Abstract
China possesses vast saline-alkaline water resources, necessitating their utilization. Macrobrachium nipponense, an economically important freshwater shrimp with notable salinity tolerance, is a candidate for saline aquaculture. This study determined the 96-h LC50 of salinity for the genetically improved "Taihu No. 3" strain juveniles across a gradient (0–30 parts per thousand) and investigated associated stress responses. Morphological, physiological, and molecular responses were analyzed via antioxidant enzyme activity (glutathione peroxidase (GPx), glutathione reductase (GR), catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA)) and immune gene expression in hepatopancreas and gills. Results showed the 96-h LC50 was 11.841 ppt, significantly lower than wild populations, suggesting enhanced energy allocation towards growth over osmoregulation in "Taihu No. 3". Acute and chronic stress significantly elevated GPx, GR, CAT, SOD activities and MDA levels (P < 0.05), indicating their critical role in mitigating oxidative damage and maintaining homeostasis. Salinity ≥ 10 ppt induced structural damage, including hepatopancreatic basement membrane disruption and gill alterations (enlarged interlamellar spaces, epithelial swelling). Chronic exposure significantly upregulated immune genes (CAT, Mn-SOD, Cu/Zn-SOD) in both tissues (P < 0.05), demonstrating their involvement in saline acclimation. These findings define measurable salinity tolerance limits and elucidate key immune response mechanisms in "Taihu No. 3", providing a scientific basis for its cultivation in saline-alkaline aquaculture.
Similar content being viewed by others
Introduction
China possesses extensive saline-alkali water resources, with low-lying saline-alkali water areas covering 4.6 × 1011 m2, widely distributed across 19 provinces in the Northeast, North, and Northwest regions of the country1. Saline-alkali water is characterized by high carbonate alkalinity, high pH, complex water quality, and imbalanced ratios of major ions. Due to physicochemical indices that exceed standard thresholds, it is unsuitable for direct consumption or agricultural irrigation2. Owing to the escalating scarcity of freshwater resources, the development of salt-tolerant aquaculture technologies for aquatic species is critically needed to alleviate pressure on limited freshwater supplies and safeguard the sustainable development of the aquaculture sector.
As a key environmental variable, salinity governs aquatic organism distribution and physiological activities3. It constitutes a major environmental factor affecting both prokaryotic4 and eukaryotic organisms5,6. Currently, increasing salinity in inland waters results in decreased biodiversity, functional degradation of ecosystems, economic losses, social conflicts, and ecological risks. Consequently, establishing saline-alkaline aquaculture systems represents a crucial solution. Therefore, identifying aquatic species with wide salinity tolerance is particularly important.
As euryhaline organisms, crustaceans are widely distributed in brackish water zones, intertidal areas, and salt marshes7,8,9, exhibiting strong tolerance to salinity changes. For example, Penaeids exhibit broad salinity adaptability ranging from 3 to 50‰. The adult Portunidae can withstand salinity fluctuations between 1.4 and 4‰10. However, their early developmental stage (zoea larvae) survives only within salinities of 20–35‰11. As a key environmental factor, salinity affects crustaceans’ survival rate, growth performance, reproductive efficiency, and development processes. The mechanisms underlying saline tolerance in crustaceans include molecular regulatory pathways, physiological compensation, behavioral adaptation, and ecological strategies12.
Gills are important organs in crustaceans performing various physiological functions, including regulating ion transport, maintaining acid–base balance, and excreting ammonia. The ion exchange function of gills plays a key role in osmoregulation. Crustaceans employ osmoregulatory mechanisms via their gills to maintain internal homeostasis in low-salinity environments. In high-salinity conditions, they actively take up ions from water and eliminate excess ions to maintain physiological homeostasis13.
The hepatopancreas performs digestion (enzyme secretion), metabolic regulation, and immune defense functions, playing essential roles in stress response14,15,16. It also stores energy and participates in lipid metabolism, storing lipids from food and synthesizing new lipids. Studies show that energy metabolism regulation is crucial for saline acclimation in non-isotonic environments. The greater the deviation of environmental salinity from the isotonic point, the higher the energy consumed for osmoregulation, resulting in growth inhibition17,18. Recent studies indicate that Litopenaeus vannamei hepatopancreas consumes fatty acids to support osmotic regulation and ion transport19.
Macrobrachium nipponense is an economically important freshwater crustacean aquaculture species in China. Its annual production exceeds 2 × 105 tons, ranking third among freshwater economic shrimp species. Studies show that the 96-h median lethal salinity (LC50) for M. nipponense larvae is 25 parts per thousand (ppt), and their growth and weight gain rates peak at salinities < 12 ppt20. Under acute salinity exposure, adult specimens maintain normal physiological functions within 7–20 ppt21. However, the effects of salinity on gill and hepatopancreas immune responses remain unclear. “Taihu No. 3”, a selectively bred variety of M. nipponense (approved by the Ministry of Agriculture and Rural Affairs), is characterized by faster growth rate and stronger adaptability than wild populations.
This study investigated acute and chronic salinity effects on antioxidant enzyme activity, morphology, and gene expression in M. nipponense “Taihu No. 3” gills and hepatopancreas. Acute exposure (96 h) employed salinity levels of 0, 5, 10, 15, 20, and 30 ppt, while chronic exposure (30 days) used 4 ppt (low salinity) and 8 ppt (high salinity). The findings provide valuable evidence for the culture and potential expansion of this species into environments with elevated salinity, supporting its sustainable development.
