Abstract
In AD research, word-learning tests are often used interchangeably despite using distinct learning protocols. This study verified the equivalence of the Rey Auditory Learning Test (RAVLT) and Free and Cued Selective Reminding Test (FCSRT) when investigating medial temporal lobe (MTL) changes and AD-related tau pathology. We obtained the FCSRT and RAVLT immediate and delayed free recalls from 286 participants aged 51+. We segmented MTL regions to obtain the volume and tau-PET signal using the [18F]MK-6240 tracer. Tau-PET Braak stages and plasma p-tau181, p-tau217 and p-tau231 quantifications were also acquired. Using partial correlations, we compared FCSRT to RAVLT as well as their ability to detect the cognitive status the AD biomarker results. FCSRT and RAVLT were strongly correlated to one another (R > 0.779), with similar differentiation of cognitively impaired and cognitively unimpaired individuals (AUC > 0.810). Both predicted MTL volume, MTL tau-PET accumulation, plasma p-tau and Braak stages similarly, with no significant effect size differences. For all tests, a subtle memory impairment was found at tau-PET Braak stage III, while more robust impairments were found at stage IV onward. Despite their differences, both the RAVLT and FCSRT are equivalent at detecting AD-related pathology and symptoms, suggesting that, in these contexts, they may be used interchangeably. However, these results should be interpreted with care since the sample is not representative of a global population.
Similar content being viewed by others
Background
Word-learning tests employ a variety of strategies to identify memory impairments and isolate them from other cognitive deficits. Most word list learning tests such as the Rey Auditory Verbal Learning Test (RAVLT) rely on learning by the repetition of the word list over several trials1,2,3. These tests rely on a single sensory modality (e.g. auditory) and follow a rapid pace, which might limit the ability of participants to properly encode the stimuli. The Free and Cued Selective Reminding Test (FCSRT) aims to control encoding to avoid interference from sensory limitations or limited non-memory cognitive abilities4,5. To do so, learning is achieved by asking participants to name pictures, which are then associated with semantic categories. Encoding is therefore confirmed and reinforced with visual, auditory and semantic components, which putatively allows the FCSRT to better isolate memory deficits. While the conceptual differences between tests have been discussed in detail within the field of neuropsychology, the nuances are not generally considered in biomedical fields6. For example, the Preclinical Alzheimer Cognitive Composite employed different word learning tests interchangeably, with mixed results7,8.
Memory tests attempt to capture the function of the medial temporal lobe (MTL), which is understood as a crucial substrate of memory consolidation and retrieval9. The perirhinal and entorhinal cortices store and process high-level object representations while the hippocampus associates these representations to contexts10,11. Further, verbal information is more specifically processed in the left MTL while visual information is processed in the right hemisphere12,13. Importantly, this hemispheric lateralization was not consistently found with word learning tests, potentially due to the tests being best solved by a combination of visual and verbal strategies14,15. Still, MTL-associated functions can be linked with the volume of the regions that support them16. However, these associations are limited by other cognitive processes such as executive strategies, attention and sensory processing, which may affect memory test performances independently of the memory capacity itself17,18. As such, a controlled encoding like that found in the FCSRT may better represent the memory consolidation and retrieval by the MTL regions.
In addition to normal MTL function variability, it is also affected in Alzheimer’s disease (AD). The memory impairment has been associated with AD pathology19,20,21. More specifically, memory test scores are well correlated with tau pathology in the MTL in AD-enriched populations20. Tau pathology builds up early in the AD continuum, which means that memory tests would benefit from a high sensitivity to mild, or even subclinical memory impairment22,23. One would expect such sensitivity to differ between memory tests using distinct approaches. Word learning tests using the repetition (such as the RAVLT) approach and the controlled encoding of the FCSRT have been compared to one another24, although they have not been compared over their ability to identify MTL atrophy or tau pathology.
The goal of this study was to compare the ability of the memory tests to identify MTL atrophy and tau pathology. To do so, we obtained the immediate and delayed free recall scores from the FCSRT and the RAVLT. Tau pathology was measured using plasma phosphorylated tau (p-tau) 217, 181 and 231 as well as [18F]MK-6240 positron emission tomography (PET) imaging. This PET imaging was used in conjunction with a lateralized MTL segmentation to obtain both regional MTL tau accumulations and regional volumes. Based on their similarity, we expected FCSRT and RAVLT measures to strongly overlap, although the FCSRT was expected to be more discriminative of the cognitively impaired and cognitively unimpaired participants. We hypothesized that both tests would be similarly associated with volumes of MTL regions, with no significant lateralization. In addition, we hypothesized that both tests would be similarly associated with tau-PET and plasma p-tau biomarkers.
