Abstract
Upper limb dysfunction is highly prevalent among patients in the chronic stage of stroke. Brain-computer interface (BCI) technology, which creates a direct link between the brain’s electrical signals and external devices, stroke patients with motor disabilities are able to perform BCI tasks for clinical rehabilitation. However, traditional BCI applications are often limited in their capacity to monitor the brain function of patients. In this study, functional near-infrared spectroscopy (fNIRS) was employed to observe changes in brain cortex activation patterns before and after BCI use in ischemic stroke patients with upper limb dysfunction. Thirty-four ischemic stroke patients with upper limb dysfunction meeting the inclusion criteria were selected and randomly assigned to either a treatment group or a control group using a random number table, with 17 patients in each group. During the study, 4 participants dropped out, leaving 30 patients for the final statistical analysis, 15 in each group. Both groups received routine upper limb rehabilitation training. Additionally, the treatment group underwent daily BCI training for 30 min, 5 days a week, for 4 consecutive weeks. Upper limb function was evaluated using the Fugl-Meyer assessment for upper extremity (FM), and daily living activities were assessed with the modified barthel index (MBI). The six regions of interest (ROIs) in the cortex for fNIRS measurement were the ipsilesional and contralesional primary motor cortex (PMC), supplementary motor area (SMA), and somatosensory motor cortex (SMC). The three time points of measurement were baseline (prior to any treatment), 2 weeks of treatment, and 4 weeks of treatment. fNIRS was used to detect the oxygenated hemoglobin values (HbO) in six ROIs at each time point. After treatment, both groups exhibited improvements in FM and MBI scores. The treatment group demonstrated significantly greater functional gains than the control group at both 2 and 4 weeks, as reflected in FM (T1T0: 5.867 ± 3.482 vs. 3.200 ± 2.077, P < 0.01, d = 0.93; T2T0: 13.533 ± 5.705 vs. 7.133 ± 2.503, P < 0.05, d = 1.45) and MBI scores (T1T0: 13.400 ± 7.129 vs. 8.133 ± 4.357, P < 0.05, d = 0.89; T2T0: 27.867 ± 10.106 vs. 16.467 ± 7.010, P < 0.05, d = 1.31). fNIRS data revealed that after 4 weeks, the treatment group showed significantly increased oxygenated hemoglobin levels in PMC and SMA compared to baseline (PMC: P < 0.001, d = 0.62; SMA: P < 0.001, d = 0.89), along with a more pronounced PMC activation and higher brain network efficiency relative to the control group (PMC: 0.019 ± 0.017 vs. 0.007 ± 0.005, P < 0.01, d = 1.01; network efficiency: P < 0.05). Moreover, improvements in brain network efficiency were positively correlated with gains in both FM and MBI scores across the cohort. Our study suggests that BCI treatment combined with conventional medical and rehabilitation therapy can effectively enhance motor function and activities of daily living in stroke patients with upper-limb dysfunction. Additionally, it can promote cortical activation in the ipsilesional PMC and SMA regions and improve the network efficiency between brain regions.
Similar content being viewed by others
Introduction
According to reports1, stroke was the second leading cause of death globally in 2019, which will increase the economic burden in various countries, especially in low-income countries; ischemic stroke accounts for a high proportion of this2. Statistics show that Approximately 15 million people worldwide are affected by ischemic stroke each year, of which nearly one-third ultimately lose their lives, and in some countries, ischemic stroke has become one of the main causes of disability and death3. Cerebrovascular disease is mainly caused by an inadequate blood supply to the brain, usually due to blockage or narrowing of the cerebral arteries. Once the local blood supply is insufficient, brain cells begin to die rapidly, causing severe neurological damage. The risk factors for ischemic stroke include hypertension, hyperlipidemia, diabetes, smoking, unhealthy diet, lack of exercise, and heart disease, all of which are common worldwide4.