Materials and methods
Ethics declarations
All animal experiments were approved by the Institutional Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center at Chinese Academy of Fishery Sciences (Wuxi, China) (Authorization NO.20240610006, 10 June 2024).
The study was conducted in strict accordance with the Institutional Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center.
This study was conducted in accordance with the editorial policies of Scientific Reports for research involving experimental subjects.
This study was reported in full compliance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments).
Experimental animal
All M. nipponense “Taihu No. 3” specimens used in this study were provided by the Dapu M. nipponense Breeding Base (120°13 44′′ E, 31° 28′ 22′′ N). A total of 1,800 specimens were collected, with a mean body weight of 0.36 ± 0.05 g and a mean body length of 20.03 ± 1.22 mm. They were temporarily reared in aerated freshwater (water temperature of 28.0 ± 2.0 ℃, dissolved oxygen of ≥ 6 mg/L) for 3 days and fed twice daily.
Identification of 96-h LC 50 salinity for “Taihu No. 3”
A total of 1,080 specimens were exposed to six salinity levels (0, 5, 10, 15, 20, and 30 ppt) for 96 h to assess mortality, under controlled conditions with water temperature of 28.3 ± 1.26 ℃, pH ranging from 7.81 to 8.32, and dissolved oxygen ≥ 6.0 mg/L. Six discrete salinity gradients (0 [control, NaCl-free], 5, 10, 15, 20, and 30 ppt) were prepared by dissolving NaCl into artificial aquatic systems. Salinity was quantified using an optical salinometer (Sunrising Optronic Co., Ltd., Beijing, China). Each salinity treatment was conducted in triplicate, each containing 60 prawns. The 96-h mortality rate for each replicate tank was calculated, and the 96-h LC50 of M. nipponense “Taihu No. 3” was analyzed using Probit Analysis.
Sample collection
Tissue collection after acute salinity stress
The hepatopancreas and gill samples for acute salinity stress analysis were collected from the specimens described in Section "Identification of 96-hour LC50 salinity for “Taihu No. 3”". From each salinity group, five hepatopancreas and five gill tissues were pooled to form one biological replicate for antioxidant enzyme activity measurement, with three biological replicates prepared per group. All collected tissues were immediately flash-frozen and stored at –80 ℃. Additionally, three hepatopancreas and gill tissues from each salinity group were collected after 96 h of exposure for histological observation. These samples were fixed in 4% paraformaldehyde and stored at room temperature until processing for histological sectioning. Due to the absence of surviving M. nipponense “Taihu No. 3” specimens beyond 96 h at salinities of 20 and 30 ppt, tissue samples from these groups were not obtained.
Tissue collection after chronic salinity stress
A total of 720 specimens were reared for 30 days under chronic salinity stress. Salinity levels of 4 ppt (low salinity) and 8 ppt (high salinity) were prepared by dissolving NaCl into artificial aquatic systems. Each salinity group included three replicates with 120 shrimp per tank. The hepatopancreas and gills were respectively collected from each group after 0, 1, 4, 7, 15 and 30 days of salinity exposure. The hepatopancreas and gill tissues from five individuals were pooled to form one biological replicate, and three biological replicates were prepared for the determination of antioxidant enzyme activity and qPCR analysis. Due to complete mortality of M. nipponense occurring before day 15 and day 30 under 8 ppt salinity, samples at these time points were not available.
Measurement of antioxidant enzyme activity
The activities of antioxidant enzymes were measured in the hepatopancreas and gill tissues of M. nipponense “Taihu No. 3” using commercial kits (Nanjing Jiancheng Bioengineering Institute). The parameters analyzed included superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA). All measurements were performed using a Bio-Rad iMark microplate reader (San Francisco, USA), in accordance with the manufacturer’s protocols.
Hematoxylin and eosin (HE) staining of gills and hepatopancreas
The morphological changes of the hepatopancreas and gills were assessed using hematoxylin and eosin (HE) staining following acute salinity exposure. The detailed procedures for HE staining have been described in previous studies22,23. Briefly, samples were dehydrated through a graded ethanol series. The dehydrated gill and hepatopancreas tissues were cleared in xylene and embedded in paraffin wax. Subsequently, the embedded tissues were sectioned into 5 μm slices using a microtome (Leica, Wetzlar, Germany). The sections were stained with HE for 3–8 min, respectively. Morphological changes in gills and hepatopancreas induced by salinity exposure were observed under an Olympus SZX16 microscope (Olympus Corporation, Tokyo, Japan).
qPCR analysis
Total RNA was extracted from hepatopancreas and gill tissues of M. nipponense “Taihu No. 3” subjected to chronic saline stress. The RNA quality was evaluated using 1.2% agarose gel electrophoresis, and its concentration was determined using an ultraviolet spectrophotometer (Eppendorf, Germany). The first-strand cDNA was synthesized using an M-MLV reverse transcriptase kit (TaKaRa) and subsequently stored at − 20 °C until use. Gene expression levels were quantified using the Ultra SYBR Mixture (CWBIO, Beijing, China) on a Bio-Rad iCycler iQ5 Real-Time PCR System, in accordance with the manufacturer’s protocol. All qPCR primers are listed in Table 1. Eukaryotic translation initiation factor 5A (Eif) was selected as the internal reference gene for normalization, as validated in previous research24. Previous studies have found that the expression level of the Eif remains stable under various environmental stresses and across different tissues, therefore it was selected as a reference gene. Relative expression levels were calculated using the 2–ΔΔCt method25.
