Abstract
To evaluate ultrasound’s impact on braised rabbit legs, rabbit leg meat was treated at frequencies of 0 (control), 30, 60, 90, and 120 kHz during the low-temperature braising process. A multi-dimensional analytical approach—incorporating scanning electron microscopy (SEM), texture profile analysis (TPA), water-holding capacity (WHC) assessment, flavor compound profiling, and oxidation analysis—was employed to systematically investigate ultrasound’s effects on braised rabbit meat. SEM revealed ultrasound-induced muscle fiber contraction and structural disruption, which directly improved water-holding capacity and texture. Quantification of sodium chloride and amino acids in sample cores demonstrated enhanced mass transfer, particularly at higher frequencies (60–120 kHz). Lipid oxidation (TBARS) and fatty acid profiling (GC-MS) confirmed ultrasound-promoted oxidation, generating significantly increased flavor-active aldehydes. Concurrently, HPLC-MS/MS analysis showed elevated nucleotide levels, indicating accelerated hydrolysis of flavor precursors. Collectively, these results suggest that ultrasound may offer a viable approach to energy-efficient braising while improving texture and flavor profiles towards sustainable meat processing.
Similar content being viewed by others
Introduction
Braising is a traditional Chinese food processing technology, which provides characteristic flavors from spices and extends storage time. Moreover, secondary metabolites from spices enhance braised products’ nutritional profiles through bioactive functions—particularly demonstrated antimicrobial efficacy against foodborne pathogens1, alongside anti-inflammatory2 and antioxidant activities3. However, industrial application of braising is limited by drawbacks: including (1) severe textural degradation due to uncontrolled protein denaturation resulting in toughness, and (2) excessive energy consumption during prolonged cooking durations. The food processing sector requires innovative braising technologies that achieve cost efficiency while preserving textural integrity.
Ultrasound addresses these limitations through cavitation-induced effects including enhanced mass transfer and tenderization4. Pretreatments with ultrasound have improved tenderness and water-holding capacity (WHC) in various meats, demonstrating its utility in mitigating braising-induced texture loss5,6,7. Furthermore, ultrasound promotes mass transfer and accelerates marination of beef, pork, rabbit meat, and fish8,9,10,11, indicating its potential in reducing braising time and energy consumption. Besides these findings, ultrasound has various applications in food processing, including emulsification, degassing, sterilization, among others12. Although ultrasound has been extensively utilized in food processing, its multidimensional impacts on textural, microstructural, and flavor profiles during meat products’ braising remain unexplored.
This study investigated the impact of ultrasound frequency on microstructure, texture, water-holding capacity and flavor compound formation during rabbit meat braising. Rabbit leg meat was selected as the material for its dual relevance: (i) nutritional advantages, characterized by high protein bioavailability, favorable lipid profiles (low fat, high polyunsaturated fatty acids), and rich mineral content (e.g., iron, selenium); (ii) structural sensitivity, where fine muscle fibers of rabbit meat increase susceptibility to texture degradation during thermal processing. SEM-based muscle fiber imaging, texture analysis, water-holding capacity quantification and low-field nuclear magnetic resonance were performed to evaluate the influences of ultrasound on braised rabbit leg meat. TBARS-based lipid oxidation analysis and flavor compound quantification via GC–MS and HPLC–MS/MS elucidated flavor formation mechanisms in ultrasound-assisted braising. Additionally, sodium chloride and amino acids in the braised rabbit leg meat were measured to assess ultrasound-assisted mass transfer during braising. Overall, this study aimed to find influences of ultrasound in braising and provide theoretical and practical references for manufacturers.
Materials and methods
Rabbit legs cooking
Rabbit hind legs ranging from 245 to 255 g were purchased from Ningde., Co Ltd, Jinan, Shandong, vacuum-packaged and stored at -20 ºC after fatty and connective tissues were removed. Raw rabbit legs were pickled for 1 h in a vacuum rolling equipment with the following formulation: 0.1% ginger powder, 4% cooking wine, 0.1% pepper powder, 1% Sichuan pepper, 0.1% shallot powder, 10% soybean oil, 0.1% starch.
Afterwards, rabbit legs were braised in brine at 85 °C for 44 min with the following formulation: 100 g rabbit legs, 5 ml light soy sauce, 4 ml dark soy sauce, 15 ml cooking wine, 1.5 g salt, 5 g sugar, 2.5 g scallion, 2.5 g ginger slices, 1 g Sichuan pepper, 0.5 g star anise, 0.5 g cinnamon, 0.03 g clove, 0.25 g Chinese parsley, 0.1 g cumin, 0.25 g bay leaves, 0.21 g amomum tsaoko, 0.1 g licorice and 0.5 g caramel in 350 ml water were cooked. Ultrasound of 0 kHz (control), 30 kHz, 60 kHz, 90 kHz and 120 kHz was applied in braising.
The ultrasonic braising system comprised a steam-jacketed kettle with integrated ultrasonic transducers located within the jacket layer (custom-designed by Lilan Intelligent Equipment Co., Ltd., Suzhou, China). A temperature probe (accuracy ± 0.1℃) inside the cooking chamber provided real-time feedback to a digital controller, which regulated steam inlet valves to ensure constant temperature (85 ± 0.5 ℃) throughout braising. Ultrasound was in continuous mode. For 0 kHz group, the ultrasound generator remained switched off during braising. The generator’s total electrical power output ranged from 600—900 W across frequencies (manufacturer-calibrated under nominal load).
Shear force and texture analysis
After cooking, rabbit legs were cooled to ambient temperature. Muscle square columns (cross section = 1 cm*1 cm) were cut parallel to the muscle fibers orientation and shear force was quantified with a Texture Analyzer (Stable Micro Systems Ltd) according to methods described by Chen13. Texture parameters were detected following a previous study with some modifications: pre-test speed :60 mm/min, test speed: 120 mm/min, test shape variable: 60%, trigger force :0.4 N, test interval: 1 s.