Methods
Participants
Data from 286 participants, aged 51 to 85, were analyzed from the Translational Biomarkers in Aging and Dementia (TRIAD) cohort based in Montreal, Canada. Established in 2017, TRIAD focuses on AD and other neurodegenerative conditions, tracking their progression from preclinical to advanced dementia stages. The cohort employs diagnostic tools including biomarkers, genetic testing, PET tracers, MRI, and clinical and neuropsychological assessments25. Participants were divided into two groups based on cognitive status based on the Clinical Dementia Rating (CDR): cognitively unimpaired (CU) individuals, and cognitively impaired (CI), which includes participants diagnosed with a mild cognitive impairment (MCI) or an AD dementia (ADD) based on the clinical assessment26. Those with non-amnestic dementia or motor neurodegenerative diseases were excluded. All participants underwent MRI, PET imaging with [18 F]NAV4694 (amyloid-β) and [18 F]MK-6240 (tau), phlebotomy and neuropsychological testing. Informed consent was obtained according to the Declaration of Helsinki, and the protocol was approved by the local ethics committee. All methods were performed in accordance with the relevant guidelines and regulations.
Memory assessment
Immediate and delayed recall scores were evaluated using the Rey Auditory Verbal Learning Test (RAVLT)3 and the Free and Cued Selective Reminding Test (FCSRT)5. In the RAVLT, participants listened to a sequence of 15 commonly used words (list A), read aloud at a pace of one word per second. Immediately after the list was read, participants were asked to recall as many words as they could. This process was repeated across five trials using the same list of words in the same order. Afterward, a new set of words (list B) was introduced, followed by a trial where participants attempted to recall list A without hearing it again, known as the immediate recall trial (RAVLT-IR). After a 20-minute break, participants were asked to remember the words from list A again in the delayed recall trial (RAVLT-DR)3.
The FCSRT was initiated with a learning phase where participants were shown sheets containing four pictures at a time, each from a distinct category. Participants were asked to point to the picture corresponding to a category and then name it (e.g. Experimenter: “what is the fruit?” Participant: “The grape”). After this, they were required to recall the word associated with the picture. In the event of failure to recall a word, they are told what the word was and asked to repeat it. This process continues over four sheets, for a total of 16 words. Following a short interference task, participants underwent three immediate recall trials. For each trial, they were first asked to recall as many words as possible from the list (free recall). If they failed to recall a word, they were cued with the word’s category (total recall). If they still failed to recall the word, they were reminded of it. The sum of the words recalled freely over the three recalls was the immediate free recall score (FCSRT-IR). Approximately 20 min later, the participants were asked to freely recall the words again during a delayed recall phase (FCSRT-DR). Following this, any remaining missing words are cued to give the participants a second opportunity to remember them27. Here, we used the free recall scores since they would more closely match with the RAVLT-IR and RAVLT-DR scores. None of the four memory measures had a significant ceiling or floor effects in this study (Supplementary Fig. 1).
Medial temporal lobe segmentation
Using a 3T Siemens Magnetom Prisma scanner at the Montreal Neurological Institute, each TRIAD participant had a 1 mm isotropic voxel T1-weighted (T1w) MRI using an Ultrafast Gradient Echo 3D sequence (TR: 2300 ms, TE: 2.96 ms, FoV: 256 mm, flip angle: 9°). Using the method described by Bedford et al.28, one rater (E.A.) evaluated the T1w images’ quality on a 4-point rating system, where 1 denotes good quality and 4 denotes very bad quality. Scans were included if their quality was rated as either 1 or 2.
The Automatic Segmentation of Hippocampal Subfields (ASHS) open-source program was used to segment the left and right MTL on the T1w images, using the same method described in detail elsewhere29. In short, the ASHS-PMC-T1 atlas was used to label the anterior hippocampus, the posterior hippocampus, the entorhinal cortex, Brodmann Area (BA) 35, BA36, and the parahippocampal cortex. Four independent raters (E.A., B.J.H., T.C., and L.T.) then evaluated the final segmentations for quality using a 5-point rating system ranging from 0 to 1. Segmentations assessed as 0.75 or 1 were directly included in the study, while segmentations assigned a 0.5 were manually adjusted and those assigned a 0.25 or 0 were discarded.
Amyloid-β and tau-PET imaging
We assessed amyloid-β and tau loads with a Siemens High Resolution Research Tomograph (HRRT) using [18F]NAV4694 and [18F]MK-6240 tracers, respectively. [18F]MK-6240 imaging sessions were 90–110 min after injection, while [18F]NAV4694 sessions were 40–70 min after injection. We used the ordered subset expectation maximisation algorithm on a 4-dimensional volume to reconstruct the images in 300-second frames (four frames for [18F]MK-6240 and six frames for [18F]NAV4694)30. Each PET imaging session was followed by a 6-minute transmission scan with a rotating point of 137Cs source to correct for attenuations. Images underwent corrections for dead time, decay, random, and scattered coincidences.
Amyloid-β- and tau-PET images were first aligned on the participant’s T1w MRI. The T1w images were next linearly and nonlinearly registered to the standard ADNI template space using Advanced Normalization Tools31. We then applied these transformations to the PET images to carry them to the ADNI space. PET images were spatially smoothed using an 8 mm full-width half-maximum Gaussian kernel. The standardised uptake value ratio (SUVR) was calculated using the inferior cerebellar grey matter as a reference region for [18F]MK-6240 images and the whole cerebellar grey matter as a reference region for [18F]NAV4694 scans30,32.