Statistical analysis
All data in this study were statistically analyzed using SPSS 27.0 and are presented as mean ± standard deviation (SD). Differences among stress durations under the same salinity condition and in mortality rates under different saline concentrations were analyzed using one-way ANOVA followed by Duncan’s test. A significance threshold of p < 0.05 was applied, as established in previous studies.
Results
Identification of 96-h LC 50 salinity for “Taihu No. 3”
Mortality of M. nipponense “Taihu No. 3” exhibited a concentration-dependent increase with salinity during the 96-h acute exposure. As shown in Fig. 1, mortality increased from 3.33% at 0 ppt to 100% at 30 ppt. In contrast, no significant difference in mortality was detected between the 0 ppt (control) and 5 ppt groups (P > 0.05). Complete mortality (100%) was observed at both 20 ppt and 30 ppt after 96 h of exposure. The 96-h LC50 for M. nipponense “Taihu No. 3” was calculated to be 11.841 ppt.3.2 Measurement of the Activities of Antioxidant Enzymes after the acute salinity exposure.
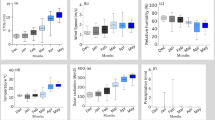
The activities of antioxidant enzymes in the hepatopancreas (Fig. 2A) and gills (Fig. 2B) of M. nipponense “Taihu No. 3” were assessed after 96-h acute salinity exposure to a gradient of salinities. CAT activity in the hepatopancreas exhibited no significant differences across salinity levels (P > 0.05). The activities of GSH-Px and GR reached their maximum at 10 ppt and 15 ppt, respectively (P < 0.05), while MDA levels increased significantly at 5 ppt and then gradually decreased with further increases in salinity. SOD activity remained relatively stable at 0, 5, and 10 ppt (P > 0.05), but decreased markedly at 15 ppt (P < 0.05).
(A) The activity of antioxidant enzymes in hepatopancreas of M. nipponense “Taihu No. 3” after 96 h of acute stress with different salinity. (B) The activity of antioxidant enzymes in gills of M. nipponense “Taihu No. 3” after 96 h of acute stress with different salinity. CAT: catalase; GR: glutathione reductase; GSH-Px: glutathione peroxidase; MDA: malondialdehyde; SOD: superoxide dismutase. Data are shown as mean ± standard deviation (SD) of tissues from three biological replicates. Letters indicate the difference in antioxidant enzymes activities between different saline concentrations.
CAT activity ecxhibited no significant differences in the gill tissues of M. nipponense “Taihu No. 3” at 0, 5, and 10 ppt (P > 0.05), while increased significantly at 15 ppt (P < 0.05). GR activity was significantly elevated in the 10 and 15 ppt groups compared to the 0 and 5 ppt groups (P < 0.05). However, no significant differences were observed between 0 and 5 ppt or between 10 and 15 ppt (P > 0.05). GSH-Px activity peaked at 5 ppt, while salinity exposure resulted in a reduction in MDA levels. SOD activity showed a significant increase from 0 to 5 ppt (P < 0.05) and remained stable at 5–15 ppt (P > 0.05).
Histological observations after the acute salinity exposure for 96 h
The morphological changes of the hepatopancreas (Fig. 3) and gills (Fig. 4) after 96 h under salinity exposure were observed by hematoxylin–eosin staining. Histological observations revealed that the normal hepatopancreas structure comprised secretory cells, basement membrane, lumen, storage cells and vacuoles. There was no obvious damage observed in the hepatopancreas after 96 h exposure under the salinity of 5 ppt. In contrast, salinities exceeding 10 ppt resulted in structural damage to the basement membrane and an enlarged lumen.
Histological observation of hepatopancreas of M. nipponense “Taihu No. 3” under different saline concentrations. B: secretory cells of type B; BM: basement membrane; L: lumen; R: storage cells of type R; V: vacuoles. (a) Histological observation of hepatopancreas of M. nipponense “Taihu No. 3” after 0 ppt 96 h stress; (b) Histological observation of the hepatopancreas of M. nipponense “Taihu No. 3” after 5 ppt 96 h stress; (c) Histological observation of the hepatopancreas of M. nipponense “Taihu No. 3” after 10 ppt 96 h stress; (d) Histological observation of the hepatopancreas of M. nipponense “Taihu No. 3” after 15 ppt 96 h stress; (e) Histological observation of the hepatopancreas of M. nipponense “Taihu No. 3” after 20 ppt 96 h stress; (f) Histological observation of the hepatopancreas of M. nipponense “Taihu No. 3” after 30 ppt 96 h stress.
Histological observation of gills of M. nipponense “Taihu No. 3” under different saline concentrations. HC: hemocytes; HV: hemolymph vessel; M: membrane; MC: marginal channel. (a) Histological observation of gill of M. nipponense “Taihu No. 3” after 0 ppt 96 h stress; (b) Histological observation of gill of M. nipponense “Taihu No. 3” after 5 ppt 96 h stress; (c) Histological observation of gill of M. nipponense “Taihu No. 3” after 10 ppt 96 h stress; (d) Histological observation of gill of M. nipponense “Taihu No. 3” after 15 ppt 96 h stress; (e) Histological observation of gill of M. nipponense “Taihu No. 3” after 20 ppt 96 h stress; (f) Gill histological observation of M. nipponense “Taihu No. 3” after 30 ppt 96 h stress.