Scanning electron microscopy
Rabbit meat cylinders (0.5 cm × 0.5 cm × 0.3 cm) were collected from the center of cooked rabbit legs, fixed in 2.5% glutaraldehyde at 4 ℃ for 14 h, and then washed three times with 0.2 M PBS buffer (pH = 7.4) for 30 min each time. Gradient dehydration with 50%, 70%, 80% and 90% ethanol for 15 min each time was performed. Final dehydration was performed with 100% ethanol three times (30 min each). Replacement was done in tert-butanol for 30 min and repeated twice. The samples were pre-cooled at -80 °C for 8 h and freeze-dried for 10 h. A metal film (10 nm) was coated on the surface of the sample with an ion sputtering instrument. Samples were imaged with a scanning electron microscope (VEGA 3 SBU–EasyProbe, TESCAN, Shanghai) at a voltage of 15.0 kV.
Pressure loss
Pressure loss was measured to evaluate water-holding capacity of samples14. Briefly, muscle cylinders (diameter 2.5 cm, thickness 1 cm) were cut parallel to the muscle fiber orientation and the mass was recorded as M1 immediately. The muscle cylinders were wrapped with filter paper and placed onto a pressure platform. A press of 343 N was applied to the sample for 3 min. The mass of pressed sample was recorded as M2 after removal of filter paper. The pressure loss was calculated using Eq. (1).
Cooking loss
Cooking loss was measured to evaluate amounts of water lost in braising. The mass of raw rabbit hind legs before cooking was recorded as W1. After pickling and braising, residual liquid on the surface was removed with filter paper and the weight was recorded as W2. The cooking loss was calculated using Eq. (2).
NMR transverse relaxation (T2) measurements
Muscle cubes (2.5 cm × 5 cm × 1 cm) were collected from braised rabbit legs, wrapped with cling film and placed into specialized sample tubes. The low-field NMR spin–spin relaxation measurements were performed on an NMR analyzer (MesoMR23-060H-I, Niumai Analysis Instruments, Co., Ltd, Suzhou, China). The analyzer was operated at a resonance frequency of 10 MHz at 36 °C. The relaxation time was measured by Ti-T spectroscopy with IR-CPMG sequence. The parameters were set as follows: receiving gain 120, echo interval 0.2 ms, sampling number 3000, scanning times 4, interval 2 s. The measurements were repeated 4 times for each sample, and the inversion of the attenuation curve was performed15.
Evaluation of brine permeating
The contents of sodium chloride in cooked rabbit legs were measured according to methods from China National Standard GB 5009.44-2016. Lightness (L), redness (a) and yellowness (b) were measured with a colorimeter (Minota cr-200, Monita, Japan).
Quantification of nucleotides
Nucleotides were extracted with 50% methanol. Briefly, 500 μL ice-cold 50% methanol was added to 0.1 g rabbit meat tissue. The sample was vortexed to mix thoroughly, sonicated for 20 min, then incubated with shaking for 2 h at 4 ℃. Afterwards, the sample was centrifuged for 10 min at 12,000 rpm at 4 ℃, and the supernatant was collected for further quantification via HPLC–MS/MS.
Nucleotide extracts were injected onto a reverse-phase column (Acquity UPLC HSS T3 column, 100 Å, 1.8 µm, 2.1 mm × 100 mm). Injection volume was 3 μL. Flow rate was 0.3 mL/min. The initial eluent (from 0 to 3 min) was 95% buffer A (0.1% formic acid) and 5% buffer B (acetonitrile). The content of buffer B was raised to 95% until 6 min, held at 95% until 8 min, reduced to 5% until 8.1 min and held at 5% until 10 min. Ion source: ESI. Positive mode. MRM mode. Quantification was performed with an external standard curve according to mass transition and declustering potential parameters listed in Table 1.
Lipid analysis
Lipid oxidation was determined using a spectrophotometric thiobarbituric acid reactive substance (TBARS) method with minor modifications16. To 1.0 g of rabbit leg meat, 5 mL of 7.5% TCA solution supplemented with 0.1% EDTA was added. The sample was vortexed three times to mix thoroughly. The homogenate was centrifuged at 12,000×g for 5 min to obtain the supernatant. To the mixture, 2 mL of 0.02 mol/L 2-thiobarbituric acid (TBA) was added. The sample was vortexed again to mix thoroughly and incubated in water at 100 °C for 40 min. After cooling to room temperature, the absorption value at 538 nm was measured against the reagent blank with a spectrophotometer (UV-160, Beijing Beifen-Ruili Analytical Instrument Co., Ltd). The lipid oxidation product malondialdehyde (MDA) was quantified with the standard curve. MDA stock solution was prepared by dissolving MDA equivalent 1,1,3,3-Tetraethoxypropane in deionized water. The MDA stock solution was serially diluted and the absorption at 538 nm was recorded to prepare the standard curve. A linear standard curve was established over the range of 0–10 mg/L MDA equivalents (Y = 0.2681X-0.0158, R2 > 0.99), where Y = Absorbance, X = MDA concentration in mg/L. Results were expressed as mg MDA per kg meat.
Fatty acids were profiled via GC-MS17. Briefly, ~ 10 g of rabbit leg meat was cut into pieces with a scissor and 14ml of chloroform: methanol (2:1, v/v) was added. The samples were incubated in a water bath of 45 °C with gentle shaking for 2 h. 30 ml of saturated sodium chloride solution was added to obtain phase separation. Lower phase (chloroform phase) was collected, and residual water was removed with anhydrous sodium sulfate. Solvent was evaporated in water bath of 45 °C to obtain fatty tissue. 3 mL of benzene: petroleum (1:1, v/v) was added to dissolve lipids. Then 2 mL of 14% boron fluoride methanol (Sigma-Aldrich) was added. The sample was mixed thoroughly and incubated in water bath of 45 °C for 30 min to derivatize fatty acids to fatty acid methyl esters. To the sample, 1 ml of n-hexane and 1 ~ 2 ml of saturated sodium chloride solution were added to obtain phase separation. The upper phase was transferred to GC–MS sample vial for analysis via GC–MS.
