Abstract
Currently, there is relatively limited research regarding the relationship between the red blood cell distribution width to platelet ratio (RPR) and the prognosis of patients with acute ischemic stroke (AIS). Therefore, this study aims to investigate the association between RPR and the incidence of unfavorable functional outcomes in AIS patients. This study utilized a prospective cohort design and included 1,682 patients who had been diagnosed with AIS and were treated at Shenzhen Second People’s Hospital from January 2022 to June 2024. To evaluate the relationship between RPR and the incidence of 90-day unfavorable outcomes, a binary logistic regression model was employed. Furthermore, an additional logistic regression model that included cubic spline functions was utilized to investigate possible nonlinear associations between them. A range of sensitivity analyses and subgroup analyses were conducted to strengthen the reliability of the results. After adjusting for confounding variables, the binary logistic regression analysis demonstrated that for each 0.1 unit increase in RPR, the incidence of unfavorable outcomes at 90 days for AIS patients increased by 45.5% (OR = 1.455, 95% CI: 1.268–1.669). Additionally, the study found a nonlinear relationship between RPR and 90-day unfavorable outcomes, with an inflection point occurring at RPR = 0.33. On the left side of the inflection point, the OR for the relationship between RPR (per 0.1 unit) and 90-day unfavorable outcomes was 1.708 (95% CI: 1.403–2.080). On the right side of the inflection point, the OR for their relationship was 0.942 (95% CI: 0.630–1.410). Sensitivity analysis further confirmed the reliability of these results. This study identifies a distinct positive link between RPR and 90-day unfavorable outcomes in patients with AIS. Additionally, a non-linear relationship was observed in the relationship between them. Specifically, when the RPR value falls below 0.33, a significant positive association is noted. These findings offer valuable insights for improving rehabilitation strategies and enhancing clinical management for AIS patients.
Similar content being viewed by others
Introduction
Acute ischemic stroke (AIS) is a major global cause of disability and mortality, representing a significant socioeconomic challenge1,2. Despite notable progress in the acute treatment and rehabilitation of AIS, accurately predicting neurological outcomes for affected individuals remains a considerable challenge3. The identification and application of dependable prognostic indicators are essential for effective risk stratification, tailoring treatment approaches, and enhancing patient outcomes4. Currently, key prognostic factors commonly acknowledged in AIS include age, hypertension, the underlying cause of the stroke, and diabetes mellitus (DM)5,6,7.
The inflammatory response is believed to be crucial during various pathological and physiological phases of AIS. When brain tissue is damaged, pro-inflammatory chemical mediators are released, initiating a robust inflammatory response8,9,10. Research indicates that the intensity of this inflammation is significantly linked to the clinical prognosis for patients with AIS11,12. Recently, the ratio of red blood cell distribution width (RDW) to platelet count (RPR) has gained attention as a new inflammatory marker. Studies showed that RPR is often significantly elevated and is closely related to the severity of the inflammatory response, in several acute or chronic inflammatory diseases (such as sepsis, acute pancreatitis, systemic lupus erythematosus, etc.)13,14,15. At the same time, it has been shown to predict outcomes in several conditions, including ST-segment elevation myocardial infarction, liver fibrosis, malignancies, and chronic liver disease16,17,18. In addition, elevated RDW levels have been confirmed as a reliable indicator of systemic inflammatory status, and early studies have shown that higher RDW levels are positively correlated with poor prognosis in AIS patients19,20. Moreover, platelet counts appear to provide a protective effect, showing a significant negative association with poor functional outcomes21,22. Therefore, we hypothesize that a potential positive relationship may exist between RPR and unfavorable outcomes in AIS patients.
Regrettably, there is currently limited research examining the association between RPR and the risk of unfavorable outcomes in AIS patients. One study involving 235 AIS patients treated with intravenous thrombolysis23 and another with 286 AIS patients who underwent mechanical thrombectomy24—identified a significant association between elevated RPR and short-term negative outcomes. However, both investigations utilized small sample sizes and focused on specific patient populations, and the potential nonlinear association between RPR and unfavorable outcomes was not explored. Furthermore, the studies differed in several aspects, including study design, the range of RPR values, demographic distributions of sex, definitions of functional outcomes, and adjustment variables. As a result, the association between RPR and short-term prognosis in the broader AIS population in China remains uncertain. Therefore, a prospective cohort study has been launched to explore the relationship between RPR and the risk of unfavorable outcomes in patients with AIS, which may provide valuable insights for developing rehabilitation strategies.
