = 0.784, 95% CI = 0.714–0.854). Comparing AUC values among the different biomarkers, we found that CD3+PD-1+(%) in combination with APACHE II, and SOFA score presented the highest AUC value (Fig. 4). Internal validation using 1000 bootstrap resamples showed stable performance, with AUC remaining at 0.80 (95%CI: 0.71–0.89) and classification accuracy of 75.5% (Kappa = 0.43), indicating reproducible discrimination and moderate agreement with outcomes (Suppl. Table 1, Suppl. Table 2).
To assess each obtained marker comprehensively, sensitivity, specificity, cut-off point, and Youden index were calculated. It was found that CD3+PD-1+(%) + APACHE II had the greatest sensitivity(90.3%), while CD3+PD-1+(%) + SOFA had the greatest specificity(84.5%). In addition, the combination of CD3+PD-1+(%) + APACHE II + SOFA had the highest Youden index (0.553). Taken together, it was the best combination in predicting, discriminating, and reclassifying 30-day mortality(Table 5).
Survival analysis of sepsis patients
Based on the cut-off values by the ROC curve analysis, patients were stratified into groups, and survival analyses were conducted. Stratified by the 36.56% PD-1+CD3+ T cell threshold, there were significantly different survival rates among septic patients, with patients having a high level of PD-1+CD3+ T cells exhibiting lower survival rate compared to those with a lower percentage (HR = 2.481, P < 0.001) (Fig. 5A).
Moreover, we calculated predictive probabilities using a combination of three independent risk factors: PD-1+CD3+ T cells, APACHE II score, and SOFA score. Patients were stratified based on the predictive probability cut-off value at 0.41. The 30-day mortality rate was significantly higher in patients with a predictive probability above this threshold compared to those below it (HR = 6.516, P < 0.001) (Fig. 5B).
Kaplan-Meier curves of septic patients (A) Sepsis patients with PD-1+ CD3+ T cell levels ≥ 36.56% have higher 30-day mortality than those with lower levels. (B) Sepsis patients with a predicted probability of CD3+PD-1+(%) + APACHE II + SOFA ≥ 0.41 have higher 30-day mortality than those with lower levels.
Discussion
Sepsis is a rapidly progressive disease that is characterised by dysfunction of immune response to infection. Delays in diagnosis and treatment can lead to severe deterioration and increased mortality. Identifying sepsis biomarkers, especially those related to immune function, is critical for early detection, appropriate management and improved patient outcomes19,20. This study was designed to determine whether the proportion of PD-1+CD3+ T cells independently predicts 30-day mortality in sepsis and to quantify its incremental value to SOFA and APACHE II scores.
T lymphocytes play a critical role in the pathophysiology of sepsis, but there is relatively little research on the CD3+ T cell population as a whole. CD3+ T cells represent the entire spectrum of mature T lymphocytes, encompassing various T cell subsets and providing a direct assessment of overall T cell function21,22. The advantage of studying CD3+ T cells lies in their ability to offer a comprehensive evaluation of T cell activity and quantity. This approach simplifies the testing process, reduces costs, and shortens the time required for testing, thereby enabling the rapid identification of immune function abnormalities.
In the present investigation, we found significant differences in the proportions of CD3+ and CD4+ T cell between survivors and non-survivors, with non-survivors having a significant lower percentage. This is consistent with the findings of Drewry, who noted that persistent lymphopenia related to higher death rates in sepsis patients23. Possible mechanisms include sepsis-induced immune suppression, increased lymphocyte apoptosis and impaired function24,25. Differently, when comparing the two groups, there was no significant difference in CD8+ T cell percentages, which may indicate differences in the sensitivity and response to immune injury of lymphocyte subpopulations in sepsis.
We further conducted an assessment of the relationship with the severity of the disease and found that the percent of CD3+ T cells was negatively related to APACHE II and SOFA scores. The patient’s disease and organ failure worsened as the percentage of T cells decreased. This was consistent with increased APACHE II and SOFA values, both of which predicted a worse prognosis26,27.
In addition, our study shows significantly increased levels of PD-1 expression on the surface of CD3+ T cells in non-survivors of sepsis, in contrast to survivors. Previous researches have indicated that patients with sepsis had a generally increased PD-1 expression on T-lymphocyte subsets10,28.Binding between PD-1 and PD-L1 is a key immunomodulatory mechanism that is essential for maintaining immune system homeostasis by inhibiting the activation, proliferation and function of T cells. This interaction is also thought to be a key factor in the reduction in the number and function of T lymphocytes, which can impair the body’s ability to mount an immune response against pathogens, leading to a poor prognosis7. Interesting, PD-1 expression on different T-cell subpopulations does not consistently reflect immunosuppression and prognosis in sepsis, which requires further investigation29,30,31.
In the next step, we identified independent predictors of 30-day mortality in patients with sepsis using univariate and multivariate logistic regression analyses. The results showed that the level of PD-1 expression on the surface of CD3+ T cells, SOFA score and APACHE II score were significantly associated with poor patient prognosis. Although previous studies had shown that CD3+ T cells level can predict disease severity and mortality32,33, the percentage of CD3+ T cells did not emerge as an independent risk factor in the anglyses. The reason for the difference may be due to the insufficient sample size.