GC–MS conditions: TSQ 8000, Thermo fisher, USA. J&W 122–7062 column, 60 m × 250 µm × 0.25 µm. Split ratio: 5:1. Injection volume: 5µL. Carrier gas: helium. Flow rate: 3.0 mL/min. Detector temperature: 260 °C. Column temperature: initial temperature 100 °C for 5 min, 4 °C /min to 240 °C, 240 °C for 30 min. Relative contents (%) were calculated through area normalization.
Flavor precursor analysis
Total sugar of rabbit legs was quantified according to China National Standard GB/T 9695.31–2008 (Meat products- Determination of total sugars content). Briefly, 1.0 g of rabbit leg meat was sampled and incubated in a boiling water bath for 30 min. Total volume was adjusted to 500 mL with water. The sample was filtered, and the supernatant was retained for further analysis. To 1 mL of supernatant, 1 mL of 5% phenol and 5 mL of concentrated sulfuric acid were added. The mixture was vortexed thoroughly, incubated at room temperature for 20 min. Absorbance at 470 nm was observed with a spectrophotometer (UV-160, Beijing Beifen-Ruili Analytical Instrument Co., Ltd).
Amino acids from rabbit legs were quantified with an autonomous amino acid analyzer (Biochrom 30 + , England)15. Loading buffer flow rate: 25 mL/h; reaction flow rate: 10 mL/h; Na+ cationic resin chromatographic column, 200 mm × 4.6 mm, particle size 8 μm. UV detection wavelength: 570 nm and 440 nm. Column temperature: 55-65-77 °C program heating; reaction tank temperature: 138 °C; injection volume: 20 μL.
Volatile analysis
Total volatiles were isolated via headspace extraction and characterized through GC–MS18. The extraction column was preheated to 250 ℃ for 1 h to remove residual compounds from the last sampling. 2.0 g of rabbit leg meat was cut into pieces, collected in a sample vial and 3 mL of saturated sodium chloride solution was added. The sample vial was incubated in a water bath of 60 ℃ during extracting. Headspace extraction was performed for 40 min.
GC–MS conditions: TSQ 8000, Thermo fisher, USA. J&W 122–7062 column, 60 m × 250 µm × 0.25 µm. Carrier gas: helium. Flow rate: 1.5 mL/min. Detector temperature: 250 ℃. Column temperature: initial temperature 40 °C for 3 min, 5 °C /min to 150 °C, 150 °C for 1 min. 15 °C /min to 180 °C. 10 °C /min to 250 °C. 250 °C for 5 min. Ion source temperature: 230 °C. Positive mode. 70 eV. Relative contents (%) were calculated through area normalization.
Data processing and analyzing
Each experiment was conducted in triplicate, and the results were expressed as mean ± standard deviation (SD). Data were analyzed by one-way ANOVA followed by Tukey’s honestly significant difference (HSD) post hoc test; statistical significance was defined as p < 0.01. Different italic letters above the bars indicate significant differences, while identical italic letters indicate non-significance. Raw data were recorded with Microsoft Office Excel. Curves, bar charts and significance analysis were produced and performed with Prism 10. Distinct sample cohorts were used for each analytical test (texture profile analysis, WHC, SEM, GC–MS/HPLC–MS/MS, TBARS, etc.). Following braising, samples from each treatment group were randomly allocated to specific test batches.
Results
Microstructure of rabbit legs
Altered muscle fiber microstructure critically influenced texture and WHC. Figure 1a shows that muscle fibers in control (0 kHz) are tightly bound with few spaces among fibers. Muscle fibers in 30 kHz exhibit comparable morphology to the control. At 60 kHz, distinct inter-fiber gaps emerge. At 120 kHz, fibers exhibit severe shrinkage and structural disruption, generating macroscopic cavities.
Influence of ultrasound frequency on microstructure, water-holding capacity, and texture of rabbit leg meat. (a) Microstructure of rabbit muscle imaged by scanning electron microscope; (b) Shear force, hardness, chewiness, springiness, and resilience; (c) Pressure loss and cooking loss; (d) Low-field NMR spin–spin relaxation times T2; (e) Distribution of low-field NMR transverse relaxation T2 times; (f) Low-field NMR spin–spin proportions P2. Mean ± SD, 3 biological replicates. One-way ANOVA test, p < 0.01. Different italic letters above the bars indicate significant differences.
Texture
As shown in Fig. 1b, ultrasound-assisted braising reduces shear force, hardness and chewiness compared to the control (0 kHz). Moreover, higher frequency progressively decreases shear force, hardness, and chewiness. Besides, there are no obvious differences in springiness and resilience across frequency treatments. These findings demonstrate that ultrasound-assisted braising significantly improves texture.
Pressure loss and cooking loss
Pressure loss and cooking loss are key indicators of water-holding capacity (WHC). Traditional braised meats are cooked in boiling brine, a thermal treatment process known to reduce WHC. Ultrasound-assisted braising at lower temperatures significantly enhanced WHC (Fig. 1c). Pressure loss was highest at 0 kHz (24.44 ± 0.10%). At the optimal frequency of 30 kHz, pressure loss decreased significantly to 18.88 ± 0.30%, the lowest value observed. With increasing ultrasound frequency beyond 30 kHz, pressure loss increased, although remaining lower than at 0 kHz. Cooking loss followed a similar trend: decreasing from 33.23 ± 0.35% at 0 kHz to 27.60 ± 0.65% at 60 kHz. However, at 120 kHz, cooking loss was significantly higher than 60 kHz, yet still lower than at 0 kHz.
