Abstract
This research investigated the modulation of autophagy and protein synthesis markers in human peripheral blood mononuclear cells (PBMCs) of moderately trained men following repeated sprint exercises (RSE) performed under hypoxia (HYP, 13% FIO2), with bilateral blood flow restriction (BFR, 45% of resting arterial occlusive pressure), or normoxia. Using a crossover design, participants completed three sets of five 10-s sprints under each condition. mRNA and protein levels were assessed using qPCR and Western immunoblotting. Exercise significantly increased the microtubule-associated light chain 3B (LC3B)-II/I ratio (p < 0.001, dz = 0.58), with the effect being more pronounced in NOR (p = 0.011, dz = 0.81). Conversely, p62 protein levels were unchanged (p > 0.05). However, a tendency towards a reduced LC3B-II/I ratio was observed in HYP (p = 0.056, dz = 0.34) but not in BFR. No significant effects of exercise or conditions were found for mRNA expression of Atg4B, Beclin, and HIF1-α. Exercise increased the phosphorylation of rpS6 (p < 0.001, dz = 0.96), while the phosphorylation of p70S6K and 4E-BP1 remained unchanged (p > 0.05). These findings demonstrate, for the first time, that a 5 × 10-s RSE protocol induces the early stages of autophagy in PBMCs, whereas hypoxia tended to attenuate this effect. However, the effects on protein synthesis markers were heterogeneous, without influence of HYP and BFR.
Similar content being viewed by others
Introduction
Peripheral blood mononuclear cells (PBMCs), which include lymphocytes and monocytes, play a central role in orchestrating immune responses. PBMCs are involved in metabolic reprogramming and in the regulation of anti- and proinflammatory status to influence the direction of immune cell responses1. Throughout the last decades, significant efforts have been devoted to elucidate the mechanisms underlying the relationship between exercise and the immune system. Regular moderate exercise can boost immune function, whereas strenuous activity may temporarily weaken it and increase the risk of infection2 or possibly lead to temporary immunosuppression3. Indeed, it has been reported that leukocytes are commonly subjected to DNA damage during vigorous exercise, leading to cell death4. Previous works have also demonstrated caspase-3 cleavage in PBMCs from females following heat-stressed high-intensity exercise4.
Over the past decade, sprint training methods, characterized by their high intensity, have become increasingly popular, as highlighted in recent reviews5,6. Repeated sprint exercises (RSE) can boost repeated sprint ability in well-trained populations, and the addition of hypoxic stress may promote additional gains in performance8,9,10. However, the impact of hypoxic stress remains largely unexplored, although its combination with RSE has recently been studied for its potential to promote adaptations and improvements in physical performance5,6. The interaction between exercise-induced changes in PBMC homeostasis and the combined effects of exercise and environmental stressors warrants further investigation. Indeed, hypoxia influences immune function in a dose-dependent manner, with the extent and nature of these effects further shaped by individual variability in hypoxia responsiveness2. While the modulation of immune function by exercise is well established, data regarding the immunological consequences of hypoxia are comparatively scarce. In particular, the combined impact of exercise performed under hypoxic conditions remains insufficiently explored and poorly understood. Similar to the effects observed with severe hypoxia, bouts of intense exercise can compromise immune function and elevate the risk of infection. Performing exercise in hypoxic environments, such as hypoxic chambers or at high altitude, may further exacerbate these risks, depending on the individual’s physiological responses and tolerance to hypoxia. This underscores the need for careful adjustment of exercise parameters to mitigate potential adverse effects on immune function13,14. Alternative methods that do not involve systemic hypoxia have emerged to induce additional physiological stress during sprint protocols. While systemic hypoxia enables concurrent training of large muscular groups in a hypoxic environment, blood flow restriction (BFR) offers a distinct physiological stimulus. BFR potentially creates a localized hypoxic condition and promotes metabolite accumulation in the working muscles due to restricted blood flow11,12. This localized effect provides an alternative training stimulus compared to the systemic hypoxia approach5. Given that BFR does not systematically induce systemic hypoxic stress5, it may represent a promising strategy to promote exercise-induced adaptations while potentially having a lesser impact on PBMC homeostasis compared to HYP.
The autophagy-lysosomal system, often referred as “autophagy”, is critical for lymphocyte plasticity by ensuring protein and organelle (e.g. ribosomes, peroxisomes, mitochondria) turnover15. Autophagy is responsible for the degradation and recycling of defective or damaged cellular elements to promote cell survival, especially during high physiological stresses such as exercise, starvation or heat exposure4,16,17,18,19. Autophagy plays a crucial role in immune regulation and the maintenance of cellular homeostasis, especially under stress conditions20. Moreover, autophagy orchestrates and fine-tunes the inflammatory response by regulating both the coordinated actions of immune cells and the secretion of mediators, thereby facilitating the eradication of microbial infections and the repair of sterile tissue injuries20. Importantly, recent findings have demonstrated that autophagy is activated in PBMCs of young men during short-duration (30 min) exercise in an intensity-dependent manner, with heightened responses observed under heat exposure21. Although data in humans are limited, impaired activation of autophagy may increase cellular vulnerability and lead to greater cellular damage4. The autophagic process involves several steps, leading to the incorporation of organelles (e.g. ribosomes, mitochondria) and long-lived proteins into a double membrane vesicle (autophagosome). The last one is then degraded by lysosomal enzymes within a lysosome. Autophagy relies on autophagy-specific gene (Atg) proteins for the initial formation of autophagosomes. The formation and maturation of autophagosomes, as described by22, involve intricate molecular processes that are crucial for autophagy: the Atg12–Atg5–Atg16 complex and the microtubule-associated protein 1 light chain 3 (Atg8, LC3 in mammals) conjugation system. Additionally, two other complexes are important for the initiation of membranes formation and nucleation, unc-51 like kinase (Ulk1)/Atg1 and the Beclin1/vacuole protein sorting 34 (Vps34)/PI3K complexes, respectively23. Furthermore, protein synthesis results from activation of the mechanistic or mammalian target of the rapamycin complex 1 (MTORC1) pathway24,25,26. Phosphorylation of the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) and the ribosomal protein S6 (rpS6) is enhanced after exercise in several tissues26,27, leading to increased mRNA translation.