We obtained a global amyloid-PET load using the average SUVR from a region of interest (ROI) encompassing the precuneus, prefrontal, orbitofrontal, parietal, temporal, anterior, and posterior cingulate cortices33. An amyloid-PET uptake ≥ 1.55 was considered amyloid-β-positive (A+)34. Tau-PET SUVRs were obtained from updated ROIs corresponding to Braak stages I through VI (Fig. 129,35,36). For each of these six ROIs, a threshold was established based on 2.5 standard deviations above the mean of a subgroup in the TRIAD cohort aged 18–25 years34,36. Braak stages were then established based on the highest Braak ROI threshold surpassed, provided that all previous Braak ROI thresholds were also surpassed. Tau-PET uptake was also obtained from the MTL ROIs. These were extracted from the non-smoothed PET images aligned with the participant’s original T1w MRI.
Plasma p-tau assessment
The blood sampling followed previously described protocols37. Plasma p-tau181 and p-tau231 concentrations were measured in the Clinical Neurochemistry Laboratory at the University of Gothenburg using in-house Single molecule array (Simoa; Simoa HD-X instruments, Quanterix, Billerica, Massachusetts, USA) assays37,38. Plasma p-tau217 (also referred to as p-tau217+) concentration was measured using a Simoa assay developed by Janssen39. Plasma p-tau data was unavailable for 147 participants.
Statistical analyses
All statistical analyses were conducted in RStudio (R version 4.4.1). Linear regression models were used to assess associations between cognitive measures, tau biomarkers, and MTL volumes, adjusting for sex, age, and education, with intracranial volume (ICV) adjustment for MTL volumes specifically. Residuals to which were added the means of the original variables for visual interpretation. Standardized beta coefficients (βSTD) were extracted using the effectsize package, and p-values were corrected for multiple comparisons using the Benjamini-Hochberg False Discovery Rate (FDR) procedure.
Receiver operating characteristic (ROC) analyses evaluated the classification performance of cognitive measures (CI vs CU), reporting the area under the curve (AUC), sensitivity, and specificity. Comparison of memory measures across clinicopathologic classifications used FDR-corrected Pairwise Wilcoxon rank-sum tests and Tukey’s Honestly Significant Difference (HSD) tests from ANOVA models. Associations of memory scores with Braak staging were analyzed using Wilcoxon rank-sum tests to compare memory measure residuals across stages, with Braak stage 0 as the reference for Dunnett’s post-hoc tests. Braak stage discordant individuals were not considered in this analysis.
Results
Sample description
Among the 286 participants included in the study, 109 were classified as CI. Among those, 75 were classified as MCI and 34 were classified as ADD. All ADD participants were classified as A+. CU and CI groups did not significantly differ in terms of age and years of education, but a significantly higher proportion of males, APOE ε4 carriers and A + participants were found among the CI group (Table 1). The CI group had significantly lower memory scores on all measures. However, the variance was more unequal between CI and CU in the FCSRT measures.
Comparison between memory tests
We first compared the relationships between all four memory measures across CU and CI participants. Strong positive associations were found across all comparisons (βSTD = 0.779 to 0.948; p < 0.001), with stronger associations for within-test associations (i.e. RAVLT-IR with RAVLT-DR and FCSRT-IR with FCSRT-DR; Fig. 2). Across tests, all measures were similarly associated and were similar when comparing an immediate recall with a delayed recall. For all comparisons between FCSRT and RAVLT scores, a cluster of participants were found in the upper left quadrant. Those participants with low RAVLT scores, but normal FCSRT scores were consistent across delayed and immediate recalls and included all low RAVLT scores among the CU subgroup.
Scatterplots depicting associations among standardized memory measures. Cognitively impaired (CI) participants are shown in red and cognitively unimpaired (CU) participants in blue. All regressions were adjusted for age, sex, and years of education. Scores were standardized based on the CU participants. RAVLT = Rey Auditory Verbal Learning Test; FCSRT = Free and Cued Selective Reminding Test; IR = immediate recall; DR = delayed recall; β = standardized beta; the shaded areas represent the regressions’ 95% confidence intervals.
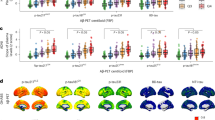
ROC curves were used to assess the ability of cognitive tests to differentiate between CU and CI participants. All four tests demonstrated strong classification performance, with AUC values ranging from 0.810 to 0.824 (DeLong’s test: p > 0.05). Delayed recall measures tended to show slightly higher AUCs (Fig. 3a). Sensitivity and specificity were similar across measures (Table 2). Both tests also performed similarly at detecting amyloid pathology 0.712 to 0.755 (DeLong’s test: p > 0.05; Fig. 3b). All tests were effective at distinguishing CU from MCI and ADD, whether they be amyloid positive or negative (Fig. 3C).
Detection of cognitive impairment (CI) using memory measures. Receiver operating characteristic (ROC) curves illustrating the discriminative ability of Rey Auditory Verbal Learning Test (RAVLT) and Free and Cued Selective Reminding Test (FCSRT) immediate (IR) and delayed recall (DR) measures across CI and cognitively unimpaired (CU) groups (a) and across amyloid-positive and negative (A + and A-) groups (b). Area under the curve (AUC) values indicate classification performance for each test. (c) boxplots depicting group differences in memory measures (FCSRT-IR, FCSRT-DR, RAVLT-IR, and RAVLT-DR) across clinical status categories (CU A-, CU A+, MCI A-, MCI A+, and ADD). A + indicates amyloid-beta positivity, defined as a global amyloid-PET SUVR > 1.55, while A- indicates Aβ negativity. All analyses were adjusted for age, sex, and years of education. Displayed p-values indicate significant differences between groups and were derived from FDR-corrected Pairwise Wilcoxon rank-sum tests and Tukey’s HSD tests from ANOVA models. Although all pairwise comparisons were performed, only comparisons with adjacent stages or within clinical groups are displayed here.