Water-holding capacity
Low-field nuclear magnetic resonance (LF-NMR) is a rapid and nondestructive technology for characterizing water distribution and water-matrix interactions with biological samples. Transverse relaxation time T2 of rabbit leg meat after ultrasound cooking was measured. As shown in Fig. 1e, three water states were identified across all treatment groups: bound water (T2b), immobilized water (T21) and free water (T22) . Bound water, tightly associated with hydrophilic groups of muscle proteins, is hence also termed protein-associated water. Bound water lacks solvent properties and rarely participates in biochemical reactions. Immobilized water, primarily located in intra-myofibril spaces, constitutes the main water form in muscle tissues and critically influences meat water-holding capacity and texture. Free water resides in the sarcoplasmic area and exhibits high environmental sensitivity. Free water is only held by capillary forces19. Shorter relaxation times indicate tighter binding between water and food matrix15. From 0 to 60 kHz, T2b decreased significantly, reaching its minimum value at 60 kHz. While frequencies higher than 60 kHz resulted in an increase of T2b. At 120 kHz, T2b exceeds the control. T21 at 60 kHz was the lowest. While there is no obvious T21 differences among other frequencies. T22’s trend was opposite to T2b: T22 reaches its peak at 60 kHz, while at 120 kHz it was even lower than the control. These results demonstrate that ultrasound at 60 kHz could strengthen interaction between bound/immobilized water molecules and the food matrix and weaken interaction between free water and the food matrix. The dominant type of water in rabbit leg meat is immobilized water, reaching 70% (Fig. 1f). It is interesting to note that at 60 kHz, the proportion of immobilized water (P21) was higher than other groups. This is in accordance with observations in Fig. 1d.
Mass transfer
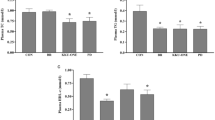
Figure 2 shows that sodium chloride contents in meat increase significantly with ascending frequency. This demonstrates that cavitation effects generated by high-frequency ultrasound can enhance salt diffusion. Appearance is a critical visual quality indicator for customers. As shown in Fig. 2, brightness (L* value) of rabbit leg meat decreases significantly with increasing ultrasound frequency. This is due to accelerated penetration of soybean sauce into the meat matrix during braising. Soybean sauce is a traditional liquid condiment with brown appearance widely used in east Asia regions, including China, Japan, and Korea. Regarding color parameters, redness (a* value) decreased progressively with frequency (Fig. 2), whereas yellowness (b* value) remained relatively stable across treatment groups. The observed reduction in redness may be attributed to ultrasound-induced acceleration of myoglobin oxidation processes.
Lipid oxidation and fatty acid compositions
Lipid oxidation, measured by TBARS values, increased significantly in ultrasound-treated rabbit meat compared to the control group (Fig. 3a). While all ultrasound frequencies elevated oxidation, the 120 kHz treatment yielded slightly but significantly lower TBARS values than both 60 kHz and 90 kHz. Similarly, ultrasound induced substantial degradation of fatty acids across all identified species (Fig. 3b). Notably, total fatty acid levels at 120 kHz were lower than those in the control yet higher than those observed at 60 kHz. This nonlinear response suggests that very-high-frequency ultrasound (120 kHz) partially mitigates fatty acid degradation relative to intermediate frequencies (60–90 kHz).
Quantification of lipid oxidation and fatty acid compositions. (a) The thiobarbituric acid reactive substance (TBARS) value. (b) The fatty acid composition. Fatty acids were extracted with solvents, derivatized into fatty acid methyl esters in boron fluoride methanol and quantified via GC–MS. SFA, total saturated fatty acids. MUFA, total monounsaturated fatty acids. PUFA, total polyunsaturated fatty acids. Mean ± SD, 3 biological replicates. One-way ANOVA test, p < 0.01. Different italic letters above the bars indicate significant differences.
Flavor precursors
Reducing sugars are key substrates in the Maillard reaction, contributing both sweetness and reactive carbonyl groups essential for developing characteristic browning, aromas, and flavors. As shown in Fig. 4a, ultrasound treatment significantly reduced total reducing sugar content in rabbit meat, with a pronounced frequency-dependent effect. Sugar content decreased progressively with increasing ultrasound frequency, reaching approximately 50% of control (0 kHz) levels at 120 kHz. This substantial reduction strongly suggests that ultrasound accelerates Maillard reaction kinetics by enhancing the depletion of reducing sugars.
Flavor precursors. (a) Total sugar was measured according to the phenol method in China National Standards. (b) Free amino acids were detected with an autonomous amino acid analyzer. Gln, glutamine; Asn, asparagine; Tyr, tyrosine; Pro, proline; Asp, aspartic acid; Ser, serine; Arg, arginine; Ala, alanine; Gly, glycine; Glu, glutamic acid; Trp, tryptophan; Met, methionine; Val, valine; Lys, lysine; Phe, phenylalanine; Leu, leucine; Lle, isoleucine; Thr, threonine. Mean ± SD, 3 biological replicates. One-way ANOVA test, p < 0.01. Different italic letters above the bars indicate significant differences.
Free amino acids are critical flavor sources of meat products. Figure 4b demonstrates that ultrasound treatment increased the concentration of most free amino acids in the meat with increasing frequency. This likely reflects enhanced diffusion of amino acids from the brine into the muscle tissue matrix due to ultrasonic cavitation. A notable exception was glutamate, whose content decreased sharply to ~ 40% of control levels in all ultrasound-treated groups.
Nucleotides
Nucleotides represent a major source of umami taste, with amino acids constituting another key contributor. Food-derived nucleotides originate primarily from the hydrolysis of ATP and RNA. As shown in Fig. 5, IMP is the dominant nucleotide in rabbit meat, followed by GMP and AMP. All three nucleotides exhibited increasing concentrations with ascending ultrasonic frequency (30–90 kHz), but showed a significant, dramatic decline at 120 kHz.