Understanding the immune consequences of different exercise modalities, particularly high-intensity exercise combined with environmental stressors such as hypoxia, is essential for optimizing training protocols and safeguarding athlete health. In this study, we examined whether markers of protein turnover in PBMCs are modulated during RSE performed under hypoxia or with BFR, compared to RSE under normoxia. The maximal stress levels for both HYP and BFR protocols were carefully determined based on preliminary experiments conducted in our laboratory, to ensure they remained within the safe tolerance limits of our participant cohort. We hypothesized that HYP would elicit greater metabolic stress than the other conditions, leading to increased autophagic flux, as evidenced by a higher LC3B-II/LC3B-I ratio and a reduction in p62 protein content19. Given the absence of data concerning PBMC homeostasis under BFR, we theorized that BFR would promote lower cellular stress and autophagy, as BFR does not systematically induce systemic hypoxia. Based on the available data from skeletal muscle studies5,28, we further postulate that BFR may favor the activation of protein synthesis markers (i.e. p70S6K, rpS6 and 4E-BP1) after exercise compared to systemic hypoxia due to reduced integrative stress. Investigating the combined effects of RSE, hypoxia, and BFR on PBMC protein turnover is critical. Such studies will help formulate evidence-based recommendations aimed at optimizing training protocols while minimizing immune-related risks in athletes.
Materials and methods
Participants
Fifteen physically active men (running or cycling, 8 ± 2 h per week) were enrolled in the study (age 25 ± 3 years, weight 73 ± 1 kg, height 179 ± 5 cm, body fat percentage 12 ± 2%). The participants did not follow RSE in their regular training and had not been exposed to altitude within the previous six months. The participants were asked not to consume alcohol, medication, or dietary complements and supplements one month before and during the period of the study. On the day preceding each experiment, a standardized diet (55% carbohydrate, 15% protein, and 30% fat) was provided. Participants were informed in detail about data collection, procedures, and associated risks before beginning the present study. The study procedures conformed to the Declaration of Helsinki on human experimentation and were approved by the ethics committee “Commission cantonale d’éthique de la recherche sur l’être humain – Canton de Vaud”: CER-VD 2022–00238. Participants completed written informed consent forms. The inclusion criteria were as follows: (i) not to consume alcohol or dietary supplements four weeks before and during the period of the study, (ii) not to take any medication (iii) to be aged 18–30 years old, (iv) to be free of any chronic desease. Moreover, they also completed the PAR-Q (Physical Activity Readiness questionnaire). Finally, the participants were asked to follow a standardized diet composed of approximately 15% protein, 30% fat, and 55% carbohydrate.
Study design
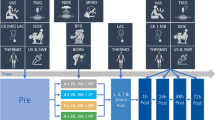
Figure 1 illustrates the study design, which followed a randomized crossover protocol. Participants have been instructed to follow RSE for three days (interspaced by at least 72 h) in a random order: NOR (above sea level), HYP (~ 4000 m, 13% FIO2), and bilaterally-cuffed BFR at 45% of the resting femoral arterial occlusive pressure. BFR was applied during sets at sea level. Normobaric systemic hypoxia was generated using a hypoxic chamber (ATS altitude training, Australia) with hypoxia induced prior to the warm-up and maintained throughout the RSE. To ensure maximal effort, verbal encouragement was provided to the participants during RSE. The day of the first visit, body composition was assessed using bioelectrical impedance analysis (InBody 770, Cheonan, Chungcheongnam, South Korea). Participants were instructed not to carry out intense efforts between the sessions and at least 48 h before the first RSE. All RSE sessions were conducted at a room temperature of approximately 24 °C with a relative humidity of around 55%
Schematic representation of the protocol. The exercise consisted of a 10-min warm-up performed on ergocycle at 100 W, followed by short recovery periods (i.e. 54 s) preceding two sprints of six seconds. After four min of passive recovery, participants achieved three sets of five 10-s sprint exercises, interspaced with 20 s of passive recovery. According to a crossover study, participants performed the same exercise in normoxia, hypoxia or with blood flow restriction (BFR). Blood was collected to isolate peripheral blood mononuclear cells (PBMCs) for RNA (N = 15) and protein (N = 11) extraction, in order to evaluate both mRNA and protein expression. The grey bars represent the sprints. FiO2: fraction of inspired oxygen.
Repeated sprint exercises protocol
Participants were asked to achieve RSE on a cycling stationary ergometer (Lode Excalibur Sport ergometer, The Netherlands) operating in constant torque mode, with a torque setting of 0.8 Nm.kg−1. After a 10-min warm-up at 100 W, followed by 54 s of passive recovery, participants achieved two six-second sprint exercises at maximal velocity. After four minutes of recovery, athletes carried out three sets (with 5 min of recovery between each set) of 5 × 10-s sprints at maximal velocity interspaced by 20 s of passive recovery. A three-second countdown was given before each starting. Saddle contact was maintained to ensure reproducibility and participants remained seated on the ergometer during all the exercise session.