The normal gill structure of M. nipponense “Taihu No. 3” was characterized by the presence of a marginal channel, hemocytes, a hemolymph vessel, and a membrane. Gill tissues exhibited a normal architecture at 5 ppt salinity. However, with increasing salinity, the gill lamellae showed progressive dilation, and noticeable swelling occurred from 10 ppt onward.
Measurement of the activities of antioxidant enzymes after the chronic salinity exposure
The activities of five antioxidant enzymes in the hepatopancreas and gills of M. nipponense “Taihu No. 3” were measured after 30 days of chronic salinity exposure at 4 ppt and 8 ppt. Significant variations in antioxidant enzyme activities were observed in response to the different saline concentrations. In the hepatopancreas tissues of M. nipponense “Taihu No. 3” (Fig. 5A, B), the activity of CAT increased over time under both 4 ppt and 8 ppt conditions. GSH-Px and SOD activities reached their peaks on Day 1 at 4 ppt, whereas GR and MDA activities peaked on Day 15 and Day 7, respectively. Under 8 ppt exposure, the activities of CAT, GR, SOD, and MDA all peaked on day 7, while GSH-Px activity exhibited a distinct pattern, reaching its maximum on day 4.
(A) Antioxidant enzyme activities in the hepatopancreas of M. nipponense “Taihu No. 3” under chronic 4 ppt exposure. (B) Antioxidant enzyme activities in the hepatopancreas of M. nipponense “Taihu No. 3” under chronic 8 ppt exposure. (C) Antioxidant enzyme activities in the gills of M. nipponense “Taihu No. 3” under chronic 4 ppt exposure. (D) Antioxidant enzyme activities in the gills of M. nipponense “Taihu No. 3” under chronic 8 ppt exposure. CAT: catalase; GR: glutathione reductase; GSH-Px: glutathione peroxidase; MDA: malondialdehyde; SOD: superoxide dismutase. Data are shown as mean ± standard deviation (SD) of tissues from three biological replicates. Letters indicate the difference in antioxidant enzymes activities between different exposure times.
In the gill tissues of M. nipponense “Taihu No. 3” (Fig. 5C, D), CAT activity was significantly higher on days 4 and 30 under 4 ppt compared to other time points (P < 0.05), and GSH-Px activity peaked on day 7. GR and MDA activities increased significantly from day 0 to day 4, peaking on day 4, while SOD activity peaked on day 15. Under 8 ppt exposure, CAT and GR activities peaked on day 1, and GSH-Px and MDA activities peaked on day 4, after which their activities gradually declined over time. SOD activity varied significantly across time points (P < 0.05), reaching its peak on day 7.
qPCR analysis
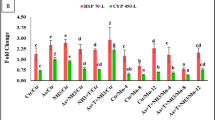
qPCR analysis was performed to evaluate the expression changes of immune-related genes in the gills and hepatopancreas of M. nipponense “Taihu No. 3” under chronic salinity exposure (4 ppt and 8 ppt). As shown in Fig. 6, the expression of Cat in hepatopancreas and gill tissues under 4 ppt reached peak levels on day 4 and day 15, respectively (P < 0.05). Under 8 ppt exposure, Cat expression in the hepatopancreas on day 1 was significantly higher than at other time points (P < 0.05). In gill tissues under 8 ppt, Cat expression remained stable on days 0, 1, and 7 (P > 0.05) and peaked on day 4 (P < 0.05).
A: Expression of three genes in the hepatopancreas of M. nipponense “Taihu No. 3” under chronic 4 ppt exposure. B: Expression of three genes in the hepatopancreas of M. nipponense “Taihu No. 3” under chronic 8 ppt exposure. C: Expression of three genes in the gills of M. nipponense “Taihu No. 3” under chronic 4 ppt exposure. D: Expression of three genes in the gills of M. nipponense “Taihu No. 3” under chronic 8 ppt exposure. Dates are shown as mean ± standard deviation (SD) of tissues from three biological replicates. Letters indicate differences in gene expression at different time points under the same salinity exposure.
The expression of Cu/Zn-SOD peaked on day 4 under 4 ppt (P < 0.05), while under 8 ppt, its expression in the hepatopancreas peaked on day 1 (P < 0.05). In gill tissues, no significant differences in Cu/Zn-SOD expression were observed between day 1 and day 4 (P > 0.05) or between day 0 and day 7 (P > 0.05). However, expression levels on days 1 and 4 were significantly higher than those on days 0 and 7 (P < 0.05).
The expression of Mn-SOD in the hepatopancreas peaked on day 4 under 4 ppt (P < 0.05). Under 8 ppt, no significant differences were detected among days 0, 4, and 7 (P > 0.05), or among days 0, 1, and 4 (P > 0.05). In gill tissues under 4 ppt, Mn-SOD expression decreased from day 0 to day 4, increased significantly on days 7, 15, and 30, and remained stable thereafter (P > 0.05). Under 8 ppt, Mn-SOD expression in gills peaked on day 4 (P < 0.05).