Nucleotide contents. Nucleotides were extracted with 50% methanol, identified and quantified with LC–MS/MS. GMP, 5’-guanosine monophosphate; AMP, 5’-adenosine monophosphate; IMP, inosine-5 -monophosphate. Mean ± SD, 3 biological replicates. One-way ANOVA test, p < 0.01. Different italic letters above the bars indicate significant difference.
Volatiles
As shown in Fig. 6, hexanal and octanal constitute the dominant aldehydes in braised rabbit leg meats. Hexanal contributes a characteristic “green” odor note, while octanal imparts a distinct "citrus-like" character20. Ultrasonic treatment significantly diversifies the aldehyde profile, generating unique flavor—active compounds including 2-heptenal, piperonal, and 2-octenal—exclusively detected in ultrasound-treated groups. Quantitatively, concentrations of most aldehydes peak at 60 kHz, declining progressively with higher frequencies (90–120 kHz). Notably, this trend aligns with overall volatile compound dynamics, indicating that intermediate-frequency ultrasound (60 kHz) optimally promotes fatty acid oxidation and Maillard reactions. These findings corroborate the frequency-dependent patterns observed in prior analyses of lipid oxidation (Fig. 4) and nucleotide hydrolysis (Fig. 5).
Volatile composition in percentage. Head space-solid micro extraction was performed to collect volatile compounds from braised rabbit legs. The relative content was calculated with the area normalization method. Mean ± SD, 3 biological replicates. One-way ANOVA test, p < 0.01. Different italic letters above the bars indicate significant differences.
Discussion
Ultrasound changes muscle microstructure and improves water-holding capacity (WHC) and texture
Figure 1a shows ultrasound-assisted cooking leads to muscle shrinkage and disruption, producing extracellular gaps that enhance water-holding capacity (WHC). This aligns with reports by Chang et al. showing that ultrasound could promote muscle fibers shrinking and separating from each other6. This enlarged extracellular space enhances meat water-holding capacity (WHC), which could be seen in Fig. 1c. Similarly, Xiong et al. also found that ultrasound could significantly improve water-holding capacity by measuring cooking loss in braising5. Pressure loss reflects meat’s water-holding ability under pressure. Higher pressure loss means more water is lost when pressure is applied to meat. On the other hand, cooking loss reflects the amount of water lost during cooking. Higher cooking loss indicates poorer water retention. As shown in Fig. 1, the pressure loss at 90 kHz and 120 kHz is significantly higher than at 30 kHz and 60 kHz. Also, there is a slight cooking loss increase at 120 kHz, compared to 60 kHz. This phenomenon suggests that WHC is partly impaired at high frequency, potentially due to ultrasound-induced protein secondary structure damage. Consequently, the binding of water molecules and muscle fiber is weakened, leading to compromised WHC19. Figure 1d supports this explanation, showing that relaxation time T2b at 120 kHz is higher than at other frequencies, indicating the weakest interaction between water and food matrix. The unfolding effects of ultrasound on proteins have been reported by Su and Cavaco-Paula21. Furthermore, Ma et al. demonstrated that protein unfolding leads to exposure of buried hydrophobic amino acid residues, which in turn leads to impaired WHC22. These earlier reports supported our findings, showing that ultrasound with high frequency (120 kHz) could reduce WHC.
Many studies have reported that ultrasound has important influences on meat texture, which result from microstructure changes. Ultrasound can significantly lower shear force and consequently tenderize chicken and beef in processing5,6,23. Jayasooriya et al. further found that hardness was significantly affected by ultrasound23. These findings align with Fig. 1b, demonstrating that ultrasound could significantly improve textural properties of rabbit meat.
Ultrasound facilitates substance movement in braising
Salt, pigments and flavor compounds from spices continuously penetrate the meat surface and bind with meat tissues during braising. In this study, the influence of ultrasound frequency on braised rabbit meat was explored by measuring salt content, brightness, redness and yellowness. Mass transfer is strongly facilitated by ultrasound. As shown in Fig. 2, ascending ultrasound frequency leads to increasing salt content in the center of meat. Carcel et al. also found that ultrasound with frequency higher than 30 kHz produces higher salt content in pork. The increase in salt content was proportional to the frequency24. Similar observations were reported in Ozuna’s research25. Figure 4b further shows that amino acid levels in rabbit legs increase with ascending frequency. These findings demonstrate that ultrasound could promote mass transfer in braising. Moreover, the movement-promotion effect could be intensified by even higher frequency. Extrapolating from ultrasound-accelerated marination and curing, the enhanced solute diffusion demonstrated in this study suggests that ultrasound-assisted braising could substantially reduce processing time in industrial settings—achieving target salt penetration and flavor development faster than conventional methods.
Figure 2 also shows that brightness and redness are significantly affected by ultrasound frequency. The reduction of brightness arises from accelerated diffusion of soybean sauce, whose ability to reduce brightness was proven in chicken marination26. The reduction in redness is attributed to the oxidation of myoglobin into the brown pigment metmyoglobin27. Critically, higher ultrasound frequencies intensify this transition through enhanced cavitation energy dissipation, promoting heme–iron oxidation via reactive oxygen species generation.
Ultrasound promotes lipid oxidation and Maillard reaction
Lipid oxidation is an important source of flavor compounds and quality deterioration. Malondialdehyde (MDA), a major secondary product of lipid oxidation, was quantified to evaluate lipid oxidation. The results were expressed as TBARS (2-thiobarbituric acid reactive substances) content. As shown in Fig. 3a, TBARS content in the control (0 kHz) is merely 0.19 ± 0.02 mg/kg. While in ultrasound-treated rabbit meat, the lowest TBARS reaches 0.51 ± 0.01 mg/kg, which is over twice that of the control. This enormous increase demonstrates that significant lipids were oxidized in cooking, which could be verified by Fig. 3b. GC–MS profiling of fatty acids from rabbit leg meats shows that the amounts of 3 fatty acid families (saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids) in ultrasound-treated rabbit legs are all lower than the control, namely 0 kHz. This indicates that cavitation produced by ultrasound can promote fatty acids oxidation regardless of the existence or non-existence of double bonds. Kang et al. also reported that ultrasound could promote lipid oxidation in beef during curing28, consistent with our results.