Paralysis of limbs is a common sequelae faced by stroke patients, especially the impairment of upper limb motor function is more common5. In recent years, an increasing number of studies6 have focused on the recovery of upper limb function after stroke, aiming to provide more effective rehabilitation methods. For example, Wang A, et al.7 demonstrated that 12 weeks of upper limb robotic rehabilitation significantly improved the range of motion in stroke patients’ affected limbs. This therapy utilizes robotic arms or exoskeletons to deliver passive, active, or resistance training, thereby promoting neural remodeling and muscular recovery. These methods, including upper limb robots and electrobiological feedback therapy, simulate normal movement trajectories, stimulate the brain’s neural network, and provide real-time feedback to promote muscle recovery and the improvement of motor function, not only enhance the upper limb motor ability of patients but also improve their quality of life and social self-care ability. Over the past decade, with the advancements in neuroscience and computer technology, Brain-computer interface (BCI) technology has made remarkable progress and opened up new avenues for post-stroke neurorehabilitation8. BCI technology establishes a direct link between the brain’s electrical signals and external feedback devices. This allows patients to manipulate the devices through voluntarily generated brain commands, a mechanism that has been recognized as a promising approach for neurorehabilitation9. A number of studies indicate that BCI technology shows promising effects for treating post-stroke upper limb dysfunction. Shou YZ et al.10 demonstrated that the subacute phase is likely the optimal and critical period for implementing BCI-based rehabilitation therapy. Furthermore, for patients with relatively mild conditions, BCI intervention can lead to greater improvement in upper limb function11. Hu YQ et al.12 demonstrated that motor imagery training engages patients in active movement imagination, enhancing the reconstruction of brain motor-related neural networks. When integrated with BCI technology, this process converts brain signals into executable commands and, combined with multimodal feedback, forms a closed-loop system that effectively improves motor function. Liu L et al.13 carried out in-depth monitoring research with the help of functional near-infrared spectroscopy technology and finally found that BCI-robot training can cause substantial changes in the cortical activation pattern of patients, when performing upper limb movement tasks, the activation intensity and range of the areas in the brain closely related to movement are significantly enhanced, and the functional connection between the cerebral hemispheres is also significantly enhanced. This research provides evidence that BCI-robot training can effectively stimulate neuroplasticity, contributing to the reorganization of the motor control network and subsequent improvements in upper limb function. These findings support the technology’s potential as a valuable tool in future stroke rehabilitation strategies.
This study focuses on the upper limb motor dysfunction after ischemic stroke. It innovatively combines BCI treatment with conventional rehabilitation therapy, leveraging the advantages of both to construct a more effective treatment model. During the research, the non-invasive functional near-infrared spectroscopy (fNIRS) technology is utilized to comprehensively monitor the activation changes of the brain motor cortex during the movement of the affected upper limb, including the data comparison before and after BCI treatment. The study objectives encompass two primary aspects. Firstly, it aims to evaluate the comprehensive effectiveness of the combination of BCI technology and traditional rehabilitation therapy on the recovery of upper limb motor function in stroke patients. Although there is already a research foundation for this combined therapy, there are still knowledge gaps in the optimal parameters of BCI training, such as intensity, frequency, and duration, as well as the interaction between these factors and individual characteristics of patients, such as disease course and severity. Moreover, this improvement will be accompanied by significant changes in the cortical activation pattern detected by fNIRS, specifically manifested as an increase in the activation intensity and range of the brain areas related to movement, as well as enhanced functional connectivity between the two cerebral hemispheres.
Materials and methods
General information
Inclusion criteria
① First onset, meeting the diagnostic and treatment criteria for ischemic stroke in the “China Acute Ischemic Stroke Diagnosis and Treatment Guidelines 2018“14, and confirmed without misdiagnosis by cranial CT or MRI; ② Within one month of onset, stable vital signs, clear consciousness, ability to cooperate with examination; ③ involvement of one hemisphere of the brain, Brunnstrom stage II - IV for upper limb and hand hemiplegia; ④ ability to sustain a sitting position for ≥ 20 min; ⑤ willingness to cooperate with treatment, signed informed consent by themselves or their families.
Exclusion criteria
① history of epilepsy or currently taking antiepileptic drugs; ② impaired important organ functions such as the heart, lung, liver, and kidney; ③ Unable to understand or comprehend testing instructions due to severe cognitive and communication disorders, inability to complete test tasks and movements; ④ wearing a pacemaker, having intracranial or other internal metal implants, or having cranial bone defects; ⑤ deterioration of the patient’s condition, appearance of new infarctions, or other brain diseases.
Treatment methods
Basic treatment
Both the groups received basic pharmacological treatment in the neurology department and underwent routine rehabilitation. In addition to these treatments, the treatment group also received upper-limb brain-machine interface therapy. Basic pharmacological treatment in the neurology department involved taking medications that improved circulation, nerve and platelet aggregation, controled blood pressure and blood sugar, and stabilized plaques. Routine rehabilitation included training for proper limb positioning in bed, position conversion, muscle strength and joint mobility training for the upper limb, standing and walking training, and neuromuscular electrical stimulation therapy. All the treatments were performed once daily for 40 min, five days a week, for four weeks.
Brain-machine interface therapy
In this study, during the BCI treatment, the Rehabilitation-III-Plus (RHB-III-Plus) upper-limb BCI therapy instrument of Shenzhen Ruihan Medical Technology Co., Ltd. was adopted. This instrument was mainly composed of a brain-electrical signal amplifier, an electroencephalogram (EEG) cap, an exoskeleton robotic hand, a working display screen of the brain-computer interface training system, and a computer for data acquisition and control.