Discussion
China possesses 46 million hectares of saline-alkali land. The sustainable utilization of saline-alkali water resources remains a key research focus26. Certain aquatic crustaceans exhibit broad salinity adaptability. Phylogenetic studies indicate that M. nipponense originated from marine ancestors and subsequently migrated to freshwater habitats, where it has become fully adapted27. Consequently, M. nipponense possesses strong saline tolerance and a broad salinity tolerance range. Cultivating M. nipponense in saline-alkaline water regions is therefore essential for sustaining the development of its aquaculture industry. To the best of our knowledge, the specific effects of salinity exposure on the immune response of M. nipponense remain unclear. “Taihu No. 3”, a newly certified M. nipponense variety selected for enhanced growth performance, has been officially recognized by the Ministry of Agriculture and Rural Affairs of China. Consequently, elucidating its salinity stress response mechanisms is crucial for promoting its cultivation in saline-alkaline waters. The present study aimed to investigate the immune response to salinity changes in M. nipponense, using “Taihu No. 3” as the model organism. This research establishes a theoretical foundation for culturing M. nipponense “Taihu No. 3” in brackish water, thereby supporting the sustainable development of its industry.
Salinity is a critical environmental factor governing physiological processes in crustaceans, including behavior, metamorphosis, growth, and reproduction. However, all organisms exhibit defined salinity tolerance limits; exceeding these thresholds triggers osmoregulatory failure28. Previous studies determined the LC50 salinity for juvenile M. nipponense (body length 2.0–2.5 cm) at 24, 48, 72, and 96 h as 30.71, 26.66, 26.31 and 25.80 ppt, respectively20. Similarly, the 24–96 h LC50 for Procambarus clarkii (15.15 ± 2.12 g) was 31.74, 27.21, 26.45 and 26.09 ppt, respectively29. and for Palaemonetes sinensis it was 37.07, 35.86, 35.04 and 34.31 ppt, respectively30. Notably, these crustacean species demonstrate superior salinity resilience compared to the teleost fishes. In contrast, the present study determined a 96-h LC50 value of only 11.841 ppt for juvenile M. nipponense from the “Taihu No. 3” population. This reduced tolerance may be attributed to the significantly faster growth rate of “Taihu No. 3” prawns compared to wild populations, which likely demands higher energy allocation towards growth, consequently reducing the energy available for environmental stress adaptation.
Environmental stress induces reactive oxygen species (ROS) production. Excessive ROS accumulation causes oxidative stress31,32. In response, organisms activate antioxidant defenses to maintain homeostasis33. Changes in antioxidant enzyme activity serve as key indicators of an organism’s or cell’s physiological state under stress, reflecting the level of external stress exposure or damage. Key antioxidant enzymes include CAT, SOD, GR, and GSH-Px. However, different aquatic animal taxa exhibit significant variations in their antioxidant defense responses. For example, elevated salinity significantly inhibits CAT activity in the liver of Acanthopagrus latus but enhances GPX and SOD activities34. Conversely, high salinity stimulates CAT and GSH activities in the liver of Coris julis, while SOD activity remains unchanged35. Under acute salinity stress, SOD activity in Marsupenaeus japonicus shows dual regulation by stress intensity and duration, demonstrating dose-dependent responses.36. Significant fluctuations in serum SOD activity were also observed in Litopenaeus vannamei under acute salinity exposure37. Consistent with these tissue-specific variations, this study reveals that M. nipponense “Taihu No. 3” exhibits distinct antioxidant responses in hepatopancreas and gills following acute salinity stress. Specifically, in hepatopancreas, high salinity significantly increased MDA content and GSH-Px/GR activities, decreased SOD activity, while CAT activity remained stable. In gills, MDA content decreased with increasing salinity, whereas GSH-Px, GR, and CAT activities increased significantly. The effects of chronic salinity stress on crustaceans are also well-documented. For instance, high salinity suppresses SOD activity and elevates MDA accumulation in the hepatopancreas of juvenile Eriocheir sinensis38. In Litopenaeus vannamei, SOD and CAT activities exhibit an initial increase followed by a decrease under chronic salinity stress. Furthermore, low salinity exposure has been shown to dose-dependently inhibit SOD and GPx activities, while promoting MDA accumulation in both hepatopancreas and serum of Litopenaeus vannamei.30. This study further demonstrates that chronic exposure to different salinity regimes induces temporally distinct peak activation patterns of SOD, CAT, GR and GSH-Px in both hepatopancreatic and gill tissues of M. nipponense “Taihu No. 3”, with progressive enzymatic enhancement observed throughout the exposure period. Collectively, these findings indicate that M. nipponense “Taihu No. 3” activates antioxidant enzyme systems in both tissues to effectively alleviate salinity stress-induced oxidative damage and maintain physiological stability. The antioxidant response in M. nipponense is intrinsically linked to its salinity adaptation capacity and ultimately survival outcomes. The tissue-specific modulation of key enzymes (SOD, CAT, GR, GSH-Px) and MDA levels under both acute and chronic salinity stress demonstrates a coordinated defense mechanism against oxidative damage. Notably, the observed progressive enhancement of enzymatic activities during chronic exposure in "Taihu No. 3" suggests physiological acclimation, where the sustained activation of antioxidant systems correlates with improved salinity tolerance. Conversely, the suppression of SOD activity accompanied by elevated MDA accumulation in hepatopancreas—as seen in high-salinity exposed juvenile Eriocheir sinensis36 and low-salinity stressed Litopenaeus vannamei28—is often associated with increased mortality. Thus, the efficiency of the antioxidant response serves as a critical determinant of survival: effective ROS scavenging maintains cellular homeostasis and supports acclimation, whereas inadequate response leads to irreversible oxidative damage and organism death. The ability of M. nipponense “Taihu No. 3” to differentially regulate antioxidant defenses across tissues underscores its adaptive advantage, contributing to reduced mortality under fluctuating saline conditions.