Methods
Study design and population
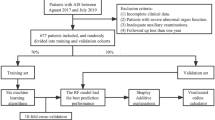
This study was a prospective cohort study. During the period from January 2022 to June 2024, patients who presented to the Stroke Center of Shenzhen Second People’s Hospital and were diagnosed with AIS by computed tomography (CT) or magnetic resonance imaging (MRI) and were aged over 20 years totaled 2,106 people. Among them, 147 people refused to participate in this study, and initially, a total of 1,959 AIS patients were included.
The criteria for exclusion included the following: (i) Participants with AIS onset of more than one week(n = 91); (ii) Participants without follow-up at 3 months post-discharge, refused to participate in follow-up, or those for whom the 90-day modified Rankin Scale (mRS) score could not be assessed during the follow-up(n = 149); and (iii) participants with incomplete data on RDW or platelet count (n = 25), as well as those exhibiting extreme and abnormal RPR values (deviation exceeding three standard deviations from the mean) (n = 12). In total, 1,682 participants were included in the final analysis. Figure 1 illustrated the participant selection process.
Ethical approval and consent
Approval for the study was granted by the ethics review board of Shenzhen Second People’s Hospital (Ethics Approval Number: 2023-305-01PJ), and the research was carried out following the ethical principles established in the Declaration of Helsinki of 1964 and its later modifications, along with other relevant ethical guidelines. Written informed consent was secured from all participants included in this study.
Variables
Red blood cell distribution width to platelet count ratio
The RPR value is calculated using the following formula: RPR = RDW/platelet count25. In this formula, RDW refers to the standard deviation of red blood cell volume, measured in femtoliters (fL), and the platelet count is expressed in 10⁹/L. Both RDW and platelet count are measured within 24 h after hospital admission.
Assessment of clinical outcome and follow-up
At 90 days following the onset of AIS, trained follow-up personnel, who have a thorough understanding of the mRS scoring criteria, assessed patients through face-to-face interviews or telephone interviews to collect data on patients’ functional status. During these assessments, we employed the mRS to evaluate functional outcomes, which is a commonly used tool for evaluating functional recovery and independence in daily living among patients with stroke or other neurological diseases. The mRS score ranges from 0 to 6, reflecting various functional states from no symptoms to death26. The primary endpoint of this study was the neurological functional outcome at 90 days, classified into two categories: unfavorable (mRS ≥ 3) and favorable (mRS < 3) outcomes27,28. To ensure the accuracy of follow-up, we established a patient information contact database and conducted multiple phone calls to remind patients to participate in the follow-up, thus reducing the occurrence of follow-up loss.
Covariates
Covariates were chosen based on our clinical expertise and prior studies4,29,30,31. The variables identified for inclusion as covariates included: (i) categorical variables including coronary heart disease (CHD), sex, history of prior stroke or transient ischemic attack (TIA), pneumonia, hypertension, atrial fibrillation (AF), DM, smoking status, and stroke etiology; and (ii) continuous variables such as neutrophil count (NEU), age, lymphocyte count (LYC), total cholesterol (TC), the initial score on the National Institutes of Health Stroke Scale (NIHSS) upon admission, triglycerides (TG), fibrinogen (FIB), fasting plasma glucose (FPG), body mass index (BMI), homocysteine (HCY), serum albumin (ALB), low-density lipoprotein cholesterol (LDL-C), hemoglobin concentration (HGB), high-density lipoprotein cholesterol (HDL-C), and D-dimer levels.
Data gathering and measurement
At the time of admission to the hospital, specialized research coordinators systematically gathered baseline data pertaining to patients’ demographic information and medical histories. This data included information on prior strokes, smoking status, AF, CHD, hypertension, and DM. Neurologists assessed stroke severity at admission using the NIHSS. Stroke subtypes were categorized based on the criteria established by the Trial of Org 10,172 in Acute Stroke Treatment (TOAST). Blood samples were gathered within 24 h following patient admission and were later examined at the laboratory of Shenzhen Second People’s Hospital. Qualified technicians adhered to strict quality control protocols during laboratory evaluations, ensuring the confidentiality of patients’ baseline data.
Addressing missing data
In this study, certain covariates exhibited missing data, with the corresponding counts and percentages of absent entries listed as follows: FIB (5, 0.30%), FPG (21, 1.25%), TG (40, 2.38%), HDL-c(40, 2.38%), LDL-c (40, 2.38%), TC (41, 2.44%), HCY (119, 7.07%), and NIHSS score (207, 12.30%). Missing data can undermine the statistical validity of the sample analyzed during the modeling phase. To minimize the bias resulting from these missing variables, we utilized multiple imputation techniques to address the unavailable data32,33. The covariates utilized in the imputation model included age, NEU, LYC, NIHSS score at the time of admission, HGB, RDW, platelet count, BMI, FIB, D-dimer, HCY, TG, HDL-c, ALB, FPG, TC, LDL-c, sex, history of previous stroke or TIA, DM, hypertension, AF, pneumonia, CHD, smoking status, and stroke etiology. The imputation procedure was executed using a linear regression method across ten iterations. The analysis of the missing data was based on the assumption of missing at random (MAR)33.