We then combined the clinical status with the amyloid-β biomarker status to compare clinicopathologic groups over memory measures. Comparable group differences were observed across cognitive impairment groups (CU, MCI, and ADD) and amyloid-β status (A + vs A−) for FCSRT-IR, FCSRT-DR, RAVLT-IR, and RAVLT-DR (Fig. 3b). In all cases, memory scores were significantly lower in MCI and ADD participants compared to CU, no matter the amyloid-β status (p < 0.001). However, there was one exception, where MCI A + were significantly more impaired at the RAVLT-DR than the MCI A-. These results indicate that memory performance declines with increasing clinical impairment, with poor detection of Amyloid-β-positive individuals within the diagnostic categories.
The relationship between the memory measures and MTL volumes.
Next, we performed linear regression analyses between cognitive test performance and MTL volumes. Across all memory measures, we found positive associations with the anterior hippocampus, posterior hippocampus, entorhinal cortex, and transentorhinal cortex (βSTD = 0.32 to 0.48; p < 0.001; Fig. 4; Supplementary Table 1). These coefficients were marginally different across memory measures. The perirhinal and parahippocampal cortex volumes showed weaker associations across all memory measures (βSTD = 0.22 to 0.29; p < 0.001; Fig. 4; Supplementary Table 1). FCSRT and RAVLT were not significantly associated with any MTL volume (supplementary Table 2). When splitting MTL regions between the left and right hemispheres, we did not find a clear lateralization of the associations (Supplementary Table 3).
To further examine the relationship between MTL volumes and memory measures, we conducted subgroup regression analyses for A+, CI and CU participants. Significant associations between memory scores and all MTL volumes were observed in the CI group and A + group (βSTD = 0.158 to 0.55; p < 0.05; Supplementary Tables 4 & supplementary Fig. 2). In contrast, these associations were non-significant in the CU group (βSTD = -0.058 to 0.189; p > 0.05; Supplementary Table 5). This suggests that the relationship between memory performance and MTL atrophy emerges more clearly in the presence of a cognitive impairment.
Detection of tau biomarkers using memory measures
The next part of our analysis examined the relationship between memory performance and tau burden in MTL regions and the plasma samples. Higher tau burden in all MTL subregions was strongly associated with poorer memory performance across all memory measures (βSTD = -0.60 to -0.69; p < 0.001; Fig. 5; Supplementary Table 6). All plasma p-tau biomarkers were also significantly negatively correlated with memory performance, consistent with regional tau-PET findings (βSTD = -0.32 to -0.62; p < 0.001; Fig. 5; Supplementary Table 6). No memory measure was distinctly associated with tau burden measures.
To further examine the relationship between tau burden and memory measures, we conducted subgroup regression analyses for A+, CI and CU participants. Significant associations between memory measures and tau burden were observed in the A + and CI groups (βSTD = -0.253 to -0.609; p < 0.038; Supplementary Tables 7 & Supplementary Fig. 3), except for p-tau231, which was non-significantly associated with memory tests in the A + subgroup (βSTD = -0.108 to -0.172; p > 0.168). In contrast, these associations were non-significant in the CU group (βSTD = 0.004 to -0.206; p > 0.071; Supplementary Table 8).
Standardized-β heatmap for regressions between memory measures and medial temporal lobe (MTL) volumes. All regressions were adjusted for, age, sex, years of education and intracranial volume (ICV). βSTD from linear regression models are displayed, with color intensity reflecting effect sizes. All of the partial regressions are significant at pcorr < 0.001. RAVLT = Rey Auditory Verbal Learning Test; FCSRT = Free and Cued Selective Reminding Test; IR = immediate recall; DR = delayed recall.
Standardized-β heatmap of regressions between memory measures and tau burden. This burden was obtained in medial temporal lobe (MTL) regions for tau-PET, or plasma p-tau assays. All regressions were adjusted for age, sex and years of education. βSTD from linear regression models are displayed, with color intensity reflecting effect sizes. All of the partial regressions are significant at pcorr < 0.001. RAVLT = Rey Auditory Verbal Learning Test; FCSRT = Free and Cued Selective Reminding Test; IR = immediate recall; DR = delayed recall.
Memory measures across Braak stages
To further investigate when memory deficits become apparent as the AD-related tau pathology is at more advanced stage, we examined how memory measures varied across Braak stages. Progressive memory impairment with increasing Braak stage was found across all memory measures (Fig. 6). All measures showed a trend for cognitive impairment at stage III (p < 0.091) with robust group differences emerging at Braak stages IV to VI (p < 0.001). Only these robust differences were significant among the A + subgroup (Supplementary Fig. 4).