Previous studies have documented that salinity stress induces morphological alterations in the gills and hepatopancreas of aquatic animals to facilitate adaptation and prevent mortality39. Histopathological studies confirm that salinity stress induces hemocytic swelling in branchial tissues and marked structural deformation of hepatic tubules in Exopalaemon carinicauda40. Research on Portunus trituberculatus has shown that low salinity stress leads to hemocoelic dilation, hemocytic proliferation, and epithelial layer degeneration41. Additionally, low salinity-exposed Portunus trituberculatus showed reduced R-cells and increased numbers of B-cell transport vesicles42. Similarly, in the present study, acute salinity stress (≥ 10 ppt) induced significant histopathological alterations in the hepatopancreas of M. nipponense “Taihu No. 3”, primarily characterized by basement membrane disintegration and luminal dilation. Furthermore, although gill morphology retained structural integrity after salinity exposure, branchial tissues exhibited epithelial hypertrophy accompanied by expansion of interlamellar distances, representing compensatory adaptations to osmotic variation.
Previous transcriptome analysis of M. nipponense “Taihu No. 3” revealed significant salinity-induced alterations in the expression of antioxidant genes Cat, Mn-SOD, and Cu/Zn-SOD in hepatopancreas and gills, highlighting their essential roles in regulating salinity acclimation in this species43. Supporting the role of these enzymes, studies show that subjecting Pampus argenteus to specific low-salinity stress stimulates SOD gene expression to counteract oxygen free radical damage44. Similarly, as salinity decreases, SOD enzyme activity increases in the renal tissue of Larimichthys crocea, demonstrating its response pattern across salinities45. Moreover, chronic salinity stress significantly upregulates Cat gene expression in both hepatopancreas and gill tissues of Litopenaeus vannamei46. Functionally, the Cat gene encodes catalase, an essential antioxidant enzyme that catalyzes hydrogen peroxide decomposition, thereby mitigating oxidative stress. SOD, encoded by SOD genes, serves as the primary defense against oxidative stress by dismasting superoxide radicals, constituting a core component of cellular antioxidant systems. SOD enzymes are classified into four distinct types based on structural features, subcellular distribution, and metal cofactors: Cu/Zn-SOD, Mn-SOD, Fe-SOD, and Ni-SOD47. Specifically, Mn-SOD predominantly localizes to the mitochondrial matrix to scavenge superoxide radicals48, whereas Cu/Zn-SOD functions in cytoplasmic antioxidant defense49. In line with this, qPCR analysis in this study demonstrated rapid upregulation of Cat and Cu/Zn-SOD genes in response to high salinity, with both activated on day 1. Mn-SOD expression in gill tissue recovered during the late phase of low salinity stress. These results confirm that salinity stress induces the upregulation of these three genes (Cat, Cu/Zn-SOD, Mn-SOD) in the hepatopancreas and gill tissues of M. nipponense “Taihu No. 3”, which mediate immune responses to counteract the detrimental effects of hyperosmotic stress and oxidative damage.
Conclusion
In this study, the 96-h LC50 salinity for M. nipponense “Taihu No. 3” was determined to be 11.841 ppt. Exposure to acute and chronic salinity stress significantly upregulated key antioxidant defense enzymes (CAT, SOD, GR, GSH-Px) and elevated the oxidative stress marker MDA, emphasizing the crucial role of these four key antioxidant enzymes in alleviating oxidative damage under saline conditions. Furthermore, salinities ≥ 10 ppt induced severe histopathological alterations, including disruption of hepatopancreatic basement membranes and gill epithelial swelling. qPCR analysis revealed that salinity stress significantly induced the expression of antioxidant-related genes encoding Cat, Mn-SOD, and Cu/Zn-SOD in both gill and hepatopancreatic tissues (P < 0.05), highlighting their essential roles in mediating osmotic adaptation and maintaining ionic homeostasis under saline conditions. This study provides empirical evidence to support the brackish water aquaculture of M. nipponense “Taihu No. 3” by investigating its optimal salinity range and antioxidant mechanisms in response to saline stress.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
References
Yongxin, L., Hui, F., Qifang, L. & Liqun, L. The current state and development strategy for China’s saline-alkaline fisheries. Chin. J. Eng. Sci. https://doi.org/10.15302/j-sscae-2016.03.012 (2016).
Liang, L., Ren, B., Chang, Y., Tang, R. & Zhang, L. Inland brackish (alkaline-saline) water resources and fisheries utilization in China. Chin. Fish. Econ. 31, 138–145 (2013).
Deane, E. E. & Woo, N. Y. Differential gene expression associated with euryhalinity in sea bream (Sparus sarba). Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1054–R1063 (2004).
Ambily Nath, I. & Loka Bharathi, P. Diversity in transcripts and translational pattern of stress proteins in marine extremophiles. Extremophiles 15, 129–153 (2011).
Telesh, I., Schubert, H. & Skarlato, S. Life in the salinity gradient: Discovering mechanisms behind a new biodiversity pattern. Estuar. Coast. Shelf Sci. 135, 317–327 (2013).
Parida, A. K. & Das, A. B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 60, 324–349 (2005).
Bayly, I. Salinity tolerance and osmotic behavior of animals in athalassic saline and marine hypersaline waters. In Annual Review of Ecology and Systematics, 233–268 (1972).