Determination of femoral arterial occlusive pressure
Athletes were seated on a chair with their right leg at a 90° angle. A cuff (SC12D Rapid Version Cuff, D.E. Hokanson Inc., United States) was placed around the most proximal part of the limb. Using a rapid inflation system (AG101 Cuff Inflator Air Source, D.E. Hokanson Inc., United States), the cuff pressure was initially set to 90 mmHg and gradually increased until complete ischemia was detected via Doppler ultrasound (EchoWave II 3.4.4., Telemed Medical Systems, Italy). For reproducibility, the trial was repeated at least twice, with a one-minute recovery period between trials. The cuff dimensions were 13 × 85 cm, and the same system was used throughout the trial. For the RSE protocol, 45% of the highest obtained value was utilized. Previous experiments highlighted that continuous BFR was painful and did not allow the completion of the protocol11.
Blood collection and extraction of peripheral blood mononuclear cells
Blood samples (12 mL) were collected 10 min before and 10 min after each session into four 3-mL K3-EDTA tubes (Blood collection tubes EU ref: 368,499, BD Vacutainer, UK). PBMCs were separated from blood samples as previously described29,30. Briefly, 3 mL of blood was gently layered over 3 mL of Histopaque-1077 solution (Sigma-Aldrich, St. Louis, MO). The obtained two-phase system was centrifuged at 400 g for 30 min at 10 °C. Following the collection of the white band of mononuclear cells, the sample was washed three times using a sterile phosphate-buffered saline (PBS) solution (#D837, Sigma-Aldrich, St. Louis, MO) by centrifugation at 250 g for 10 min. After the last wash, PBMCs were frozen at − 80 °C for further analysis.
RT-qPCR analysis
For RNA extraction, three milliliters of blood were collected from each of the 15 participants. The samples were then processed using TRIzol reagent, following the manufacturer’s instructions. RNA concentration and quality (260/280 ≥ 1.8) were assessed by spectrophotometry (BioDrop, Fisher Scientific). Total RNA (1 μg) was reverse-transcribed into cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). To evaluate the specificity of each primer, both agarose gel electrophoresis and melting curve analyses were conducted. qPCR was performed using a commercially available kit (SensiFAST Probe HI-ROX Kit, Bioline USA Inc., USA) and a real-time thermocycler (StepOne Real-Time PCR System, Applied Biosystems, USA). Each well contained a mixture of 7.5 µL of SensiFAST Probe Hi-ROX Mix 2X, 1.2 µL of 10 µM forward and reverse primer mix, 2 µL of complementary DNA, and 4.3 µL of ultra-pure water, for a final volume of 15 µL. Each sample was duplicated with primers targeting the housekeeping β-actin complementary DNA (forward: 5′-CCTCGCCTTTGCCGATCC-3′, reverse: 5′ ATCTTCATGAGGTAGTCAGTC-3′. The efficiency of these primers was tested using serial dilutions, and the result obtained was 101.9%. The qPCR conditions were as follows: 2 min of initial activation of the polymerase enzyme, 40 cycles of denaturation (5 s) and hybridation (60 °C). The primers for the genes HIF1-α (forward: 5′-TATGAGCCAGAAGAACTTTTAGGC-3′, reverse: 5′-CACCTCTTTTGGCAAGCATCCTG-3′, efficiency: 104.9%), ATG4B (forward: 5′-GATGGAGGAAATCAGAAGGTTG-3′, reverse: 5′- CGCAGGGGAATGAGAAGTA-3′, efficiency: 91.3%) and Beclin (forward: 5′-CACATCTGGCACAGTGGACA-3′, reverse: 5′-CGGCAGCTCCTTAGATTTGT-3′, efficiency: 109.8%) were designed using Primer-BLAST NCBI). The relative expression of these three genes was calculated using the delta-delta Ct (threshold cycle) method: gene expression = 2−ΔΔCt. Where ΔΔCt corresponds to ΔCtpost − ΔCtpre. Of note, ΔCt is the Ct difference between β-actin and the target gene.
Western immunoblotting
PBMCs from 11 participants were homogenized in lysis buffer [20 mM MOPS, pH 7; 1% Triton X-100; 2 mM EGTA; 5 mM EDTA; 30 mM NaF; 1 mM dithiothreitol; 60 mM β-glycerophosphate; 1 mM sodium orthovanadate; 20 mM sodium pyrophosphate; and protease inhibitors (Roche, #11836153001)]. The homogenates were sonicated for four cycles of 10 s on ice to fragment nuclear DNA. Then, the samples have been centrifuged at 15,000 g (10 min at 4 °C). The supernatant was collected, and protein concentration was determined using the Bradford method (Bio-Rad, #5000001). Subsequently, 50 µg of protein was diluted in Laemmli sample buffer and resolved under denaturing conditions by SDS-PAGE. The separated proteins were then transferred onto a 0.2 µm nitrocellulose membrane. The membranes were stained with Ponceau S to verify transfer efficiency, then blocked 2 h with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) to prevent non-specific antibody binding. Membranes were incubated overnight (4 °C) with primary antibodies in either 5% bovine serum albumin (BSA) or non-fat dry milk in TBST (0.1% Tween) following supplier’s recommendations. After three washes in TBST, the membranes were incubated for one hour at room temperature with the secondary antibody. After three washes, the membranes were developed using a horseradish peroxidase solution (SuperSignal™ West Femto Maximum Sensitivity Substrate, Thermo Fisher Scientific, USA). Membrane imaging was performed using a ChemiDoc Touch Imaging System (Bio-Rad Laboratories, USA), and the bands were quantified using software (Image Lab version 6.1, Bio-Rad Laboratories, USA). Normalization of total and phosphorylated protein levels was carried out using Ponceau S staining as a loading control31.