Residualized memory measures across Braak stages. All residuals were adjusted for age, sex, and years of education. Displayed p-values indicate significant differences between groups and were derived from Dunnett’s post-hoc test, comparing each Braak stage to Braak 0. RAVLT = Rey Auditory Verbal Learning Test; FCSRT = Free and Cued Selective Reminding Test; IR = immediate recall; DR = delayed recall.
Discussion
This study aimed to compare the ability of two of the most frequently used memory tests to predict MTL volumetric changes as well as local and systemic tau accumulation. We were expecting both tests strongly overlap, but with a superior detection of cognitive impairment by the FCSRT. This was not confirmed, as both tests were equivalent. Secondly, hypothesized similar associations with MTL volumes across memory scores, which was confirmed. Thirdly, we found equivalent associations of FCSRT and RAVLT scores with either cerebral tau or plasma p-tau pathology biomarkers, confirming our last hypothesis. This study shows that, despite differences between both tests, the FCSRT and RAVLT are equivalent when predicting MTL volumes and AD-related tau pathology.
The FCSRT was designed to best capture memory performance through its learning protocol16,40. Despite this, we did not find evidence of the FCSRT’s superiority over the RAVLT. Plus, we found very high correlations between any combination of both tests’ IR and DR, with the highest correlations between the scores from the same tests. This suggests that differences between IR and DR are outweighed by the test differences41,42. These differences may be related to the differences in variance between CI and CU, as we observed smaller variance in the CU group and larger variance than in the CI group on the FCSRT. Those distinct variances could be explained by the higher specificity of the FCSRT at detecting a memory deficit, at the cost of a lower sensitivity compared to the RAVLT43; although no such difference has been confirmed in our results. Indeed, we hypothesize that the FCSRT minimizes false memory impairment due to the thorough encoding protocol relying on multiple modalities, ensuring that all of the words were properly encoded4. The RAVLT encoding, on the other hand, is less controlled and relies on working memory auditory processing or executive function44,45. As such, auditory impairment, limitations in working memory, or executive function deficits may mimic a memory deficit when using the RAVLT. These false memory deficits, putatively more readily detected by the RAVLT, may be accompanied by a normal FCSRT. The FCSRT was also specifically designed to detect memory deficits, and not the preclinical participants’ subtle decline, thus the reduced variability among CU. Supporting this, we found no discernible difference between the memory scores of the AB + and AB- CU participants in both tests. A lack of sensitivity of cognitive assessments in the preclinical stages of the disease is consistent with previous articles46. After evaluating the evidence, it appears that both the FCSRT and RAVLT have different protocols to ensure memory encoding with no clear superiority at assessing a clinically defined memory deficit in participants found in AD clinicopathological stages.
Next, we found similar associations with MTL regional volumes across memory tests. The associations were consistent with the putative role of each MTL region. The hippocampus, entorhinal and perirhinal, transentorhinal and parahippocampal cortex are all particularly important contributors to word list learning10,47. This could be explained by the hierarchical-representational model, which hypothesizes that the perirhinal and entorhinal cortices process item-level representations, connecting a word to its associated semantic knowledge10. This was supported by functional imaging studies, where the recall of novel visual stimuli learned without context specifically leads to perirhinal and entorhinal activity whereas the recall of items for which context was needed solicited the hippocampus48,49. Word list learning tests use frequent words, for which the novelty is the experimental context where the words are presented50. Additionally, learning the words requires associating them with the experimental context, which relies on the hippocampus51. Previous work from our team has found similar associations between RAVLT and hippocampal subfield volumes with no obvious lateralization29. Therefore, our results suggest that the perirhinal, entorhinal and hippocampal regions are not differentially involved in the FCSRT and RAVLT and that neither test is more specific to MTL functions.
We next found equivalent associations of FCSRT and RAVLT scores with either local or global tau pathology biomarkers. The tau pathology in all MTL regions as well as for all global biomarkers, such as tau-PET Braak stages and fluid biomarkers, showed the same strong correlations with both memory tests. More specifically, perirhinal, transentorhinal, parahippocampal tau-PET (corresponding to Braak stage III) as well as plasma p-tau217 were the most strongly associated with all memory measures. This is consistent with previous work, where perirhinal tau-PET was the strongest predictor of memory dysfunction52. Another study using the same cohort found that the perirhinal and parahippocampal tau pathology may represent the point where detectable AD-associated memory impairment begins29. To better capture this inflection point, we used an improved version of Braak stage ROIs that focuses on the early to mid-Braak stage III changes in the MTL, including perirhinal and parahippocampal cortices29. As a result, participants with Braak stage III tau-PET display worse memory scores detectable by both the FCSRT and RAVLT. Thus, we further strengthen the hypothesis that tau pathology in the perirhinal and parahippocampal cortices coincides with early memory impairment in AD. The FCSRT and RAVLT are equivalent in detecting this shift. This is consistent with previous results, where the earliest tau-PET changes were not significantly associated with RAVLT deficits20.
In addition, plasma p-tau biomarkers tend to increase throughout AD progression and are excellent predictors of early memory impairment, especially plasma p-tau21753,54,55. As such, our study confirmed that plasma p-tau217 outperforms the other p-tau variants by being more closely associated with memory impairment with almost identical correlations with FCSRT and RAVLT memory measures. Our findings suggest that FCSRT and RAVLT memory measures for cognitive impairment both correlate with similar strength to cortical tau in the MTL and plasma p-tau biomarkers.