Lignot, J.-H., Spanings-Pierrot, C. & Charmantier, G. Osmoregulatory capacity as a tool in monitoring the physiological condition and the effect of stress in crustaceans. Aquaculture 191, 209–245 (2000).
Clegg, J.S., & Trotman, C.N. Physiological and biochemical aspects of Artemia ecology. In Artemia: Basic and Applied Biology. Springer 129–170 (2002).
Leignel, V., Stillman, J., Baringou, S., Thabet, R. & Metais, I. Overview on the European green crab Carcinus spp. (Portunidae, Decapoda), one of the most famous marine invaders and ecotoxicological models. Environ. Sci. Pollut. Res. 21, 9129–9144 (2014).
Edgell, T.C., & Hollander, J. The evolutionary ecology of European green crab, Carcinus maenas, in North America. In In the Wrong Place-alien Marine Crustaceans: Distribution, Biology and Impacts. Springer 641–659 (2011).
Paital, B. & Chainy, G. Effects of salinity on O2 consumption, ROS generation and oxidative stress status of gill mitochondria of the mud crab Scylla serrata. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 155, 228–237 (2012).
Evans, D. H., Piermarini, P. M. & Choe, K. P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 85, 97–177 (2005).
Resch-Sedlmeier, G. & Sedlmeier, D. Release of digestive enzymes from the crustacean hepatopancreas: Effect of vertebrate gastrointestinal hormones. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 123, 187–192 (1999).
Qiu, L. et al. Changes of ammonia-metabolizing enzyme activity and gene expression of two strains in shrimp Litopenaeus vannamei under ammonia stress. Front. Physiol. 9, 211 (2018).
Shan, H., Geng, Z., Ma, S. & Wang, T. Comparative study of the key enzymes and biochemical substances involved in the energy metabolism of Pacific white shrimp, Litopenaeus vannamei, with different ammonia-N tolerances. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 221, 73–81 (2019).
Pillai, B. R. & Diwan, A. Effects of acute salinity stress on oxygen consumption and ammonia excretion rates of the marine shrimp Metapenaeus monoceros. J. Crustac. Biol. 22, 45–52 (2002).
Ye, L. et al. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture 290, 140–144 (2009).
Chen, K. et al. Growth and lipid metabolism of the pacific white shrimp Litopenaeus vannamei at different salinities. J. Shellfish Res. 33, 825–832 (2014).
程熙; 李家乐; 冯建彬; 聂式忠; 范益平. 日本沼虾幼虾的耐盐性研究. 大连水产学院学报, 315–317 (2008). https://doi.org/10.16535/j.cnki.dlhyxb.2008.04.014.
De Grave, S. & Ghane, A. The establishment of the oriental river prawn, Macrobrachium nipponense (de Haan, 1849) in Anzali Lagoon. Iran. Aquatic Invasions 1, 204–208 (2006).
Ma, X., Liu, X., Wen, H., Xu, Y. & Zhang, L. Histological observation on gonadal sex differentiation in Cynoglossus semilaevis Günther. Mar. Fish. Res 27, 55–61 (2006).
Bumin, S. & Zhengcong, L. Histological studies on ovarian development in Scylla serrata. J. Fish. China (China) 15, 96–103 (1991).
Hu, Y. et al. Validation and evaluation of reference genes for quantitative real-time PCR in Macrobrachium Nipponense. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19082258 (2018).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
王佳丽; 黄贤金; 钟太洋; 陈志刚. 盐碱地可持续利用研究综述. 地理学报, 66, 673–684 (2011).
Wang, W., Sun, R., Wang, A., Bao, L., & Wang, P. Effect of different environmental factors on the activities of digestive enzymes and alkaline phosphatase of Macrobrochium nipponense. Ying Yong Sheng tai xue bao= The Journal of Applied Ecology 13, 1153–1156 (2002).
李庭古. 盐度对克氏原螯虾的存活, 生长, 代谢及受精卵孵化的影响. 硕士 (2007).
李洪涛; 周文宗; 高红莉; 张硌. 盐度和碱度对克氏原螯虾的联合毒性试验. 水产养殖, 1–4 (2006).
鲁耀鹏; 钱坤; 汪蕾; 张秀霞; 王冬梅; 李军涛; 冼健安; 王安利. 养殖盐度对凡纳滨对虾抗氧化酶及免疫相关酶活力的影响. 河北渔业, 1–5+28 (2019).
Du, J. et al. Immune responses and gene expression in hepatopancreas from Macrobrachium rosenbergii challenged by a novel pathogen spiroplasma MR-1008. Fish. Shellfish. Immunol. 34, 315–323 (2013).
Meli, R., Nauser, T., Latal, P. & Koppenol, W. H. Reaction of peroxynitrite with carbon dioxide: Intermediates and determination of the yield of CO3•– and NO2•. J. Biol. Inorg. Chem. 7, 31–36 (2002).
Kailasam, M. et al. Effects of calorie restriction on the expression of manganese superoxide dismutase and catalase under oxidative stress conditions in the rotifer Brachionus plicatilis. Fish. Sci. 77, 403–409 (2011).
Mozanzadeh, M. T. et al. The effect of salinity on growth performance, digestive and antioxidant enzymes, humoral immunity and stress indices in two euryhaline fish species: Yellowfin seabream (Acanthopagrus latus) and Asian seabass (Lates calcarifer). Aquaculture 534, 736329 (2021).