Antibodies
The following primary antibodies were purchased from Cell Signaling Technology (USA): SQSTM1/p62 (#5114), LC3B (#2775), p-4E-BP1 (Thr37/46) (#2855), 4E-BP1 (#9452), p-S6 ribosomal protein (Ser240/244) (#5364), S6 ribosomal protein (#2317), p-S6K1 (Thr389) (#9206), and p70SK1(#2708). Membranes were incubated overnight at 4 °C in TBST solution with the corresponding primary antibody and 5% BSA or skimmed milk, as recommended by the manufacturer. Secondary antibodies (Anti-rabbit IgG, HRP-linked Antibody #7074 and Anti-mouse IgG, HRP-linked Antibody #7076, Cell Signaling Technologies, USA) were diluted 1:2000.
Statistics
Jamovi was used to perform statistical analyses (version 2.3.2). Western blot densitometry analysis of immunoblots was performed using Imagelab software. Data are presented as mean ± standard deviation (SD) and % variation is indicated when significant differences were found. No obvious deviations from homoscedasticity or normality were observed after inspection of residual plots. Linear mixed models (LMMs) were used to be accurate with the specificity of our experimental design. Indeed, LMMs perform subject-wise regression (subject-wise linear model). LMMs offer more accurate and robust estimates for hierarchically structured data compared to traditional repeated-measures ANOVA. The flexibility of LMMs makes them particularly well-suited for the analysis of repeated-measures data, especially when dealing with small sample sizes or missing data. These models account for both nested structures (e.g., multiple observations within the same participant under a given condition) and crossed structures (e.g., participants observed across multiple conditions)32,33,34,35. In this analysis, the fixed effects were the conditions NOR, HYP, BFR, and exercise (pre and post). Random effects were the participant number (i.e. cluster variable) and the intercept. When a main effect was observed, Post hoc analyses were employed to identify contrasts. Holms’ correction was applied for adjusting p-values. The percentage difference in autophagy marker expression between pre-exercise and post-exercise values was used to perform correlation tests with power outputs. The normality of the variables was verified using the Shapiro–Wilk test. When variables were normally distributed, Pearson correlation tests were employed; otherwise, Spearman correlation tests were utilized. The statistical significance was declared at the threshold value of 0.05. Cohen’s dz was utilized to denote effect sizes because the effects represent within-subjects comparisons36 (trivial effect d < 0.10, small effect 0.10 ≤ d < 0.50, medium effect 0.50 ≤ d < 0.80 and large effect d ≥ 0.80)37,38.
Results
Power output
A main effect of condition was found for peak power and mean power (p < 0.001 for both). Post hoc analysis showed that the peak power was lower in BFR and HYP compared to NOR (p < 0.001, dz = 0.63 and p = 0.004, dz = 0.42, respectively). Values were 12.1 ± 2.4 W/kg for BFR, 12.5 ± 2.1 W/kg for HYP and 13.1 ± 2.0 W/kg for NOR. Mean power was also lower in BFR and HYP compared to NOR (p < 0.001, dz = 0.83 and 0.88, respectively). Values were 8.7 ± 1.9 W/kg for BFR, 8.9 ± 1.7 W/kg for HYP and 9.7 ± 1.5 W/kg for NOR.
Expression of autophagy markers at the mRNA level
The expression of the two genes involved in autophagy (ATG4B and Beclin) was not affected by exercise or condition, and no interaction was found (ATG4B: p = 0.389, p = 0.544 and p = 0.508, respectively; Beclin: p = 0.278, p = 0.779 and p = 0.689, respectively). Similarly, the expression of HIF1-α was not modified (p = 0.629, p = 0.997 and p = 0.906, respectively) (Fig. 2).
Expression of autophagy markers at the protein level
Results showed an effect of condition (p < 0.001) on LC3B content (LC3B-I + LC3B-II). The levels of LC3B increased after exercise (+ 60%, p < 0.001, dz = 0.71). This variation was partly due to LC3B-I, since there was a significant effect of exercise on its expression levels (+ 25%, p = 0.038, dz = 0.36). Besides, an effect of exercise was obtained for LC3B-II (+ 74%, p = 0.003, dz = 0.57) (Fig. 3). Concerning autophagy flux proteins markers, the LC3B-II/LC3B-I ratio was upregulated by exercise (+ 60%, p = 0.004, dz = 0.58). In addition, a trend for condition and interaction exercise × condition was observed (p = 0.079 for both). Post hoc tests revealed significant interactions, with the most notable difference in the NOR condition: LC3B-II/LC3B-I ratio significantly increased in this condition (+ 93%, p = 0.011, dz = 0.81). After exercise, a trend toward higher LC3BII/I ratio values was observed in NOR compared to HYP (p = 0.056, dz = 0.34). Finally, the relative p62 protein content did not show any effect of exercise, condition or interaction (p = 0.892, p = 0.771 and p = 0.768, respectively). These results are presented in Figs. 3 and 4.