Limitations
Our results should be interpreted with caution since our associations were mostly driven by the cognitively impaired subgroup. Neither memory test appears particularly effective at identifying subclinical variability. It is possible that a composite score like the PACC, typically used in preclinical settings, might limit the noise and improve the sensitivity to subclinical memory performance variability56. The cued recall from the FCSRT could also prove useful in specific contexts. However, it was not considered in the current study due to a strong ceiling effect and lack of comparable measure in the RAVLT. Next, our sample was enriched with cognitively unimpaired and AD spectrum participants to better capture the effect of tau pathology. For this reason, the specific associations between memory scores and tau biomarkers reported in this study should be interpreted cautiously because of the study’s inclusion criteria (i.e. no amyloid-negative individuals with dementia). The RAVLT and FCSRT are also meant to distinguish AD from a pool of other conditions21. Compared to our sample, the older adult population is more diverse, with cases of Lewy body diseases, frontotemporal dementia and vascular dementia. Using a more diverse sample with biomarkers to identify these other neurodegenerative diseases should allow us to evaluate the contribution of the different pathologies to memory deficits. In addition, using a more diverse sample such as validation cohorts would allow us to investigate the differential contribution of non-memory deficits on memory test performance, which might differ between the FCSRT and the RAVLT. However, to our knowledge, no other cohort includes data from both tests, making the replication difficult.
Conclusions
In conclusion, despite differences between the FCSRT and RAVLT in terms of learning protocol, no clear differences were observed in the diagnostic accuracy of each test and thus remain equivalent at predicting tau pathology, amyloid pathology and cognitive impairment. We found an onset to the memory decline at tau-PET Braak stage III with both tests. A better specificity from the FCSRT might allow it to minimize the number of false memory impairments, but this is at the cost of a lower sensitivity. These results highlight the importance of choosing the right memory test for a given clinical or experimental setting.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADD:
-
AD dementia
- AUC:
-
Area under the curve
- BA:
-
Brodmann Area
- CDR:
-
Clinical Dementia Rating
- CI:
-
Cognitively impaired
- CU:
-
Cognitively unimpaired
- DR:
-
Delayed recall
- FCSRT:
-
Free and Cued Selective Reminding Test
- HSD:
-
Tukey’s Honestly Significant Difference
- ICV:
-
Intracranial volume
- IR:
-
Immediate recall
- MCI:
-
Mild cognitive impairment
- MTL:
-
Medial temporal lobe
- PET:
-
Positron emission tomography
- RAVLT:
-
Rey Auditory Verbal Learning Test
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SUVR:
-
Standardised uptake value ratio
- T1w:
-
T1-weighted MRI
- TRIAD:
-
Translational Biomarkers in Aging and Dementia
References
Brandt, J. Hopkins Verbal Learning Test. (1991).
Delis, D. C., Freeland, J., Kramer, J. H. & Kaplan, E. Integrating clinical assessment with cognitive neuroscience: Construct validation of the California verbal learning test. J. Consult Clin. Psychol. 56 (1), 123–130 (1988).
Rey, A. L’examen psychologique Dans les Cas d’encéphalopathie traumatique. (Les problems.). [The psychological examination in Cases of traumatic encepholopathy. Probl. Arch. Psychol. 28, 215–285 (1941).
Buschke, H. Cued recall in amnesia. J. Clin. Neuropsychol. 6 (4), 433–440 (1984).
Grober, E. & Buschke, H. Genuine memory deficits in dementia. Dev. Neuropsychol. 3 (1), 13–36 (1987).
Pelgrim, T. A. D., Beran, M., Twait, E. L., Geerlings, M. I. & Vonk, J. M. J. Cross-sectional associations of Tau protein biomarkers with semantic and episodic memory in older adults without dementia: A systematic review and meta-analysis. Ageing Res. Rev. 71, 101449 (2021).
Buckley, R. F. et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement. 14 (9), 1193–1203 (2018).
Schneider, L. S. & Goldberg, T. E. Composite cognitive and functional measures for early stage alzheimer’s disease trials. Alzheimers Dement. Diagn. Assess. Dis. Monit. 12 (1), e12017 (2020).
Ranganath, C. A unified framework for the functional organization of the medial Temporal lobes and the phenomenology of episodic memory. Hippocampus 20 (11), 1263–1290 (2010).
Cowell, R. A., Barense, M. D. & Sadil, P. S. A roadmap for understanding memory: Decomposing cognitive processes into operations and representations. eNeuro 6 (4). https://www.eneuro.org/content/6/4/ENEURO.0122-19 (2019).
Saksida, L. M. & Bussey, T. J. The representational–hierarchical view of amnesia: Translation from animal to human. Neuropsychologia 48 (8), 2370–2384 (2010).
Köhler, S. et al. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: An MR volumetry study in alzheimer’s disease. Neuropsychologia 36 (9), 901–914 (1998).
Saling, M. M. et al. Lateralization of verbal memory and unilateral hippocampal sclerosis: Evidence of task-specific effects. J. Clin. Exp. Neuropsychol. 15 (4), 608–618 (1993).