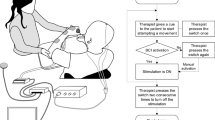
During the treatment, the patient took a sitting position on the affected side and relaxes fully. The therapist informed the patient of the training skills and precautions in advance. The EEG cap and headphones were worn for the patient, and the electroencephalogram in a quiet state for 1 min was collected as the basic data. Then, the affected forearm, wrist joint, and hand was fixed on the exoskeleton mechanical device. After selecting the upper-limb training, the screen showed the flexion and extension movements of the wrist and fingers, and the headphones gave synchronous prompts. Through visual and verbal guidance, the patients were guided to carry out the imaginary activities of the affected upper limb, so as to increase the probability of generating a correct image. The system would automatically collect and analyze the brain-electrical signals of the patient during motor imagination. Once the correct image was detected, the exoskeleton mechanical device would drive the affected upper limb of the patient to complete the same action, and the corresponding limb movement would be displayed in real-time on the screen, forming a closed-loop of motor imagination, analysis, and feedback. This brain-computer interface treatment plan was carried out once a day, 30 min each time, 5 days a week, and lasted for 4 weeks. It was expected that through systematic training, patients could make better use of motor imagery to promote the recovery of nerve and limb motor functions.
Evaluation method
Both groups of patients were evaluated for rehabilitation before treatment at two weeks and four weeks of treatment. Upper limb function was assessed using the Fugl-Meyer Assessment for Upper Extremity (FM) Score, and activities of daily living (ADL) ability was assessed using the Modified Barthel Index (MBI). Additionally, fNIRS was used to measure the concentration of oxygenated hemoglobin (HbO) in regions of interest (ROIs) in the cerebral cortex of each patient (Fig. 1).
Evaluation scales
FM score includes assessments of tendon reflexes, shoulder, elbow, wrist joint flexor, extensor synergy and dissociation movement, wrist joint stability, coordination, and speed of small joint movements (such as grasping, pinching with fingers, and finger opposition). The maximum score was 66, with higher scores indicating better upper limb function15.
MBI score assesses 10 items, including eating, bathing, dressing, grooming, toileting, chair-bed transfer, walking, stair climbing, and bladder and bowel control. The maximum score was 100, with scores above a certain threshold indicating basic self-care abilities, scores between 40 and 60 indicating moderate dependence in daily life, scores between 20 and 40 indicating severe dependence in daily life, and scores below 20 indicating complete dependence in daily life16.
fNIRS assessment
The H01B near-infrared brain function imager from China Bairuixin Intelligent Technology Co., Ltd. was used to collect 216 s near-infrared data from two patient groups. The process details are in (Fig. 2). Prior to testing, the therapist should ensure the environment remained quiet and undisturbed, instructed the patients to achieve complete physical and mental relaxation, and provided a thorough briefing on the testing procedures and necessary precautions. The test started after a 5 min break. Had patients sit steadily and comfortably. The therapist positioned the H01B imager on the patient’s head, adjusting it based on head anatomy to ensure good sensor-scalp contact for accurate data collection. Patients placed both hands on knees, stayed relaxed, and performed the upper-limb finger-nose test as directed. For the test, the subject flexed a shoulder, then the elbow, supinates the forearm, touched the nose tip with the fingers, and slowly lowered the arm. Tested both arms alternately. The subject should complete tasks to the best of their ability without using the trunk or other limbs for assistance. During data collected, patients must close eyes, stay calm, and avoid sudden head movements, facial expressions, or fidgeting. If uncomfortable, gently signaled the therapist without making large movements. Did not touch the imager during testing to avoid data interference. Ensure it was properly connected and powered on before starting. Stoped the test and notified the therapist immediately if there were abnormal lights or sounds. (Precautions: Patients should avoid caffeine, alcohol, or other stimulants 4 h before the test. If there were metal implants in the head or neck, inform the therapist in advance).
Testing instrument
The H01B type near-infrared functional imaging instrument consists of a 4 + 2 × 6 array with eight light sources, eight detectors, and a 23-channel near-infrared imaging detection system (Fig. 1). The near-infrared light sources used had wavelengths of 785 and 830 nm, with a distance of 3 cm between the adjacent light sources and detectors. The coverage area was 72cm2, acquisition time resolution was 0.1s. Regarding the detection depth, the penetration depth is affected by the thickness of an individual’s scalp and skull. As a result, there are variations in the penetration depth among different participants. It is not consistently 0.5 cm below the cortex; instead, it can be either greater or less than this value. This system uses near-infrared light imaging to detect and calculate three physiological indicators in the venous blood of the cerebral cortex: oxygenated hemoglobin (HbO), deoxygenated hemoglobin (HbR), and total hemoglobin (HbT). The unit of measurement is molar per volume (mmol/L). A simplified overview of the technical workflow for this research is illustrated (Fig. 3).
Installation of test fiber cap
The H01B type near-infrared functional imaging instrument locates brain regions using a 10–20 system. In this system, Cz (Cephalic Zero) is defined as the central reference point of the electrode positioning system, located at the intersection of the mid-sagittal line (from nasion to inion) and the coronal line (between the left and right pre-auricular points). The distance between two adjacent probes on the fiber cap was 3 cm. The positions of the regions of interest in the brain were determined outward from the point Cz., the center of detector D7 and light source L7 are Cz; the left primary motor cortex (PMC)17 area consists of channels 1, 4, 8, and 9; the supplementary motor (SMA)18 area consists of channels 2, 5, 6, and 10; the right PMC area consists of channels 3, 7, 11, and 12; the left somatosensory motor cortex (SMC)19 area consists of channels 13, 14, 15, 19, and 20; and the right PMC area consists of channels 16, 17, 18, 22, and 23 (Fig. 1).