Analysis of statistical
Baseline variables were categorized by the quartiles of RPR, facilitating the comparison of characteristics across the groups. Continuous variables with a Gaussian distribution were summarized as means and standard deviations, while non-normally distributed variables were represented using medians and interquartile ranges. Categorical data were expressed as frequencies and percentages. Both analyses of variance (ANOVA) and the Kruskal-Wallis H test were applied to continuous variables, whereas the chi-square (χ²) test was used to evaluate differences among the RPR groups for categorical variables.
This study utilized univariate and multivariate binary logistic regression analyses to develop three distinct models examining the relationship between RPR and the incidence of unfavorable outcomes 90 days after AIS. The models consisted of: (i) Model I: no covariate adjustments; (ii) Model II: adjusted for sex, age, and BMI; and (iii) Model III: adjusted for age, smoking, BMI, CHD, sex, TG, HGB, LDL-c, D-dimer, FPG, stroke etiology, hypertension, HDL-c, DM, and initial NIHSS score.
In order to enhance the reliability of the results, we carried out multiple sensitivity analyses. Initially, RPR was transformed into a categorical variable according to its quartiles, and the trend P-value was computed to evaluate the results of RPR as a continuous variable while investigating the possibility of non-linearity. Second, to address the influence of obesity, hypertension, and DM on the prognosis of AIS patients, we conducted a sensitivity analysis by excluding individuals with a BMI greater than or equal to 28 kg/m², as well as those with hypertension and DM34,35,36. In addition, we calculated the E-value to evaluate the potential impact of unmeasured confounders on the link between RPR and 90-day unfavorable outcomes37.
A logistic regression model using restricted cubic spline functions was employed to explore the potential non-linear association between RPR and 90-day unfavorable outcomes in AIS patients. A recursive approach was utilized to identify the inflection point if a non-linear association was detected. After identifying the inflection point, separate binary logistic regression models were developed for each side of this threshold. The likelihood ratio test was used to select the model that best represented the relationship.
Stratified binary logistic regression models were applied to conduct subgroup analyses across several categories, such as pneumonia, TG, sex, age, history of previous stroke or TIA, AF, FIB, ALB, hypertension, BMI, Smoking, and CHD. In this analysis, continuous variables like TG FIB, ALB, BMI, and age were categorized based on clinically relevant cutoffs. Specifically, TG was categorized using a threshold of 1.7 mmol/L, BMI by 28 kg/m², FIB by 4 g/L, and ALB by 35 g/L35,38,39. Adjustments were made for sex, LDL-c, CHD, TG, stroke etiology, age, hypertension, Scr, HDL-c, platelet count, FPG, smoking status, DM, and initial NIHSS score, while excluding the stratification variables. Likelihood ratio tests were conducted to evaluate the existence of interaction terms by comparing models that included these terms to those that did not. Finally, a receiver operating characteristic (ROC) curve was constructed to assess the predictive capability of RPR, RDW, and platelet count for unfavorable outcomes in AIS patients.
All findings were written in line with the STROBE statement40. Statistical analyses were executed using Empower® software (version 4.2) and R (version 3.4.3). A two-tailed p-value of less than 0.05 was considered statistically significant.
Results
Participants’ characteristics
Table 1 presented the demographic and clinical characteristics of the study participants. A total of 1,682 individuals were included in the final analysis, with males accounting for 62.54% of this group. RPR demonstrated an approximately normally distributed, ranging from 0.03 to 0.62, with a mean (± standard deviation, SD) of 0.19 (± 0.09)(Fig. 2). Participants were categorized into distinct subgroups based on the quartiles of RPR: Q1(<0.14), Q2(0.14–0.18), Q3(0.18–0.23), and Q4(≥ 0.23). Compared with Q1, higher levels of RDW, FIB, and FPG, as well as lower levels of Neu, platelets, ALB, and D-dimer, were observed in participants of the higher RPR quartiles. Additionally, higher proportions of males, smokers, and incidence of pneumonia were found in the higher RPR quartiles compared to Q1, while a lower incidence of hypertension was noted.