Babiloni, C. et al. Activity of hippocampal, amygdala, and neocortex during the Rey auditory verbal learning test: An event-related potential study in epileptic patients. Clin. Neurophysiol. 121 (8), 1351–1357 (2010).
Strandberg, M. et al. fMRI memory assessment in healthy subjects: A new approach to view lateralization data at an individual level. Brain Imaging Behav. 5 (1), 1–11 (2011).
Aumont, E. et al. Hippocampal subfield associations with memory depend on stimulus modality and retrieval mode. Brain Commun. 5 (6), fcad309 (2023).
Gleichgerrcht, E., Torralva, T., Martinez, D., Roca, M. & Manes, F. Impact of executive dysfunction on verbal memory performance in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 23 (1), 79–85 (2011).
Guglielmi, V. et al. Does hearing loss in the elderly individuals conform to impairment of specific cognitive domains? J. Geriatr. Psychiatry Neurol. 33 (4), 231–240 (2020).
Aumont, E. et al. Hippocampal atrophy over two years in relation to tau, amyloid-β and memory in older adults. Neurobiol. Aging 146, 48–57 (2025).
Fernández Arias, J. et al. Verbal memory formation across PET-based Braak stages of Tau accumulation in alzheimer’s disease. Brain Commun. 5 (3), fcad146 (2023).
Giuffrè, G. M. et al. Associations between free and cued selective reminding test and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. J. Alzheimer’s Dis. 100 (2), 713–723 (2024).
Sperling, R. A. et al. Toward defining the preclinical stages of alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 7 (3), 280–292 (2011).
Jack, C. R. Jr et al. Prevalence of biologically vs clinically defined alzheimer spectrum entities using the National Institute on Aging–Alzheimer’s association research framework. JAMA Neurol. 76 (10), 1174–1183 (2019).
Clerici, F. et al. Construct validity of the free and cued selective reminding test in older adults with memory complaints. J. Neuropsychol. 11 (2), 238–251 (2017).
Therriault, J. et al. Association of Apolipoprotein E ε4 With Medial Temporal Tau Independent of Amyloid-β. JAMA Neurol.77 (4), 470. https://jamanetwork.com/journals/jamaneurology/fullarticle/2757605 (2020).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 7 (3), 263–269 (2011).
Buschke, H., Sliwinski, M. J., Kuslansky, G. & Lipton, R. B. Diagnosis of early dementia by the double memory test: encoding specificity improves diagnostic sensitivity and specificity. Neurology 48 (4), 989–96. Available from: https://www.neurology.org/doi/. https://doi.org/10.1212/WNL.48.4.989 (1997).
Bedford, S. A. et al. Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol. Psychiatry 25 (3), 614–628 (2020).
Aumont, E. et al. Optimized Atlas for Early Tau-Pet Staging Via Native Space Segmentations. (Social Science Research Network, 2025). https://papers.ssrn.com/abstract=5204665.
Pascoal, T. A. et al. In vivo quantification of neurofibrillary tangles with [18F]MK-6240. Alzheimers Res. Ther. 10 (1), 74. https://alzres.biomedcentral.com/articles/https://doi.org/10.1186/s13195-018-0402-y (2018).
Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54 (3), 2033–44. https://linkinghub.elsevier.com/retrieve/pii/S1053811910012061 (2011).
Cselényi, Z. et al. Clinical Validation of18 F-AZD4694, an Amyloid-β–Specific PET Radioligand. J. Nucl. Med. 53 (3), 415–24. http://jnm.snmjournals.org/lookup/doi/https://doi.org/10.2967/jnumed.111.094029 (2012).
Jack, C. R. et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 13 (3), 205–16. https://alz-journals.onlinelibrary.wiley.com/doi/. https://doi.org/10.1016/j.jalz.2016.08.005 (2017).
Therriault, J. et al. Determining amyloid-β positivity using 18F-AZD4694 PET imaging. J. Nucl. Med. 62 (2), 247–252 (2021).
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H. & Del Tredici, K. Staging of alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. (Berl). 112 (4), 389–404 (2006).
Pascoal, T. A. et al. 18F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain 143 (9), 2818–2830 (2020).
Karikari, T. K. et al. Blood phosphorylated Tau 181 as a biomarker for alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19 (5), 422–433 (2020).
Ashton, N. J. et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. (Berl). 141 (5), 709–724 (2021).
Triana-Baltzer, G. et al. Development and validation of a high-sensitivity assay for measuring p217 + tau in plasma. Alzheimers Dement. Diagn. Assess. Dis. Monit. 13 (1), e12204 (2021).
Estévez-González, A., Kulisevsky, J., Boltes, A., Otermín, P. & García-Sánchez, C. Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of alzheimer’s disease: Comparison with mild cognitive impairment and normal aging. Int. J. Geriatr. Psychiatry. 18 (11), 1021–1028 (2003).
Arighi, A. et al. Word and picture version of the free and cued selective reminding test (FCSRT): Is there any difference? J. Alzheimer’s Dis. 61 (1), 47–52 (2018).