fNIRS data processing
In this paper, the collected signals are firstly processed by morphological filtering to remove the interference of baseline drift; then the blood oxygen signal is band-pass filtered based on the infinite impulse response filter (Infinite Impulse Response, IIR), and the frequency bands are 0.01 ~ 0.02, 0.02 ~ 0.06, 0.06 ~ 0.18, 0.18 ~ 0.7 and 0.7 ~ 2.0 Hz, respectively; Finally, in order to reduce the influence of individual differences, the blood oxygen signal of the ROI is calculated based on the entropy weight method20, in which the left premotor area includes channels 1, 4, 8 and 9, and the auxiliary motor area includes channel 2, 5, 6, and 10, the right premotor area contains channels 3, 7, 11, and 12, the left sensorimotor area contains channels 13, 14, 15, 19, and 20, and the right sensorimotor area contains channels 16, 17, 18, 22 and 23.
Statistic analysis
Statistical analyses were performed using SPSS (v27.0; IBM Corp). Data normality was assessed with the Shapiro-Wilk test. Baseline characteristics were compared using independent samples t-tests, Mann-Whitney U tests, or chi-square tests, as appropriate. For efficacy analysis, within-group (paired t-test) and between-group (independent t-test) comparisons were conducted for two endpoints (FM, MBI) at two timepoints. A Bonferroni correction was applied, setting significance at P < 0.0125 (0.05/4) for these tests. The association between functional improvement and brain network efficiency change was assessed with Pearson correlation. Effect sizes were computed as Cohen’s d; P < 0.05 was considered significant for all other analyses.
Results
Comparison of general information between two groups of patients
The patients were divided into treatment and control groups, with 17 cases in each group, but four cases were lost during the study, including two cases in the treatment group (one patient refused to cooperate in the second evaluation and one case lost contact in the later stage), two cases in the control group (two cases were not followed up and evaluated in time), and the data of 30 patients were finally included in the statistical analysis. There were no statistically significant differences between the treatment and control groups regarding sex distribution (10 males, 5 females vs. 12 males, 3 females), mean age (55.27 ± 9.93 years vs. 62.60 ± 10.99 years), average disease duration (12.40 ± 4.26 days vs. 12.00 ± 3.36 days), and affected side (8 right, 7 left vs. 10 right, 5 left) (P > 0.05).
Comparison of Fugl-Meyer upper-limb function scores before and after treatment between the two groups
Before treatment, there was no statistically significant difference in the Fugl-Meyer upper limb function scores between the two patient groups (P > 0.05). In the treatment group, patients showed a significant increase in Fugl-Meyer upper-limb function scores after 2 weeks of treatment (a1 P < 0.001, Cohen’s d = 1.68, representing a large effect size according to Cohen’s criteria) and 4 weeks of treatment (b1 P < 0.001, d = 2.37) compared to before treatment. The Fugl-Meyer upper limb function scores were significantly higher after 4 weeks of treatment than after 2 weeks in the treatment group (c1P < 0.001, d = 2.33). In the control group, patients also showed a significant increase in Fugl-Meyer upper-limb function scores after 2 weeks of treatment (a0 P < 0.001, d = 1.54) and 4 weeks of treatment (b0 P < 0.001, d = 2.85) compared to before treatment. The Fugl-Meyer upper limb function scores were significantly higher after 4 weeks of treatment than after 2 weeks in the control group (c0 P < 0.001, d = 1.99). In terms of inter-group comparison, the treatment group showed a significantly greater improvement value than the control group after 2 weeks of treatment (d P = 0.017, P < 0.01, d = 0.93). After 4 weeks of treatment, the treatment group demonstrated a significantly greater improvement than the control group, with a statistically significant difference (e P = 0.01, P < 0.05, d = 1.45). Additionally, the improvement in the treatment group was still significantly higher than that in the control group after 4 weeks of treatment compared to 2 weeks, with a statistically significant difference (f P = 0.01, P < 0.05, d = 1.38) (Table 1).
Comparison of MBI scores before and after treatment between the two groups
Before treatment, the comparison between the two groups of patients showed no statistically significant differences in MBI scores (P > 0.05). Within each group, the MBI scores of patients in the treatment group significantly increased after 2 weeks of treatment (a’1 P < 0.001, d = 1.88) and 4 weeks of treatment (b’1 P < 0.001, d = 2.76). Compared to those before treatment. The scores also significantly increased after 4 weeks of treatment compared to 2 weeks of treatment in the treatment group (c’1 P < 0.001, d = 2.53). Similarly, in the control group, the MBI scores significantly increased after 2 weeks of treatment (a’0 P < 0.001, d = 1.87) and 4 weeks of treatment (b’0 P < 0.001, d = 2.35) compared to before treatment. The scores also significantly increased after 4 weeks of treatment compared to 2 weeks of treatment in the control group (c’0 P < 0.001, d = 1.73). In terms of inter-group comparison, at 2 weeks of treatment, the improvement in the treatment group was significantly better than that in the control group (d’ P = 0.021, p < 0.05, d = 0.89). After 4 weeks of treatment, the improvement in the treatment group was significantly better than that in the control group (e’ P = 0.01, P < 0.05, d = 1.31). When comparing 4 weeks of treatment to 2 weeks of treatment, the improvement in the treatment group was still significantly better than that in the control group ( f’ P = 0.04, P < 0.05, d = 1.16) (Table 2).