LC3B protein expression after exercise. (A) Total LC3 protein content, (B) LCB-I protein expression, (C) LC3B-II protein expression, (D) LC3B-II/LC3B-I ratio (%). Total lysates were analyzed by immunoblotting using the indicated antibodies. Western blot normalizations have been made with Ponceau S staining. Data displayed in the panels represent the most representative of independent experiments. Of note, blots have been cropped from different parts of the same gel to present the most relevant data. (N = 11).
p62 protein expression after exercise. Total lysates were analyzed by immunoblotting using the indicated antibodies. Western blot normalizations have been made with Ponceau S staining. Data displayed in the panels represent the most representative of independent experiments. Of note, blots have been cropped from different parts of the same gel to present the most relevant data. (N = 11).
Protein synthesis markers
The phosphorylation levels of p70S6K and 4E-BP1 were unchanged after exercise (p = 0.125 and p = 0.104, respectively). No effect of condition (p = 383 and p = 0.167, respectively) or interaction was found neither (p = 0.383 and p = 0.166, respectively). Conversely, the phosphorylation level of rpS6 significantly increased after exercise (+ 150%, p < 0.001, dz = 0.96). However, no effect of condition or interaction was detected (p = 0.800 and p = 0.798, respectively) (Fig. 5).
Protein synthesis markers expression after exercise. Total lysates were analyzed by immunoblotting using the indicated antibodies: (A) p-p70S6K, (B) p-rpS6, (C) p-4E-BP1. Western blot normalizations have been made with Ponceau S staining. Data displayed in the panels represent the most representative of independent experiments. Of note, blots have been cropped from different parts of the same gel to present the most relevant data. (N = 11).
Association between power output and autophagy
To determine whether the observed changes in the autophagy response under HYP were associated with the reduced power output, correlations analyses were performed between power output and autophagy markers. These results, normalized to body mass, are based on data from the 11 participants for whom PBMC samples were collected (Fig. 6). The total LC3B-II/LC3B-I ratio fold change did not correlate with mean or maximal power output (R = 0.215, p = 0.254; and R = 0.113, p = 0.553, respectively). Total LC3B-II fold change was not correlated with mean or maximal power output (R = 0.127, p = 0.502; and R = 0.256, p = 0.171, respectively). Finally, no correlation was found between total p62 fold change and either mean or maximal power output (R = 0.146, p = 0.441; R = 0.161, p = 0.394 respectively).
Significant correlations between fold changes in autophagy flux markers and power output during repeated sprints exercises. (A) Spearman correlation between fold change in LC3B-II/LC3B-I ratio (%) and relative mean power output (W/kg). (B) Spearman correlation between fold change in LC3B-II/LC3B-I ratio (%) and relative maximal power output (W/kg). (C) Spearman correlation between fold change in LC3B-II (%) and relative mean power output (W/kg). (D) Spearman correlation between fold change in LC3B-II (%) and relative maximal power output (W/kg). (E) Spearman correlation between fold change in p62 content (%) and relative mean power output (W/kg). (F) Spearman correlation between fold change in p62 content (%) and relative maximal power output (W/kg). BFR, blood flow restriction; HYP, hypoxia; NOR, normoxia.
Discussion
To our knowledge, this is the first study to evaluate the modulation of protein turnover markers, especially autophagy response, in PBMCs during RSE performed in hypoxia or under BFR, in humans. In the present work, we provided new insights concerning the autophagy flux markers modifications in PBMCs after very short-duration repeated exercises. Our data showed that the current RSE protocol (i.e., repeated 10-s sprint exercises) increased the autophagic flux marker LC3BII/I ratio, with a more pronounced effect under NOR conditions, whereas this response tended to be blunted under HYP. However, gene expression remained unchanged after such a short bout of exercise. Moreover, our findings showed inconsistent results regarding protein synthesis markers.
The present results suggest that short-duration repeated sprint exercises stimulate autophagy in human PBMCs. In addition to its critical function in cell survival through the removal of inefficient and damaged cell constituents17,39,40, autophagy has also been proposed to play a role in energy homeostasis41. Indeed, autophagy may contribute to the resynthesis of ATP via the formation of monosaccharides, amino acids, and fatty acids41; that would be consistent with high intensity exercises such as RSE. Recently, McCormick and coworkers highlighted other forms of short-duration intense exercises (i.e. 30-min cycling at 70% of peak oxygen consumption) promote autophagy in PBMCs in an intensity-dependent manner4,16,21. Interestingly, these authors also reported that performing exercise in the heat (40 °C with 15% relative humidity) further stimulates the expression of autophagy flux markers. Our results extend these findings from high-intensity endurance exercise to RSE (i.e. supramaximal intensity) and suggest that exercise intensity should be a critical factor to enhance autophagy. While heat stress further enhances the autophagic response, our data highlight that hypoxic stress seems to blunt this response at the protein level.
In line with the observed attenuation of autophagy under HYP, no significant correlation was found between either peak or mean power output and the expression of key autophagy markers, including the LC3B-II/LC3B-I ratio, LC3B-II levels, and p62 expression. These findings indicate that the reduced power output measured during HYP is unlikely to be the primary driver of the observed autophagy impairment. Notably, our results revealed a modulation of the LC3B-II/I ratio following exercise, while p62 expression remained unchanged. This pattern is consistent with previous findings by McCormick et al., who also reported no significant changes in p62 expression at lower exercise intensities. Taken together, our data support the hypothesis that an early autophagic response is triggered immediately after exercise, as evidenced by changes in LC3B processing, whereas downstream events such as lysosomal degradation—typically indicated by a decrease in p62 expression—may require a longer timeframe to become detectable, particularly in PBMCs.