Moradi, E., Hallikainen, I., Hänninen, T. & Tohka, J. Rey’s auditory verbal learning test scores can be predicted from whole brain MRI in alzheimer’s disease. NeuroImage Clin. 13, 415–427 (2017).
Grande, G. et al. Free and cued selective reminding test predicts progression to alzheimer’s disease in people with mild cognitive impairment. Neurol. Sci. 39 (11), 1867–1875 (2018).
Grober, E., Sanders, A. E., Hall, C. & Lipton, R. B. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis. Assoc. Disord. 24 (3), 284–290 (2010).
Afthinos, A. et al. The contribution of working memory areas to verbal learning and recall in primary progressive aphasia. Front. Neurol. 13. https://www.frontiersin.org/journals/neurology/articles/. https://doi.org/10.3389/fneur.2022.698200/full (2022).
Liu, S. et al. Predicting amyloid beta accumulation in cognitively unimpaired older adults: Cognitive assessments provide no additional utility beyond demographic and genetic factors. Alzheimers Dement. 21 (3), e70036 (2025).
Aggleton, J. P. & Brown, M. W. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 22 (3), 425–444 (1999). discussion 444–489.
Ross, D. A., Sadil, P., Wilson, D. M. & Cowell, R. A. Hippocampal engagement during recall depends on memory content. Cereb. Cortex 28 (8), 2685–2698 (2018).
Sanders, D. M. W. & Cowell, R. A. The locus of recognition memory signals in human cortex depends on the complexity of the memory representations. Cereb. Cortex. 33 (17), 9835–9849 (2023).
Atucha, E., Karew, A., Kitsukawa, T. & Sauvage, M. M. Recognition memory: Cellular evidence of a massive contribution of the LEC to familiarity and a lack of involvement of the hippocampal subfields CA1 and CA3. Hippocampus 27 (10), 1083–1092 (2017).
O’Keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Clarendon Press; Oxford University, 1978).
Rani, N. et al. Tau PET burden in Brodmann areas 35 and 36 is associated with individual differences in cognition in non-demented older adults. Front. Aging Neurosci.15. https://www.frontiersin.org/journals/aging-neuroscience/articles/. https://doi.org/10.3389/fnagi.2023.1272946/full (2023).
Ashton, N. J. et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat. Med. 28 (12), 2555–2562 (2022).
Fernández Arias, J. et al. Plasma phosphorylated tau217 strongly associates with memory deficits in the Alzheimer’s disease spectrum. Brain awaf033. (2025).
Pereira, J. B. et al. Plasma markers predict changes in amyloid, tau, atrophy and cognition in non-demented subjects. Brain J. Neurol. 144 (9), 2826–2836 (2021).
Sperling, R. A. et al. Trial of Solanezumab in preclinical Alzheimer’s disease. N. Engl. J. Med. 389 (12), 1096–1107 (2023).
Acknowledgements
The authors thank psychometricians Vanessa Pallen and Emilie Thomas for their contribution to the data collection and processing. The authors are indebted to the participants and the administration personnel of the TRIAD cohort for their time and dedication.
Funding
PRN and the McGill University Research Centre for Studies in Aging receive support from the Weston Bain Institute, the Canadian Institutes of Health Research [MOP-11-51-31; RFN 152985, 159815, 162303], the Canadian Consortium of Neurodegeneration and Aging (CCNA; MOP-11-51-31 -team 1), the Alzheimer’s Association [NIRG-12-92090, NIRP-12-259245], the Brain Canada Foundation (CFI Project 34874; 33397), and the Fonds de Recherche du Québec – Santé (FRQS; Chercheur Boursier, 2020-VICO-279314). EA received funding from a FRQS postdoctoral training scholarship. PRN and SG are members of the CIHR-CCNA Canadian Consortium of Neurodegeneration in Aging. Colin J. Adair Charitable Foundation funded this project.
Author information
Authors and Affiliations
Contributions
EA contributed to project conception, analyses data interpretation and was a major contributor in writing the manuscript. KA curated the data, performed data analyses, created the figures and contributed to writing the manuscript. DOL contributed to data interpretation, writing and revising the manuscript. JFA and MM both contributed to study conception and revising the manuscript. GB, BJH, LT, TC JS, SS, ACM, SAH and JT all contributed to data acquisition and processing as well as manuscript revision. NR was cohort administrator. PV and NMP worked on neuropsychological data acquisition and interpretation. ALB, NJA, KB, TKK, GTB and HCK performed the p-tau assay data acquisition. JK and YIM oversaw the imaging acquisition and processing. PRN worked on project conception, securing funding and was a major contributor to writing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
GTB and HCK were Jansen & Jansen employees at the time of their contribution to this study. The other authors declare that they have no competing interests.
Ethical approval and consent to participate
Informed consent was obtained from all participants according to the Declaration of Helsinki, and the protocol was approved by the Research Ethics Board of the Centre intégré universitaire de santé et de services sociaux de l’Ouest-de-l’Île-de-Montréal (project 2017 − 158).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aumont, E., Amiel, K., Lopez, D.O. et al. Equivalence of the FCSRT and RAVLT to detect medial Temporal lobe atrophy and tauopathy. Sci Rep 15, 37425 (2025). https://doi.org/10.1038/s41598-025-21260-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-21260-7