Furthermore, we evaluated the predictive ability of three independent factors for 30-day mortality in patients with sepsis using ROC curves. According to the research, the APACHE II score (AUC = 0.792) had the best accuracy in prediction, closely followed by the SOFA score (AUC = 0.706) and PD-1+CD3+ T lymphocytes (AUC = 0.625). This outcome emphasized the APACHE II score’s excellence in predicting sepsis prognosis and also suggested the potential of PD-1+CD3+ T cell as a predictive indicator. Notably, when PD-1+CD3+ T cell was used in combination with APACHE II and SOFA score, the combined predictive value was superior to any single indicator, with the AUC value increasing to 0.815. In addition, the ROC curves helped us to identify the ideal cut-off values of each parameter. Although sensitivity and specificity of this multifactorial combination were average, it had the highest Youden index, suggesting the best combined performance in predicting 30-day mortality risk in patients with sepsis. These findings highlight the importance and validity of combining multiple indicators for risk assessment.
Survival analysis revealed that sepsis patients with CD3+PD-1+(%) above the cut-off value had a higher risk of death within 30 days than patients with low levels of CD3+PD-1+(%). While this confirms its prognostic value, the more important implication is that these patients represent a distinct immunological endotype - one dominated by T-cell exhaustion and functional immunosuppression. This phenotype likely explains their inability to clear primary infections and their increased susceptibility to nosocomial infections. Therefore, a high percentage of PD-1⁺CD3⁺ T cells may serve as a biomarker of unrecognized immunosuppression, potentially identifying candidates for immune checkpoint inhibitors or other adjuvant immunotherapies.
It is important to note that although our findings highlight PD-1+CD3+ T cells as a prognostic biomarker, translating PD-1-based immunomodulation into sepsis treatment remains a challenge. The dynamic nature of immune dysfunction in sepsis, from hyperinflammation to immunosuppression, complicates the timing of intervention. In addition, PD-1/PD-L1 inhibitors, while effective in oncology treatment, may exacerbate the early hyperinflammatory response in sepsis.
This study has several limitations. Its single-center design may limit the statistical power and generalizability of our findings. The analysis captured PD-1 expression at a single time point, precluding assessment of its dynamic changes during sepsis. In addition, the exclusion of patients with chronic comorbidities limits the applicability to these high-risk populations. Finally, the lack of plasma cytokine data prevents correlative analysis with T-cell immunophenotypes. Future multicenter studies with larger, more inclusive cohorts including patients with chronic comorbidities, longitudinal follow-up, and integrated cytokine profiling are needed to address these limitations.
In conclusion, by exploring the correlation between PD-1+CD3+ T and prognosis of cellular sepsis, we propose a risk assessment index with promising clinical applications: by combining the APACHE II and SOFA scores, the PD-1+CD3+ T cell ratio can more accurately predict the risk of 30-day mortality in sepsis patients. This could provide a potential tool for sepsis endophenotyping to guide future trials of immune-targeted therapies and individualized treatments in high-risk patients, although prospective validation is needed before clinical implementation.
Data availability
The study’s data, containing sensitive participant information, aren’t publicly accessible but can be requested from the corresponding author.
References
Cavaillon, J., Singer, M. & Skirecki, T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 12, e10128 (2020).
Rhee, C. & Klompas, M. Sepsis trends: increasing incidence and decreasing mortality, or changing denominator? J. Thorac. Dis. 12, S89–S100 (2020).
Torres, L. K. & Pickkers, P. Van der Poll, T. Sepsis-induced immunosuppression. Annu. Rev. Physiol. 84, 157–181 (2022).
Wiersinga, W. J. & Van Der Poll, T. Immunopathophysiology of human sepsis. eBioMedicine 86, 104363 (2022).
Patil, N., Guo, Y., Luan, L. & Sherwood, E. Targeting immune cell checkpoints during sepsis. Int. J. Mol. Sci. 18, 2413 (2017).
Chen, W. et al. Long noncoding RNA HOTAIRM1 promotes immunosuppression in sepsis by inducing T cell exhaustion. J. Immunol. 208, 618–632 (2022).
Jubel, J. M., Barbati, Z. R., Burger, C., Wirtz, D. C. & Schildberg, F. A. The role of PD-1 in acute and chronic infection. Front. Immunol. 11, 487 (2020).
Costa, P. A. C. et al. Plasmodium Vivax infection impairs regulatory T-cell suppressive function during acute malaria. J. Infect. Dis. 218, 1314–1323 (2018).
Sossou, D. et al. Regulatory T cell homing and activation is a signature of neonatal sepsis. Front. Immunol. 15, 1420554 (2024).
Yan, L., Chen, Y., Han, Y. & Tong, C. Role of CD8 + T cell exhaustion in the progression and prognosis of acute respiratory distress syndrome induced by sepsis: a prospective observational study. BMC Emerg. Med. 22, 182 (2022).