Autophagy plays a crucial role in the activation and function of T lymphocytes within PBMCs and is essential for their survival. It is also required for the development and maintenance of B lymphocytes, as well as the long-term survival of plasma cells42. Therefore, a recurrent impairment of autophagy in PBMCs under HYP conditions warrants further investigation, particularly given the observed trend toward autophagy dysregulation in this context. Thus, repeated impairment of autophagy in PBMCs should be further of interest, since we observed a tendency of alteration of autophagy under HYP. In addition, McCormick and coworkers also reported that biological sex and ageing differentially alter autophagy response in PBMCs, probably due to hormonal modulations (i.e. 17-ß estradiol and testosterone). Indeed, it was suggested that older women are expected be most exposed to risks of impaired cellular function during high-intensity exercise conducted in the heat due to a defective autophagic response in PBMCs43. However, this question remains to be addressed for exercise performed under hypoxia or BFR. Thus, the autophagic response to RSE needs to be evaluated in female and older people to compare responses between sexes and young versus old subjects. Finally, no effect has been observed regarding gene induction, likely due to the timing inherent to this type of protocol. Indeed, the current RSE protocol has a very short duration, which probably does not allow sufficient time for enhanced mRNA production.
Furthermore, the results on proteosynthesis markers did not allow clear conclusions to be drawn. Indeed, although rpS6 phosphorylation increased following exercise, no change was observed in p70S6K and 4E-BP1 phosphorylation at this time point. Notably, divergent phosphorylation kinetics between p70S6K and its target rpS6 have been previously reported44. While it is well established that, in many cellular contexts including skeletal muscle, p70S6K1 is the main kinase responsible for rpS6 phosphorylation downstream of mTORC1, the situation in PBMCs encompassing lymphocytes and monocytes appears to be more complex. Some studies have demonstrated that rpS6 can also be phosphorylated by alternative kinases, notably the p90 ribosomal S6 kinases, which are activated via the MAPK/ERK pathway. This alternative pathway for rpS6 phosphorylation has been shown to be particularly relevant in immune cells during cytokine stimulation or under certain stress and inflammatory conditions45,46. To our knowledge, no studies have explored this differential response in PBMCs in response to exercise or metabolic stress. However, the mechanistic possibility and some indirect experimental evidence suggest that such a phenomenon is plausible, especially under conditions that activate stress- or cytokine-responsive MAPK/ERK/RSK signaling alongside or instead of mTORC1. On the other hand, 4E-BP1 phosphorylation facilitates protein translation by promoting the formation of the pre-initiation complex47. However, the phosphorylation of this protein remained unchanged in the current study, suggesting low or unchanged protein synthesis rates. It is nonetheless plausible to speculate that during an energy stressful situation such as high-intensity exercise, PBMCs prioritize catabolic processes such as autophagy over energy-requiring anabolic processes. Alternatively, these results could be explained by differences in kinetics of protein phosphorylation response in PBMCs. To gain a comprehensive overview, it would be valuable to assess the activity of other key players involved in protein translation, such as glycogen synthase kinase 3 (GSK3), which is notably targeted by Akt48, and to measure protein synthesis rates using puromycin incorporation. Finally, although the role of rpS6 phosphorylation in the regulation of protein synthesis remains controversial, it has been proposed that rpS6 contributes to modifications of ribosome function, thus promoting the translation of mRNAs encoding sarcomeric proteins in skeletal muscle49. Thus, this potential role of rpS6 remains to be elucidated in PBMCs.
Importantly, because regular exercise at moderate intensities (< 70% maximal oxygen consumption) is believed to boost the immune system, intense exercises can be followed by immunosuppression3. Thus, studies on the effect of RSE combined with other environmental stressors (e.g. hypoxia, BFR, and heat exposure) on PBMCs protein turnover (protein synthesis versus degradation) must be encouraged. Such studies will help formulate evidence-based recommendations for optimizing training protocols while reducing risks to immune health. According to our data, HYP and BFR did not exhibit a supplementary effect on proteosynthesis markers in PBMCs. One hypothesis concerning BFR is that this method does not induce systemic hypoxia but rather local stress within the exercising muscles. Consequently, BFR should promote lower stress in PBMCs while maintaining a comparable effect on protein turnover. The current study also indicates that HYP may suppress the autophagy response, potentially due to overwhelming cellular stress. Further investigation is required to validate this hypothesis and to examine additional facets of protein dynamics, such as ribosome activity, biogenesis, and mitochondrial health maintenance. These findings will play a pivotal role in formulating evidence-based approaches to mitigate disease risks and optimize wellness in both athletic and clinical populations.
Limitations
The study of autophagy in humans is complex and limitations must be acknowledged. The LC3B-II/LC3B-I ratio reflects the formation of autophagosomes and the relative expression of p62 serves as an indirect indicator of lysosomal degradation. Notwithstanding, it would also be interesting to examine certain intermediate stages, such as the maturation of autophagosomes. In this regard, two studies suggest that LC3B is mainly involved in phagophore elongation, whereas GABARAP (Gamma-aminobutyric acid receptor-associated protein) would intervene later, playing a key role in the expansion and closure of phagophore extremities50. Furthermore, the autophagic flux was not directly measured because this would require the use of autophagy inhibitors such as Bafilomycin A1, preventing autophagosome-lysosome fusion thus making it possible to measure the accumulation of autophagosomes. However, this is not feasible for in vivo human experimentation.