Near infrared detection data
Intra-group comparisons showed that the oxygenated hemoglobin level in the PMC region of the affected side before treatment in the treatment group was 0.011 ± 0.009 and that in the PMC region of the affected side in the treatment group was 0.019 ± 0.017 after 4 weeks of treatment, and the difference between the two groups was statistically significant (a P = 0.001, P < 0.01, d = 0.62). The oxygenated hemoglobin level in the SMA of the affected side before treatment was 0.007 ± 0.005 in the treatment group. Oxygenated hemoglobin in the SMA of the affected side was 0.015 ± 0.012 in the treatment group after 4 weeks of treatment, and the difference between the two groups was statistically significant (b P < 0.001, d = 0.89). No statistically significant differences were observed in the other ROI areas. Compared with before treatment, oxygenated hemoglobin in PMC region significantly increased after 4 weeks of treatment in both groups. After 4 weeks of treatment in the treatment group, oxygenated hemoglobin in PMC region on the affected side (0.019 ± 0.017) and after 4 weeks of treatment in the control group (0.007 ± 0.005). There was a statistically significant difference between the two groups (c P = 0.004, P < 0.01, d = 1.01), and there was no significant difference in oxygenated hemoglobin in other ROI regions between the two groups (Fig. 4A, B,a, b; Table 3).
Comparison of brain network parameters in two groups of patients
Further analysis of the data from near-infrared brain functional imaging technology showed that the within-group comparison showed that the brain network efficiency of the treatment group increased significantly after 4 weeks of treatment compared with before treatment (P = 0.004, P < 0.01). The between-group comparison revealed a significantly greater increase in brain network efficiency (a quantitative measure derived from computational analysis of brain network parameters) in the treatment group after 4 weeks of intervention compared to baseline, relative to the control group (P = 0.014, P < 0.05) (Fig. 4C, c).
Visual diagram of the change in HbO concentration in the test of the two groups of patients. (A) Patients with left hemisphere involvement in the treatment group. Before treatment, significant inhibition of activation in the left PMC and SMA was observed, while activation in various areas of the right hemisphere was evident. After treatment, activation in the left PMC and SMA was significantly enhanced. (B) Patients with left hemisphere involvement in the control group. Before treatment, significant inhibition of activation in the left PMC and SMA was observed. After treatment, there was also a certain degree of activation in the left PMC and SMA. (a,b) Oxygenated hemoglobin concentration in different brain regions in the two groups. (C) The color of the line represents the strength of the connection. (c) Brain network parameter values of two groups.
Further correlation analysis showed that the improvement in MBI of the two groups of patients was positively correlated with changes in brain network connectivity efficiency. In the control group, T1 compared to T0, P = 0.0394, T2 compared to T0, P = 0.0437, T2 compared to T1, P = 0.0271. In the treatment group: T1 compared to T0, P = 0.0185. T2 compared to T0, P = 0.0129. T2 compared to T1, P = 0.0124. Similarly, the improvement in FM was also positively correlated with changes in brain network connectivity efficiency.In the control group: T1 compared to T0, P = 0.0136. T2 compared to T0 P = 0.0180. T2 compared to T1 P = 0.0206. In the treatment group, T1 compared to T0, P = 0.0136. T2 compared to T0, P = 0.0024. T2 compared toT1, P = 0.0196 (Fig. 5).
Correlation between changes in network efficiency and functional improvement values at different time points before and after treatment in the two groups. Correlation between the improvement of Fugl-Meyer upper-limb function score and the improvement value of MBI score and the change in network efficiency.
Discussion
This study demonstrated that the BCI-based intervention yielded substantial effects in improving upper limb motor function in stroke patients, with the improvements being particularly pronounced after 4 weeks of treatment (ΔFM: 13.533 ± 5.705 vs. 7.133 ± 2.503, P < 0.05, d = 1.45). Similarly, a significantly greater improvement was observed in the treatment group for the Modified Barthel Index (ΔMBI: 27.867 ± 10.106 vs. 16.467 ± 7.010, P < 0.05, d = 1.31). These findings were not only statistically significant but also reflected large effect sizes, indicating considerable clinical benefits of the intervention. Furthermore, functional near-infrared spectroscopy (fNIRS) data revealed that after 4 weeks, the treatment group exhibited significantly higher activation in the ipsilesional primary motor cortex (PMC) compared to the control group (P < 0.05), with a large effect size (d = 1.01). Additionally, within the treatment group, significant increases in activation from baseline to 4 weeks were observed in both the ipsilesional PMC and supplementary motor area (SMA) (P < 0.05 and P < 0.01, respectively), accompanied by moderate (d = 0.62) and large (d = 0.89) effect sizes. These results suggest that 4 weeks of BCI therapy can lead to significant improvements in both motor function and activities of daily living in patients with early-stage ischemic stroke, paralleled by a reduction in inhibition and enhanced activation in key motor regions such as the affected PMC and SMA.