The pro-oxidative effects of ultrasound in braising arise through two interconnected mechanisms: cavitation-induced thermal/pressure effects and metal catalyst release. Ultrasonic cavitation generates transient microbubbles whose collapse produces localized extreme temperatures (> 5000 K) and pressures (> 1000 atm). These conditions directly accelerate lipid oxidation by (a) activating oxygen molecules through thermal energy29,30, and (b) inducing physical disruption of lipid membranes, consistent with pressure-driven oxidation pathways observed in high-pressure processing31,32. Concurrently, ultrasound denatures hemeproteins (e.g., myoglobin), liberating iron ions from its prosthetic groups. This is critical as iron—particularly in its free ionic form—acts as an active catalyst for lipid peroxidation chain reactions33.
It should be noted that the TBARS content at 120 kHz is obviously lower than at 60 kHz and 90 kHz, and fatty acid contents at 120 kHz are also higher than at 60 kHz and 90 kHz. This demonstrates that the acceleration of fatty acid oxidation is partly inhibited when ultrasound intensity exceeds a threshold. One explanation is that ultrasound could decrease oxygen solubility in aqueous solutions, which in turn reduced substrate amount available for lipid oxidation34.
16:0 and 18:0 fatty acids dominate in rabbit meat, followed by 18:1, 18:2 and 20:4 fatty acids. The levels of 18:2 and 20:4 fatty acids are ~ 150% and 50% of 18:1 fatty acid, respectively. Guo et al. found that in pork, the level of 18:2 fatty acid is merely ~ 50% of the 18:1 fatty acid35. In beef, the content of 18:2 fatty acid is even lower than 10% of 18:1 fatty acid, and only traces of 20:4 fatty acid could be detected36. 18:2 and 20:4 fatty acids are essential fatty acids for human nutrition. The human genome does not contain specific genes required for 18:2 biosynthesis. Inadequate intake of 18:2 fatty acids could lead to poor growth and scaly skin lesions37. This research found that rabbit meat is an excellent source of 18:2 and 20:4 fatty acids, which makes rabbit meat an ideal substitute for pork and beef.
The Maillard reaction is another source of flavor compounds in cooking. Reducing sugars and amino donors are substrates of the Maillard reaction. It should be mentioned that peptides and proteins rich in glutamine, arginine, asparagine and lysine could participate in the Maillard reaction, due to the presence of free amino groups. Figure 4 shows that the total sugar content in ultrasound-treated rabbit legs is significantly lower than the control (0 kHz), indicating that the Maillard reaction is activated by ultrasound. This could be explained by the fact that ultrasound produces local high temperatures4, while the rate of the Maillard reaction increases with temperature38. Unlike temperature, high pressure has a negative influence on the Maillard reaction39,40,41. Thus, ultrasound promotes Maillard reactivity primarily through localized heating, with pressure effects being secondary and potentially suppressive.
The major free amino acids in braised rabbit legs are glutamate, alanine and threonine. It should be noted that glutamate differs significantly from all other amino acids. Glutamate content in the control (0 kHz) is 50.1 ± 0.3 mg/100 g, which is much higher than all other amino acids, followed by alanine (14.31 ± 0.09 mg/100 g). While at 30 kHz, glutamate content dropped to ~ 50% of the control. The huge amount of glutamate in the control occurs because glutamate is a food additive in soybean sauce and cooking wine, which were used in braising. The unusual trend of glutamate occurs because glutamate is thermally unstable, and could undergo intramolecular cyclization, producing pyroglutamination42.
Ultrasound promotes formation of flavor nucleotides and aldehydes
Nucleotides are the major source of umami taste. It should be mentioned that not all nucleotides can produce flavor stimulation. Figure 5 shows that IMP is the flavor nucleotide with the highest content in rabbit leg meat. This is in line with results from other meats. Rotola-Pukkil el al. found that IMP is the dominant flavoring nucleotide in pork, followed by AMP43. Madruga et al. reported IMP to be quantitatively the most important nucleotide in raw and grilled goat meat44. Cambero et al. found that IMP is the dominant flavoring nucleotide in beef broth after heating45. The content of IMP in the control (0 kHz) is 25.5 ± 0.4 mg/100 g, which is obviously lower than at 30 kHz, 60 kHz and 90 kHz. This demonstrates that ultrasound can promote RNA hydrolysis, releasing more nucleotides. It should be noted that the content of IMP at 120 kHz is merely 8.6 ± 1.3 mg/100 g, which is significantly lower than the control. Moreover, contents of AMP and GMP are all highest at 60 kHz or 90 kHz, while they drop dramatically at 120 kHz. This demonstrates that ultrasound with a frequency higher than a threshold could damage nucleotides. This explanation could be verified by Suzuki’s research46, which claimed that radicals from cavitation effects cause release and hydroxylation of bases.
In general, aldehydes produced from lipid oxidation are considered the major flavor compounds47. The variety and amount of aldehydes in ultrasound-treated rabbit legs are significantly higher than the control (0 kHz). The higher abundance of hexanal (a diagnostic marker for linoleic oxidation) in ultrasound-treated groups confirms ultrasound’s capacity to accelerate primary lipid peroxidation, consistent with elevated TBARS values48,49. Critically, the pro-oxidative effect of ultrasound-induced cavitation constitutes a double-edged sword: higher frequencies (90 kHz and 120 kHz) promote secondary reactions that degrade aldehydes into short-chain acids, hydrocarbons or alcohols via carbon chain cleavage. Thus, ultrasonic flavor enhancement operates within a narrow kinetic window, where intermediate frequencies (60 kHz) maximize desirable volatiles before destructive oxidation dominates (Fig. 6). The authors acknowledge that formal sensory validation (e.g., consumer panels) was not conducted and will be prioritized in subsequent studies to correlate instrumental data with human perception, particularly given the complex interaction of aldehydes and nucleotides in umami-savory profiles.