Incidence of unfavorable outcomes 90-day in AIS patients
Table 2 presented the incidence of 90-day unfavorable outcomes in AIS patients. The findings reveal that 295 participants encountered unfavorable outcomes, leading to a total incidence rate of 17.78%. Particularly, the unfavorable outcome incidence rates for the first to fourth quartiles of RPR were 10.69%, 12.14%, 21.19%, and 27.08%, respectively.
Relationship between RPR and 90-day unfavorable outcomes in AIS patients
To further investigate the relationship between RPR and the risk of unfavorable outcomes in AIS patients at 90 days, three distinct binary logistic regression models were developed (Table 3). In Model I, a 0.1-unit increase in RPR is associated with a 64.9% rise in the incidence of 90-day unfavorable outcomes (OR = 1.649, 95% CI: 1.456–1.867). In Model II, each 0.1-unit increase in RPR was linked to a 49.1% higher risk of 90-day unfavorable outcomes among AIS patients (OR = 1.491, 95% CI: 1.311–1.694). Similarly, Model III indicated that a 0.1-unit rise in RPR was associated with a 45.5% increase in 90-day unfavorable outcomes (OR = 1.455, 95% CI: 1.268–1.669).
Furthermore, RPR, which was initially examined as a continuous variable, was subsequently categorized for further investigation. With the lowest quartile (Q1) serving as the reference, multivariate adjustment revealed that the OR for unfavorable outcomes was 1.186 (95% CI: 0.738–1.906) in Q2, 2.497 (95% CI: 1.635–3.813) in Q3, and 2.455 (95% CI: 1.623–3.714) in Q4. Confidence interval analysis revealed that, compared with Q1, participants in Q2 showed no statistically significant difference in 90-day unfavorable outcomes, whereas those in Q3 and Q4 exhibited a significantly higher risk of unfavorable outcomes. Additionally, the trend analysis for effect size yielded statistically significant results (P for trend < 0.05) (Table 3, Model III).
Sensitivity analysis
Multiple sensitivity analyses were conducted to enhance the validity of the findings (Table 4). First, individuals with a BMI of 28 kg/m² or higher were excluded. After adjustments for confounders, results showed a positive link between RPR and the risk of unfavorable outcomes at 90 days in AIS patients, with an OR of 1.509 (95% CI: 1.310, 1.739). Excluding those with hypertension produced similar findings, demonstrating an OR of 1.467 (95% CI: 1.198–1.797) for unfavorable outcomes at 90 days. Additionally, when patients without DM were analyzed, a significant association between RPR and 90-day unfavorable outcomes remained evident (OR = 1.708, 95% CI: 1.437–2.030).
Furthermore, the E-value was calculated to assess the potential impact of unmeasured confounding variables on the study outcomes. The determined E-value was 1.7, which surpasses the relative risk associated with unmeasured confounders and RPR (1.45). This finding suggests that unrecognized confounding factors have a negligible effect on the association between RPR and unfavorable outcomes at 90 days in AIS patients. The results from all sensitivity analyses further support the reliability of the conclusions drawn in this study.
Generalized additive model (GAM) for analyzing nonlinear association between RPR and 90-Day unfavorable outcomes
Using a logistic regression model with cubic spline functions, a non-linear relationship between RPR and 90-day unfavorable outcomes was identified in AIS patients (p for nonlinearity < 0.05, Fig. 3). The analysis adjusted for several covariates, including age, hypertension, BMI, sex, HDL-c, HGB, smoking, initial NIHSS score, stroke etiology, D-dimer, TG, FPG, CHD, DM, and LDL-c. A recursive analysis identified an inflection point for RPR at a value of 0.33. Subsequently, a piecewise logistic regression model was applied to calculate OR and CI on both sides of the inflection point. On the left of this inflection point, the OR indicating the association between RPR and the risk of unfavorable outcomes at 90 days was 1.708 (95% CI: 1.403–2.080). On the right side of the inflection point, the OR was 0.942 (95% CI: 0.630–1.410), which did not achieve statistical significance (Table 5).
Results of subgroup analysis
In all predefined or exploratory subgroup analyses (Table 6), no significant interactions were found between RPR and factors such as age, sex, TG, history of stroke or TIA, CHD, AF, FIB, ALB, hypertension, BMI, smoking, and pneumonia (all p ≥ 0.05). This suggests that these variables do not influence or modify the association between RPR and 90-day unfavorable outcomes in AIS patients.