As highlighted by Dominick et al.21, a fundamental distinction between brain-computer interface (BCI) rehabilitation therapy and conventional rehabilitation for upper limb training lies in its ability to precisely detect attempts at voluntary movement, by establishing a real-time link between robotic systems and the patient’s brain signals associated with motor execution or imagery, the BCI system can effectively assist stroke patients in completing intended actions. Research on BCI for improving upper-limb dysfunction after stroke has primarily focused on elucidating its underlying mechanisms. These include the activation of motor-related brain regions, reorganization of brain networks, induction of neuroplasticity, and regulation of neurotransmitters22,23. A study by Remsik et al.24 provided empirical support, demonstrating that BCI technology promotes motor recovery, likely through engaging neural circuits in areas such as the motor and pre-motor cortices. Building upon this mechanistic understanding, the present study applied a BCI-based intervention to patients with ischemic stroke to investigate its effect on upper-limb motor function.
The fNIRS technique, with its advantages of low cost, noninvasiveness, and continuous dynamic monitoring of cerebral oxygenation changes, can be an effective means of assessing post-stroke brain function and motor evaluation25. The use of fNIRS to detect cerebral oxygen saturation and cerebral hemodynamics has become a popular topic in post-stroke brain function imaging26,27. fNIRS and its parameters provide important tools for understanding the network mechanisms of brain function, and can detect the recovery of motor function during the rehabilitation process of stroke28. The ROIs selected to evaluate brain regions related to motor function were closely related to the preparation and execution of voluntary movements. The PMC and SMA areas are clusters of neurons activated extensively during the motor preparation stage29, which mainly involves cognition and judgment of the movement target and environment and the formulation of detailed plans for completing the movement30. The main functions of the PMC include participation in the formation of motor intentions, development of movement plans, and organization of movement commands31. The main functions of SMA include organizing and integrating movement sequences, selecting movement plans, setting movement parameters, and monitoring movements32,33. The SMC is a widely activated brain area during motor execution. The main task was to transform the movement plan into detailed procedures for each movement unit and drive muscle contractions to complete the movement using movement commands. The SMC area consists of the precentral gyrus (M1 area) for the final output pathway of movement, which conveys the movement signal and activates the movement units, and the postcentral gyrus (S1 area) for encoding and processing the sensory information34.
Prior to treatment, both groups exhibited insignificant activation or inhibition in the ROI of the affected hemisphere. This observation may be attributed to two key post-stroke recovery mechanisms: the “compensation mechanism,” where neural circuit reorganization induces experience-dependent neuroplasticity to functionally compensate for impairments32, and the “release phenomenon,” characterized by the release of tonic inhibition leading to pathological hyperactivation in distal, connected brain regions35. These findings align with established hemisphere recovery mechanisms reported in prior studies35,36. Through brain-machine interface treatment, the blood oxygen concentration in the PMC and SMA brain regions of the affected hemisphere significantly increased, improving the previously inhibited condition and facilitating the recovery of upper limb function. In addition, after four weeks of treatment, the activation of the affected hemisphere PMC area in the treatment group was greater than that in the control group, and the improvement in upper-limb motor function in the treatment group was superior to that in the control group, indicating a positive correlation between the enhancement of activation in the affected hemisphere PMC area and the recovery of upper-limb motor function. In terms of brain network parameters, the treatment group showed a greater increase in network efficiency, indicating better connectivity efficiency between brain regions, higher information transmission, wider coverage of the brain network, and greater activation of brain regions during tasks. These results demonstrate, from the perspectives of cortical activation and network parameters, that BCI treatment can effectively enhance the activation of the brain region of the affected hemisphere, improve brain network efficiency, enhance connectivity between brain regions, thereby promoting the improvement of upper-limb motor function after stroke.
This study utilized fNIRS to explore the impact of BCI technology on patients with ischemic stroke before and after treatment. The treatment effect is not only reflected in local brain areas but also involves the optimization and regulation of the entire brain network function. Future research can further investigate the influence of BCI on brain network connectivity and overall synergy, which will help us understand its mechanisms and improve treatment effectiveness.
In addition, this study still has some limitations: the number of cases included in the study is small; the differences in the activation of the cerebral cortex during upper-limb movement in patients with different stroke lesions have not been further analyzed; and further research is needed to test the changes in limb function during a longer recovery period after brain-machine interface treatment for patients. Simultaneously factors such as physiological noise, motor imagery and visual perception influence fNIRS motor testing, these limitations should be explored further in future studies.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- BCI:
-
Brain-computer interface
- fNIRS:
-
Functional near-infrared spectroscopy
- MBI:
-
Barthel index
- HbO:
-
Oxygenated hemoglobin
- ROIs:
-
Regions of interest
- HbR:
-
Deoxygenated hemoglobin
- HbT:
-
Total hemoglobin
- PMC:
-
Primary motor cortex
- SMA:
-
Supplementary motor
- SMC:
-
Somatosensory motor cortex
References
List Valery, L. et al. Global,regional,and National burden of stroke and its risk factors,1990–2019:a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 20 (10), 795–820 (2021).