Conclusions
The present study demonstrates that ultrasound treatment leads to muscle fiber shrinking and higher water-holding capacity, collectively improving textural properties. Also, movements of salts and flavor compounds in brine into muscle tissue are facilitated by ultrasound. Critically, we provide the first evidence that ultrasound during braising exerts a frequency-dependent duality on flavor chemistry: while intermediate frequencies (60–90 kHz) promote the Maillard reaction and lipid oxidation to generate flavor compounds (short-chain aldehydes and nucleotides), excessive frequency (120 kHz) triggers degradation of these key flavor components, undermining sensory benefits. This mechanistic insight bridges a fundamental gap in understanding ultrasound’s biphasic role in meat flavor modulation. The energy-saving advantage is fundamentally rooted in sub-boiling temperature operation (85 °C vs. conventional 100 °C). To summarize, ultrasound-assisted low-temperature braising demonstrates potential as an energy-efficient alternative for traditional braising in the food processing industry, with the increasing demand for green and low-carbon-emission manufacturing. While this lab-scale study confirms mechanistic benefits, industrial implementation demands transducer systems ensuring even sonication in large volumes, alongside mitigation of high-frequency-induced oxidative degradation. Potential challenges such as equipment material fatigue or localized overheating require routine monitoring and fail-safe designs. Future optimization should integrate pulsed ultrasound protocols and natural antioxidants to preserve flavor integrity under optimal frequency. Furthermore, quantifying carbon emissions by comparing energy consumed in different treatments (e.g., 85 ℃ ultrasound-assisted vs. traditional 100 ℃ braising) represents a critical next step for industrial scaling, as it would validate environmental benefits suggested by this study’s sub-boiling temperature approach.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Papadochristopoulos, A., Kerry, J. P., Fegan, N., Burgess, C. M. & Duffy, G. Natural anti‐microbials for enhanced microbial safety and shelf‐life of processed packaged meat. Foods 10 (2021).
Tsai, T.-H., Tsai, P.-J. & Ho, S.-C. Antioxidant and anti-inflammatory activities of several commonly used spices. J. Food Sci. 70, C93–C97 (2006).
Yanishlieva, N. V., Marinova, E. & Pokorný, J. Natural antioxidants from herbs and spices. Eur. J. Lipid Sci. Technol. 108, 776–793 (2006).
Wu, J. & Nyborg, W. L. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug. Deliv. Rev. 60, 1103–1116 (2008).
Xiong, G., Zhang, L., Zhang, W. & Wu, J. Influence of ultrasound and proteolytic enzyme inhibitors on muscle degradation, tenderness, and cooking loss of hens during aging. Czech J. Food Sci. 30, 195–205 (2012).
Chang, H., Wang, Q., Tang, C. & Zhou, G. Effects of ultrasound treatment on connective tissue collagen and meat quality of beef semitendinosus muscle. J. Food Qual. 38, 256–267 (2015).
Pi, X., Zhu, L., Wang, Y., Sun, F. & Zhang, B. Effect of the combined ultrasound with other technologies on food allergenicity: Ultrasound before, under, and after other technologies. J. Agric. Food Chem. 72, 16095–16111 (2024).
Gómez-Salazar, J. A., Ochoa-Montes, D. A., Cerón-García, A., Ozuna, C. & Sosa-Morales, M. E. Effect of acid Marination assisted by power ultrasound on the quality of rabbit meat. J. Food Qual. 2018, 5754930 (2018).
Kang, D. et al. Power ultrasonic on mass transport of beef: Effects of ultrasound intensity and NaCl concentration. Innov. Food Sci. Emerg. Technol. 35, 36–44 (2016).
McDonnell, C. K., Lyng, J. G. & Allen, P. The use of power ultrasound for accelerating the curing of pork. Meat Sci. 98, 142–149 (2014).
Ozuna, C., Cárcel, J. A., Walde, P. M. & Garcia-Perez, J. V. Low-temperature drying of salted cod (Gadus morhua) assisted by high power ultrasound: Kinetics and physical properties. Innov. Food Sci. Emerg. Technol. 23, 146–155 (2014).
Bhargava, N., Mor, R. S., Kumar, K. & Sharanagat, V. S. Advances in application of ultrasound in food processing: A review. Ultrason Sonochem. 70, 105293 (2021).
Chen, L. et al. Effects of high oxygen packaging on tenderness and water holding capacity of pork through protein oxidation. Food Bioproc. Tech. 8, 2287–2297 (2015).
Kauffman, R. G., Eikelenboom, G., Van Der Wal, P. G., Engel, B. & Zaar, M. A comparison of methods to estimate water-holding capacity in post-rigor porcine muscle. Meat Sci. 18, 307–322 (1986).
Wang, X. et al. Preslaughter transport effect on broiler meat quality and post-mortem glycolysis metabolism of muscles with different fiber types. J. Agric. Food Chem. 65, 10310–10316 (2017).
Zhang, W., Xiao, S., Lee, E. J. & Ahn, D. U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J. Agric. Food Chem. 59, 969–974 (2010).
Folch, J., Lees, M. & Sloane, G. H. S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Zhou, X., Chong, Y., Ding, Y., Gu, S. & Liu, L. Determination of the effects of different washing processes on aroma characteristics in silver carp mince by MMSE-GC–MS, e-nose and sensory evaluation. Food Chem. 207, 205–213 (2016).
Huff-Lonergan, E. & Lonergan, S. M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat. Sci. 71, 194–204 (2005).
Tsuzuki, S. Higher straight-chain aliphatic aldehydes: Importance as Odor-active volatiles in human foods and issues for future research. J. Agric. Food Chem. 67, 4720–4725 (2019).
Su, J. & Cavaco-Paulo, A. Effect of ultrasound on protein functionality. Ultrason Sonochem. 76, 105653 (2021).