Conclusions and perspectives
In summary, a 5 × 10-s RSE protocol with 20 s of passive recovery promoted autophagy at the protein level in PBMCs; however, HYP potentially attenuated this response, whereas BFR did not. Furthermore, RSE effects on protein synthesis markers were mixed, and neither HYP nor BFR influenced their phosphorylation level. Considering recent data from McCormick and colleagues, further studies are necessary to compare male and female responses and to investigate the impact of ageing on the autophagic response during repeated sprint exercises. The combined effects of exercise-induced changes in PBMC homeostasis and environmental stress during RSE warrant further investigation to develop evidence-based strategies for reducing disease risk and optimizing wellness in both athletic and clinical populations. To the best of our knowledge, no study has yet linked suppressed PBMC autophagy with fatigue, performance decrement, or overtraining. Therefore, these research avenues should be pursued under more comprehensive conditions to expand upon these preliminary findings.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Da Rosa, P. C., Bertomeu, J. B., Royes, L. F. F. & Osiecki, R. The physical exercise-induced oxidative/inflammatory response in peripheral blood mononuclear cells: Signaling cellular energetic stress situations. Life Sci. 321, 121440 (2023).
Nieman, D. C. & Wentz, L. M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 8, 201–217 (2019).
Peake, J. M., Neubauer, O., Walsh, N. P. & Simpson, R. J. Recovery of the immune system after exercise. J. Appl. Physiol. 122, 1077–1087 (2017).
McCormick, J. J. et al. The effect of high-intensity exercise in temperate and hot ambient conditions on autophagy and the cellular stress response in young and older females. Am. J. Physiol. Regul. Integr. Comp. Physiol. 328, R90–R101 (2025).
Solsona, R., Sabater Pastor, F., Normand-Gravier, T., Borrani, F. & Sanchez, A. M. Sprint training in hypoxia and with blood flow restriction: Controversies and perspectives. J. Sports Sci. https://doi.org/10.1080/02640414.2024.2416839 (2024).
Faiss, R., Raberin, A., Brocherie, F. & Millet, G. P. Repeated-sprint training in hypoxia: A review with 10 years of perspective. J. Sports Sci. https://doi.org/10.1080/02640414.2024.2416821 (2024).
Camacho-Cardenosa, M. et al. Haematological responses to repeated sprints in hypoxia across different sporting modalities. Res. Sports Med. 30, 529–539 (2022).
Goods, P. S. R., Dawson, B., Landers, G. J., Gore, C. J. & Peeling, P. No additional benefit of repeat-sprint training in hypoxia than in normoxia on sea-level repeat-sprint ability. J. Sports Sci. Med. 14, 681–688 (2015).
Montero, D. & Lundby, C. No improved performance with repeated-sprint training in hypoxia versus normoxia: A double-blind and crossover study. Int. J. Sports Physiol. Perform. 12, 161–167 (2017).
Faiss, R. et al. Repeated double-poling sprint training in hypoxia by competitive cross-country skiers. Med. Sci. Sports Exerc. 47, 809–817 (2015).
Solsona, R., Berthelot, H., Borrani, F. & Sanchez, A. M. J. Mechanical, cardiorespiratory, and muscular oxygenation responses to sprint interval exercises under different hypoxic conditions in healthy moderately trained men. Front. Physiol. 12, 773950 (2021).
Solsona, R., Normand-Gravier, T., Borrani, F., Bernardi, H. & Sanchez, A. M. J. DNA methylation changes during a sprint interval exercise performed under normobaric hypoxia or with blood flow restriction: A pilot study in men. Physiol. Rep. 12, e16044 (2024).
Walsh, N. P. & Whitham, M. Exercising in environmental extremes. Sports Med. 36, 941–976 (2006).
Burtscher, J. et al. Immune consequences of exercise in hypoxia: A narrative review. J. Sport Health Sci. 13, 297–310 (2024).
Arbogast, F. & Gros, F. Lymphocyte autophagy in homeostasis, activation, and inflammatory diseases. Front. Immunol. 9, 1801 (2018).
McCormick, J. J. et al. Autophagic response to exercise in peripheral blood mononuclear cells from young men is intensity-dependent and is altered by exposure to environmental heat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 323, R467–R482 (2022).
Sanchez, A. M. J. Mitophagy flux in skeletal muscle during chronic contractile activity and ageing. J. Physiol. 596, 3461–3462 (2018).
Sanchez, A. M. J., Bernardi, H., Py, G. & Candau, R. B. Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R956-969 (2014).
Sanchez, A. M. J., Candau, R., Raibon, A. & Bernardi, H. Autophagy, a highly regulated intracellular system essential to skeletal muscle homeostasis—role in disease, exercise and altitude exposure. Muscle Cell Tissue https://doi.org/10.5772/60698 (2015).
Pant, A. et al. Interactions of autophagy and the immune system in health and diseases. Autophagy Rep. 1, 438–515 (2022).
McCormick, J. J., McManus, M. K., King, K. E., Goulet, N. & Kenny, G. P. The intensity-dependent effects of exercise and superimposing environmental heat stress on autophagy in peripheral blood mononuclear cells from older men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 326, R29–R42 (2024).
Mercer, T. J., Gubas, A. & Tooze, S. A. A molecular perspective of mammalian autophagosome biogenesis. J. Biol. Chem. https://doi.org/10.1074/jbc.R117.810366 (2018).