Liu, Q. et al. Expression of PD-1 on memory T lymphocytes predicts 28-Day mortality of patients with sepsis: A prospective observational study. J. Inflamm. Res. 15, 5043–5052 (2022).
Shwetank et al. PD-1 dynamically regulates inflammation and development of brain-resident memory CD8 T cells during persistent viral encephalitis. Front. Immunol. 10, 783 (2019).
Rajamanickam, A., Munisankar, S., Dolla, C., Nutman, T. B. & Babu, S. Cytotoxic T-lymphocyte-associated antigen 4(CTLA-4)-and programmed death 1(PD-1)-mediated regulation of monofunctional and dual functional CD4 + and CD8 + T-cell responses in a chronic helminth infection. Infect. Immun. 87, e00469–e00419 (2019).
Pauken, K. E., Torchia, J. A., Chaudhri, A., Sharpe, A. H. & Freeman, G. J. Emerging concepts in PD-1 checkpoint biology. Semin Immunol. 52, 101480 (2021).
Mellinghoff, S. C. et al. T-cells of invasive candidiasis patients show patterns of T-cell-exhaustion suggesting checkpoint Blockade as treatment option. J. Infect. 84, 237–247 (2022).
Zhang, Y. et al. Predictive value of immune cell subsets for mortality risk in patients with sepsis. Clin. Appl. Thromb. 27, 10760296211059498 (2021).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801 (2016).
Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247 (2021).
Barichello, T., Generoso, J. S., Singer, M. & Dal-Pizzol, F. Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. Crit. Care. 26, 14 (2022).
Wang, A., Zhang, S., Peng, G., Tang, Y. & Yang, Y. ICU and sepsis: Role of myeloid and lymphocyte immune cells. J. Oncol. 1–7 (2022). (2022).
Liu, D., Hu, X., Chen, Z., Wei, W. & Wu, Y. Key links in the physiological regulation of the immune system and disease induction: T cell receptor -CD3 complex. Biochem. Pharmacol. 227, 116441 (2024).
Menon, A. P. et al. Modulating T cell responses by targeting CD3. Cancers 15, 1189 (2023).
Drewry, A. M. et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock Augusta Ga. 42, 383–391 (2014).
Wang, Z., Zhang, W., Chen, L., Lu, X. & Tu, Y. Lymphopenia in sepsis: a narrative review. Crit. Care. 28, 315 (2024).
Liu, J. et al. HMGB1 promotes neutrophil PD-L1 expression through TLR2 and mediates T cell apoptosis leading to immunosuppression in sepsis. Int. Immunopharmacol. 133, 112130 (2024).
Zhang, X. M. et al. Comparing the performance of SOFA, TPA combined with SOFA and APACHE-II for predicting ICU mortality in critically ill surgical patients: A secondary analysis. Clin. Nutr. 39, 2902–2909 (2020).
Li, W., Wang, M., Zhu, B., Zhu, Y. & Xi, X. Prediction of median survival time in sepsis patients by the SOFA score combined with different predictors. Burns Trauma. 8, tkz006 (2020).
Tomino, A. et al. Increased PD-1 expression and altered T cell repertoire diversity predict mortality in patients with septic shock: A preliminary study. PloS One. 12, e0169653 (2017).
Wang, L. et al. Relationship between the expression of PD-1 and CTLA-4 on t lymphocytes and the severity and prognosis of sepsis. Int. J. Gen. Med. 16, 1513–1525 (2023).
Guinault, D. et al. Expression of exhaustion markers on CD8 + t-cell patterns predict outcomes in septic patients admitted to the ICU. Crit. Care Med. 49, 1513–1523 (2021).
Zhong, W. H. et al. Elevated PD-1/CD28 ratio rather than PD-1 expression in CD8 + T cells predicts nosocomial infection in sepsis patients: A prospective, observational cohort study. Shock 58, 111–118 (2022).
Jiang, W. et al. Evaluation of a combination ‘lymphocyte apoptosis model’ to predict survival of sepsis patients in an intensive care unit. BMC Anesthesiol. 18, 89 (2018).
Liu, S., Li, Y., She, F., Zhao, X. & Yao, Y. Predictive value of immune cell counts and neutrophil-to-lymphocyte ratio for 28-day mortality in patients with sepsis caused by intra-abdominal infection. Burns Trauma. 9, tkaa040 (2021).
Acknowledgements
We would like to express our sincere gratitude to all of the individuals who have offered their help and support.
Author information
Authors and Affiliations
Contributions
YFC and LM designed the experiment. BW and SMZ conducted the experiment and analyzed the data. BW and PZ helped to drafted the paper. YCL, LM and YFC provided substantial assistance and contributed to the revision of the manuscript. All authors contributed and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, B., Zhang, P., Liu, YC. et al. Predictive value of PD-1+CD3+ T cells for 30-day mortality in patients with sepsis. Sci Rep 15, 36579 (2025). https://doi.org/10.1038/s41598-025-20299-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20299-w