Cohen-Sánchez, A. et al. Exploring the impact of high salinity and parasite infection on antioxidant and immune systems in Coris julis in the Pityusic Islands (Spain). Sci. Total Environ. 951, 175848 (2024).
陈鑫; 何杰; 张东旭; 俞学军; 平洪领; 张涛; 史会来; 李彬. 盐度渐变对日本囊对虾非特异性免疫酶,ATPase酶和抗氧化酶活力的影响. 浙江海洋大学学报(自然科学版), 43, 1–10 (2024).
叶建生; 王兴强; 马甡; 阎斌伦. 盐度突变对凡纳滨对虾非特异性免疫因子的影响. 海洋水产研究, 38–43 (2008).
陈春宇. pH和盐度胁迫对中华绒螯蟹幼蟹生理影响的初步研究. 硕士 (2023).
Carmona, R., García-Gallego, M., Sanz, A., Domezain, A. & Ostos-Garrido, M. V. Chloride cells and pavement cells in gill epithelia of Acipenser naccarii: Ultrastructural modifications in seawater-acclimated specimens. J.Fish Biol. 64(2), 553–566. https://doi.org/10.1111/j.0022-1112.2004.00321.x (2004).
张秀红. 长期盐碱胁迫对脊尾白虾生长性能和卵巢发育的影响研究. 硕士 (2023).
韩晓琳; 高保全; 王好锋; 刘萍; 陈萍; 李华. 低盐胁迫对三疣梭子蟹鳃和肝胰腺显微结构及家系存活的影响. 渔业科学进展, 35, 104–110 (2014).
Guo, H. et al. Effects of nonylphenol exposure on histological changes, apoptosis and time-course transcriptome in gills of white shrimp Litopenaeus vannamei. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2021.146731 (2021).
Xue, C., Xu, K., Jin, Y., Bian, C. & Sun, S. Transcriptome analysis to study the molecular response in the gill and hepatopancreas tissues of Macrobrachium nipponense to salinity acclimation. Front. Physiol. https://doi.org/10.3389/fphys.2022.926885 (2022).
Yin, F., Peng, S., Sun, P. & Shi, Z. Effects of low salinity on antioxidant enzymes activities in kidney and muscle of juvenile silver pomfret Pampus argenteus. Acta Ecol. Sin. 31, 55–60. https://doi.org/10.1016/j.chnaes.2010.11.009 (2011).
Wang, Y., Li, W., Li, L., Zhang, W. & Lu, W. Effects of salinity on the physiological responses of the large yellow croaker Pseudosciaena croceaunder indoor culture conditions. Aquac. Res. 47, 3410–3420. https://doi.org/10.1111/are.12788 (2016).
王芸; 李正; 段亚飞; 王珺; 黄忠; 林黑着. 红景天提取物对凡纳滨对虾抗氧化系统及抗低盐度胁迫的影响. 南方水产科学, 14, 9–19 (2018).
Kim, E. J., Chung, H. J., Suh, B., Hah, Y. C. & Roe, J. H. Transcriptional and post-transcriptional regulation by nickel of sodN gene encoding nickel-containing superoxide dismutase from Streptomyces coelicolor Müller. Mol. Microbiol. 27, 187–195. https://doi.org/10.1046/j.1365-2958.1998.00674.x (2002).
Cho, Y. S., Lee, S. Y., Bang, I. C., Kim, D. S. & Nam, Y. K. Genomic organization and mRNA expression of manganese superoxide dismutase (Mn-SOD) from Hemibarbus mylodon (Teleostei, Cypriniformes). Fish. Shellfish. Immunol. 27, 571–576. https://doi.org/10.1016/j.fsi.2009.07.003 (2009).
Chakravarthy, N. et al. Intracellular Copper Zinc Superoxide dismutase (icCuZnSOD) from Asian seabass (Lates calcarifer): Molecular cloning, characterization and gene expression with reference to Vibrio anguillarum infection. Dev. Comp. Immunol. 36, 751–755. https://doi.org/10.1016/j.dci.2011.11.002 (2012).
Acknowledgements
Thanks to the Jiangsu Province Platform for the Conservation and Utilization of Agricultural Germplasm.
Funding
This research was supported by grants from National Key R&D Program of China (2023YFD2401000); Central Public-interest Scientific Institution Basal Research Fund CAFS (2023TD39); The earmarked fund for China Agriculture Research System (CARS-48-07); The seed industry revitalization project of Jiangsu province (JBGS [2021]118).
Author information
Authors and Affiliations
Contributions
Shubo Jin (S.J.) was responsible for the conceptualization of the study. R.Z. and M.X. developed the methodology. Resources were provided by Y.X. and Sufei Jiang (S.J.). H.Q. handled the software implementation. W.Z. performed the formal analysis and data curation. R.Z. wrote the original draft. The manuscript was reviewed and edited by Shubo Jin (S.J.) and H.F. All authors have read and agreed to the final published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board
Permissions for the experiments involved in the present study were obtained from the Institutional Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Wuxi, China) (Authorization NO.20240610006, 10 June 2024).
Informed consent
Statement.
Not applicable.
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, R., Xu, M., Zhang, W. et al. Effect of salinity exposure on the antioxidant system of “Taihu No. 3” Macrobrachium Nipponense. Sci Rep 15, 35749 (2025). https://doi.org/10.1038/s41598-025-20622-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20622-5