Ma, C. et al. Study on the effects of pre-slaughter transport stress on water holding capacity of pork: Insights from oxidation, structure, function, and degradation properties of protein. Food Chem. X 24, 101913 (2024).
Jayasooriya, S. D., Torley, P. J., D’Arcy, B. R. & Bhandari, B. R. Effect of high power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat. Sci. 75, 628–639 (2007).
Cárcel, J. A., Benedito, J., Bon, J. & Mulet, A. High intensity ultrasound effects on meat brining. Meat. Sci. 76, 611–619 (2007).
Ozuna, C., Puig, A., García-Pérez, J. V., Mulet, A. & Cárcel, J. A. Influence of high intensity ultrasound application on mass transport, microstructure and textural properties of pork meat (Longissimus dorsi) brined at different NaCl concentrations. J. Food Eng. 119, 84–93 (2013).
Kim, H. W. et al. Effects of soy sauce on physicochemical and textural properties of tumbled chicken breast. Poult. Sci. 93, 680–686 (2014).
Corlett, M. T., Pethick, D. W., Kelman, K. R., Jacob, R. H. & Gardner, G. E. Consumer perceptions of meat redness were strongly influenced by storage and display times. Foods 10 (2021).
Kang, D. C. et al. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason Sonochem. 33, 47–53 (2016).
Min, B. & Ahn, D. U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 14, 152–163 (2005).
Wu, H., Richards, M. P. & Undeland, I. Lipid oxidation and antioxidant delivery systems in muscle food. Compr. Rev. Food Sci. Food Saf. 21, 1275–1299 (2022).
Medina-Meza, I. G., Barnaba, C. & Barbosa-Cánovas, G. V. Effects of high pressure processing on lipid oxidation: A review. Innov. Food Sci. Emerg. Technol. 22, 1–10 (2014).
Ganjeh, A. M. et al. Effects of pressure-based technologies on food lipids oxidation. Food Chem. 461, 140768 (2024).
Wang, D., Xiao, H., Lyu, X., Chen, H. & Wei, F. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Sci. 8, 35–44 (2023).
Laugier, F., Andriantsiferana, C., Wilhelm, A. M. & Delmas, H. Ultrasound in gas-liquid systems: Effects on solubility and mass transfer. Ultrason Sonochem. 15, 965–972 (2008).
Guo, Q. et al. Fatty acid content, flavor compounds, and sensory quality of pork loin as affected by dietary supplementation with L-arginine and glutamic acid. J. Food Sci. 84, 3445–3453 (2019).
Lee, S. et al. Comparisons of beef fatty acid and amino acid characteristics between Jeju black cattle, Hanwoo, and wagyu breeds. Food Sci. Anim. Resour. 39, 402–409 (2019).
Heird, W. C. & Lapillonne, A. The role of essential fatty acids in development. Ann. Rev. Nutr. 25, 549–571 (2005).
Martins, S. I. F. S., Jongen, W. M. F. & van Boekel, M. A. J. S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 11, 364–373 (2000).
De Vleeschouwer, K., Van der Plancken, I., Van Loey, A. & Hendrickx, M. E. The effect of high pressure−high temperature processing conditions on acrylamide formation and other Maillard reaction compounds. J. Agric. Food Chem. 58, 11740–11748 (2010).
Tamaoka, T., Itoh, N. & Hayashi, R. High pressure effect on maillard reaction. Agric. Biol. Chem. 55, 2071–2074 (1991).
Moreno, F. J., Molina, E., Olano, A. & López-Fandiño, R. High-pressure effects on maillard reaction between glucose and lysine. J. Agric Food Chem. 51, 394–400 (2002).
Ferreira, M. M. L. et al. Pyroglutamination-induced changes in the physicochemical features of a CXCR4 chemokine peptide: Kinetic and structural analysis. Biochemistry 62, 2530–2540 (2023).
Rotola-Pukkila, M. K., Pihlajaviita, S. T., Kaimainen, M. T. & Hopia, A. I. Concentration of umami compounds in pork meat and cooking juice with different cooking times and temperatures. J. Food Sci. 80, C2711–C2716 (2015).
Madruga, M. S., Elmore, J. S., Oruna-Concha, M. J., Balagiannis, D. & Mottram, D. S. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 123, 513–520 (2010).
Cambero, I., Pereira-Lima, C. I., Ordoñez, J. A. & García De Fernando, G. D. Beef broth flavour: Relation of components with the flavour developed at different cooking temperatures. J. Sci. Food Agric. 80, 1519–1528 (2000).
Suzuki, T., Yamada, K. & Inukai, M. Effects of chloride, bromide, and iodide upon decomposition of nucleosides induced by ultrasound in neutral solution. Nucleosides Nucleotides Nucl. Acids 29, 606–615 (2010).
Ying, W. et al. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chem. 190, 33–40 (2016).
Purriños, L., Franco, D., Carballo, J. & Lorenzo, J. M. Influence of the salting time on volatile compounds during the manufacture of dry-cured pork shoulder “lacón”. Meat Sci. 92, 627–634 (2012).
Corral, S., Salvador, A. & Flores, M. Salt reduction in slow fermented sausages affects the generation of aroma active compounds. Meat Sci 93, 776–785 (2013).
Acknowledgements
All authors contributed to the work and approved the final manuscript.
Funding
This research was funded by following fundings: Innovation Team Program of Sichuan University of Science and Engineering (SUSE652A009).
Author information
Authors and Affiliations
Contributions
Z.Z designed and supervised the project, applied for the funding; Z.W performed experiments and analyzed original data; X.S prepared figures and analyzed data; W.Y wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, W., Wu, Z., Song, X. et al. Ultrasonic processing in rabbit leg braising advances microstructure, water retention, and flavor development. Sci Rep 15, 36642 (2025). https://doi.org/10.1038/s41598-025-20374-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20374-2