Welten, S. J. G. C., Onland-Moret, N. C., Boer, J. M. A., Verschuren, W. M. M. & van der Schouw, Y. T. Age at menopause and risk of ischemic and hemorrhagic stroke. Stroke 52 (8), 2583–2591. https://doi.org/10.1161/STROKEAHA.120.030558 (2021).
Daniel, T. et al. Factors influencing the decline in stroke mortality:a statement from the American heart association/American stroke association. Stroke 45 (1), 315–353 (2014).
Meschia, J. F. et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American heart association/ American stroke association. Stroke 45 (12), 3754–3832. https://doi.org/10.1161/STR.0000000000000046 (2014).
Kashoo, F. Z. et al. Effect of transcranial direct current stimulation augmented with motor imagery and Upper-Limb functional training for Upper-Limb stroke rehabilitation: A prospective randomized controlled trial. Int. J. Environ. Res. Public Health 19 (22). https://doi.org/10.3390/ijerph192215199 (2022).
Hu, J. et al. Rehabilitation of motor function after stroke: A bibliometric analysis of global research from 2004 to 2022. Front. Aging Neurosci. 14, 1024163. https://doi.org/10.3389/fnagi.2022.1024163 (2022).
Zhao, M., Wang, G., Wang, A., Cheng, L. J. & Lau, Y. Robot-assisted distal training improves upper limb dexterity and function after stroke: a systematic review and meta-regression. Neurol. Sci. 43 (3), 1641–1657. https://doi.org/10.1007/s10072-022-05913-3 (2022).
Marco Franceschini, S. et al. Upper limb robot-assisted rehabilitation versus physical therapy on subacute stroke patients: A follow-up study. J. Bodyw. Mov. Ther. 24 (1), 194–198 (2020).
Smith, J. Design and assessment of a lightweight underactuated RACA hand exoskeleton for neurorehabilitation. Int. J. Neurorehabilitation. 9 (8), 3. https://doi.org/10.37421/2376-0281.2022.9.482 (2022).
Shou, Y. Z., Wang, X. H. & Yang, G. F. Verum versus Sham brain-computer interface on upper-limb function recovery after stroke: A systematic review and meta-analysis of randomized controlled trials[J]. Med. (Baltim). 102 (26), e34148. (2023).
Li, D. et al. Effects of brain-computer interface based training on post-stroke upper-limb rehabilitation: a meta-analysis. J. Neuroeng. Rehabil. 22 (1), 44. https://doi.org/10.1186/s12984-025-01588-x (2025).
Hu, Y. Q. et al. Motor Imagery-Based Brain-Computer interface combined with multimodal feedback to promote motor function after stroke: A preliminary study. Evid. Based Complement. Alternat Med. 2021, 1116126. https://doi.org/10.1155/2021/1116126 (2021).
Liu, L. et al. Brain-Computer Interface-Robot training enhances upper extremity performance and changes the cortical activation in stroke patients: A functional Near-Infrared spectroscopy study. Front. Neurosci. 16, 809657. https://doi.org/10.3389/fnins.2022.809657 (2022).
Gillian, E. et al. A systematic review and synthesis of global stroke guidelines on behalf of the world stroke organization. Int. J. Stroke. 18 (5), 499–531. https://doi.org/10.1177/17474930231156753 (2023).
Katia, D. R. et al. Fugl-Meyer assessment scores are related with kinematic measures in people with chronic hemiparesis after stroke. J. Stroke Cerebrovasc. Dis. 29 (1), 104463 (2020).
Hamza, Y. et al. Task-based mirror therapy enhances the upper limb motor function in subacute stroke patients: a randomized control trial.Eur. J. Phys. Rehabil Med. 56 (3), 265–271. https://doi.org/10.23736/S1973-9087.20.06070-0 (2020).
Frost, S. B., Barbay, S., Friel, K. M., Plautz, E. J. & Nudo, R. J. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J. Neurophysiol. 89 (6), 3205–3214. https://doi.org/10.1152/jn.01143.2002 (2003).
Pulverenti, P. T. S. et al. Neurophysiological changes after paired brain and spinal cord stimulation coupled with locomotor training in human spinal cord injury. Front. Neurol. https://doi.org/10.3389/fneur.2021.627975 (2021).
Huo, C. et al. Effectiveness of unilateral lower-limb exoskeleton robot on balance and gait recovery and neuroplasticity in patients with subacute stroke: a randomized controlled trial. J. Neuroeng. Rehabil. 10.1186/ s12984-024-01493-9 (2024).
Du, Y. et al. Optimization of extraction or purification process of multiple components from natural products: Entropy weight method combined with Plackett-Burman design and central composite design. Molecules (Basel, Switzerland) https://doi.org/10.3390/molecules26185572 (2021).