Russell, R. C. et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 15, 741–750 (2013).
Philp, A., Hamilton, D. L. & Baar, K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J. Appl. Physiol. 110, 561–568 (2011).
McGlory, C., Devries, M. C. & Phillips, S. M. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. J. Appl. Physiol. Bethesda Md 1985(122), 541–548 (2017).
Solsona, R., Pavlin, L., Bernardi, H. & Sanchez, A. M. Molecular regulation of skeletal muscle growth and organelle biosynthesis: Practical recommendations for exercise training. Int. J. Mol. Sci. 22, 2741 (2021).
Bolster, D. R. et al. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J. Physiol. 553, 213–220 (2003).
Pope, Z. K., Willardson, J. M. & Schoenfeld, B. J. Exercise and blood flow restriction. J. Strength Cond. Res. 27, 2914–2926 (2013).
Böyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 97, 77–89 (1968).
Suklek, A., Kayan, A., Rattanasrisomporn, J. & Boonkaewwan, C. Isolation of peripheral blood mononuclear cells and the expression of toll-like receptors in Betong chickens. Vet. World 13, 1372–1375 (2020).
Sander, H., Wallace, S., Plouse, R., Tiwari, S. & Gomes, A. V. Ponceau S waste: Ponceau S staining for total protein normalization. Anal. Biochem. 575, 44–53 (2019).
Muth, C. et al. Alternative models for small samples in psychological research: Applying linear mixed effects models and generalized estimating equations to repeated measures data. Educ. Psychol. Meas. 76, 64–87 (2016).
Gueorguieva, R. & Krystal, J. H. Move over ANOVA: Progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch. Gen. Psychiatry 61, 310–317 (2004).
Boisgontier, M. P. & Cheval, B. The anova to mixed model transition. Neurosci. Biobehav. Rev. 68, 1004–1005 (2016).
Baayen, R. H., Davidson, D. J. & Bates, D. M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 59, 390–412 (2008).
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863 (2013).
Cohen, J. A power primer. Psychol. Bull. 112, 155–159 (1992).
Cohen, J. A Power Primer. 284 (American Psychological Association, Washington, DC, US, 2016). https://doi.org/10.1037/14805-018.
Bjørkøy, G. et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 (2005).
Dokladny, K., Myers, O. B. & Moseley, P. L. Heat shock response and autophagy–cooperation and control. Autophagy 11, 200–213 (2015).
Yang, Q., Wang, R. & Zhu, L. Chaperone-Mediated Autophagy. in Autophagy: Biology and Diseases: Basic Science (ed. Qin, Z.-H.) 435–452 (Springer, 2019). https://doi.org/10.1007/978-981-15-0602-4_20
Cui, B., Lin, H., Yu, J., Yu, J. & Hu, Z. Autophagy and the Immune Response. Autophagy Biol. Dis. 1206, 595–634 (2019).
McCormick, J. J. et al. The effect of an exercise- and passive-induced heat stress on autophagy in young and older males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 328, R289–R299 (2025).
Roux, P. P. et al. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J. Biol. Chem. 282, 14056–14064 (2007).
Roux, P. P. & Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. MMBR 68, 320–344 (2004).
Ruvinsky, I. & Meyuhas, O. Ribosomal protein S6 phosphorylation: From protein synthesis to cell size. Trends Biochem. Sci. 31, 342–348 (2006).
Musa, J. et al. Eukaryotic initiation factor 4E-binding protein 1 (4E-BP1): A master regulator of mRNA translation involved in tumorigenesis. Oncogene 35, 4675–4688 (2016).
Hermida, M. A., Dinesh Kumar, J. & Leslie, N. R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv. Biol. Regul. 65, 5–15 (2017).
Chaillou, T., Kirby, T. J. & McCarthy, J. J. Ribosome biogenesis: Emerging evidence for a central role in the regulation of skeletal muscle mass. J. Cell. Physiol. 229, 1584–1594 (2014).
Weidberg, H. et al. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 29, 1792–1802 (2010).
Acknowledgements
The authors thank the participants, Mr Jean Peuplus and Fan Thomass for their cooperation and time.
Funding
This project was funded by the University of Lausanne, the University of Perpignan Via Domitia (Bonus Qualité Recherche). A grant from the Fédération de Recherche Energie-Environnement (FEDFREE) was obtained for this study. No external funding was perceived. Open-access funding was provided by the University of Lausanne.
Author information
Authors and Affiliations
Contributions
RS, RD, TNG, FB, HB and AMJS designed the study. RS, RD, TNG, FB, HB and AMJS performed experiments. RS, RD, FeB, TNG, FB, HB and AMJS analyzed the data. RS, RD, TNG, FB, HB and AMJS wrote the manuscript. RS, RD, FeB, TNG, FB, HB and AMJS reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Human ethics and consent to participate
The procedures related to the study complied with the Declaration of Helsinki on human experimentation and was approved by the ethics committee “Commission cantonale d’éthique de la recherche sur l’être humain – Canton de Vaud”: CER-VD 2022–00238. Participants were informed about the study procedures and risks before giving written informed consent to participate. IRB: not applicable (CER-VD 2022–00238).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Solsona, R., Deriaz, R., Boudry, F. et al. Systemic hypoxia appears to attenuate autophagy in human peripheral blood mononuclear cells during repeated sprint exercises. Sci Rep 15, 37663 (2025). https://doi.org/10.1038/s41598-025-21430-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-21430-7