Baniqued pDE, Stanyer, E. C. et al. Brain-computer interface robotics for hand rehabilitation after stroke: a systematic review. J. Neuroeng. Rehabil. 18 (1), 15. https://doi.org/10.1186/s12984-021-00820-8 (2021).
Choi, J. Y. et al. Myelin water imaging of moderate to severe diffuse traumatic brain injury. Neuroimage Clin. 22, 101785. https://doi.org/10.1016/j.nicl.2019.101785 (2019).
Opris, I. et al. Editorial: application of neural technology to Neuro-Management and Neuro-Marketing. Front. Neurosci. 14, 53. https://doi.org/10.3389/fnins.2020.00053 (2020).
Remsik, A. B. et al. Ipsilesional mu rhythm desynchronization and changes in motor behavior following post stroke BCI intervention for motor rehabilitation. Front. Neurosci. 13, 53. https://doi.org/10.3389/fnins.2019.00053 (2019).
Ferrari, M. & Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 63 (2), 921–935. https://doi.org/10.1016/j.neuroimage.2012.03.049 (2012).
Lee Friesen, C. et al. Home-based portable fNIRS-derived cortical laterality correlates with impairment and function in chronic stroke. Front. Hum. Neurosci. 16, 1023246. https://doi.org/10.3389/fnhum.2022.1023246 (2022).
Mihara, M. et al. Effect of neurofeedback facilitation on poststroke gait and balance recovery: A randomized controlled trial. Neurology 96 (21), e2587–e2598. https://doi.org/10.1212/WNL.0000000000011989 (2021).
Liu, M., Wan, C., Wang, C. & Li, X. Effects of intermittent theta burst stimulation on upper limb motor recovery in early stroke patients: an fNIRS study. Front. Neurol. 16, 1542827. https://doi.org/10.3389/fneur.2025.1542827 (2025).
Golestanirad, L. et al. RF-induced heating in tissue near bilateral DBS implants during MRI at 1.5 T and 3T: the role of surgical lead management. Neuroimage 184, 566–576. https://doi.org/10.1016/j.neuroimage.2018.09.034 (2019).
Churchland, M. M. et al. Neural population dynamics during reaching. Nature 487 (7405), 51–56. https://doi.org/10.1038/nature11129 (2012).
Bernard, G. & Schreurs Changes in cerebellar intrinsic neuronal excitability and synaptic plasticity result from eyeblink conditioning. Neurobiol. Learn. Mem. 166, 107094. https://doi.org/10.1016/j.nlm.2019.107094 (2019).
Toshi Nakajima, R. et al. Covert representation of second-next movement in the pre-supplementary motor area of monkeys. J. Neurophysiol. 101 (4), 1883–1889. https://doi.org/10.1152/jn.90636.2008 (2009).
Nishiyama, J. Genome editing in the mammalian brain using the CRISpR-Cas system. Neurosci. Res. 141, 4–12. https://doi.org/10.1016/j.neures.2018.07.003 (2019).
O’Hearn, K. et al. Representational similarity analysis reveals atypical age-related changes in brain regions supporting face and car recognition in autism. Neuroimage 209, 116322. https://doi.org/10.1016/j.neuroimage.2019.116322 (2020).
Jiang, Y., Chadwick, S. R. & Lajoie Endoplasmic reticulum stress: The cause and solution to Huntington’s disease. Brain Res. 1648 (pt B), 650–657. https://doi.org/10.1016/j.brainres.2016.03.034 (2016).
Stefanie, D. et al. Changes of cerebral network activity after invasive stimulation of the mesencephalic locomotor region in a rat stroke model. Exp. Neurol. 347, 113884. https://doi.org/10.1016/j.expneurol.2021.113884 (2022).
Funding
National Key Research and Development Program (2022YFC2009700), Suzhou University horizontal project, code H201173, Jiangsu Provincial Natural Science Foundation General project (SBK2021020630).
Author information
Authors and Affiliations
Contributions
Juan Zhang and Zhi-Yin Zhang designed the research. Juan Zhang and Yin-Long Wang provided data collection and the clinical samples. Bin Zhou and Chun-Ya Xia provided data analysis support. Juan Zhang, Zhi-Yin Zhang, Yin-Long Wang, Bin Zhou wrote the manuscript and Min Su and Chun-Guang Li revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
This study was conducted in accordance with the ethical standards of the 1975 Declaration of Helsinki and was approved by the Internal Review Committee of Dushu Lake Hospital Affiliated to Soochow University (4th Hospital Affiliated to Soochow University) (Approval No. 240005). https://www.chictr.org.cn/ is ChiCTR2400082458 identification number. Informed consent was obtained from all participants and their legal guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Zhang, ZY., Wang, YL. et al. Unveiling the upper-limb functional recovery mechanisms in stroke patients using brain-machine interfaces: a near-infrared functional imaging-based study. Sci Rep 15, 39704 (2025). https://doi.org/10.1038/s41598-025-23267-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-23267-6