The results of the ROC curve analysis
Additionally, we constructed an ROC curve to evaluate the predictive capability of RDW, platelet count, and RPR to assess the risk of unfavorable outcomes (Fig. 4). The areas under the curve (AUC) for each variable were as follows: platelet: 0.6712 < RDW:0.6732 < RPR:0.6844. The Youden indices for platelet, RDW, and RPR were 0.1982, 0.2321, and 0.2383, respectively, with corresponding best cut-off values of 196.2, 44.45, and 0.1987. The RPR demonstrated the highest Youden index and AUC, suggesting that its predictive ability for unfavorable prognosis in patients with AIS is superior to that of the other variables studied (Supplementary Table S1).
Discussion
This study identified an independent positive relationship between RPR and unfavorable outcomes at 90 days in patients with AIS. Furthermore, a nonlinear relationship was observed between them, with an inflection point occurring at an RPR value of 0.33. Different associations between RPR and unfavorable outcomes at 90 days were observed on both sides of this inflection point.
In recent years, RPR has emerged as a novel inflammatory biomarker. Various studies have demonstrated a strong link between RPR and the prognosis of multiple diseases, such as liver fibrosis, chronic liver disease, certain cancers, and ST-segment elevation myocardial infarction16,17,18. However, investigations into the impact of RPR on stroke prognosis are still limited. A cohort study involving 235 patients with AIS who received intravenous thrombolysis found that RPR is an independent risk factor for adverse outcomes (with adverse outcomes defined as an increase of 4 points or more in the NIHSS score within 24 hours after thrombolysis or death), determined through multivariate logistic regression analysis (OR = 2.031; 95% CI: 1.436–2.873; P < 0.0001)23. Similarly, another investigation involving 286 AIS patients who underwent mechanical thrombectomy found that each unit increase in RPR corresponded to a 67.1% rise of unfavorable outcomes at three months (defined as an mRS score of ≥ 3), with an OR of 1.671 (95% CI: 1.127–2.479; P = 0.011)24. Additionally, a study involving 2,673 critically ill AIS patients found that RPR was associated with a OR of 1.28 (95% CI: 1.02–1.59) for in-hospital all-cause mortality after adjusting for potential confounders41. Our study adds to the existing literature by supporting the hypothesis that elevated RPR is positively associated with short-term adverse outcomes in AIS. In contrast to prior studies, we assessed RPR as both a categorical and continuous variable, thereby reducing information loss and enabling a more precise quantification of its relationship with outcomes. Besides, we employed ROC curve analysis to evaluate the predictive performance of RPR, platelet, and RDW regarding 90-day outcomes in AIS patients. Our results demonstrate that AUC for RPR and the optimal Youden index exceed those of platelet and RDW, indicating that RPR can serve as an important predictor of adverse outcomes within 90 days post-AIS, providing critical risk assessment metrics for the development of prediction models regarding unfavorable outcomes in AIS patients. Additionally, we conducted a sensitivity analysis on participants with a BMI of less than 28 kg/m² and no history of hypertension or DM to confirm the robustness of our findings. In summary, identifying RPR as a risk factor for adverse outcomes in AIS patients and elucidating the relationship between them carries some clinical implications. Integrating RPR into routine clinical evaluations allows healthcare professionals to detect high-risk populations early, facilitating timely interventions aimed at promoting rehabilitation and ultimately reducing the incidence of unfavorable outcomes following AIS.
The specific mechanisms linking elevated RPR to poor short-term prognosis in AIS patients are not fully clear, but they likely relate to inflammation and coagulation dysfunction. Prior research has demonstrated that inflammation, coagulation abnormalities, and atherosclerosis significantly influence various pathophysiological stages of AIS42. Excessive inflammation can inflict damage on the endothelial lining of blood vessels, intensifying cerebral ischemic injury9. RDW serves as an inflammatory marker; increased RDW levels typically indicate a heightened inflammatory state in the body20. However, high RDW may compromise erythrocyte membrane integrity and increase their fragility43,44,45. Reduced erythrocyte deformability can impair microcirculation and oxygen delivery, thus further aggravating cerebral injury and affecting neuronal repair46. Studies have shown that elevated RDW values are frequently observed in AIS patients with unfavorable prognoses47. Moreover, during the pathogenesis of AIS, activated platelets adhere to the vascular wall at sites of ruptured atherosclerotic plaques and simultaneously release various inflammatory mediators, which further activate immune cells such as neutrophils, lymphocytes, and monocytes, and consequently lead to aggravated brain tissue injury4849;. Research indicates that the average platelet count in deceased AIS patients is significantly lower compared to survivors, and lower peripheral platelet counts are often linked to larger infarct sizes and increased disease severity50. Therefore, RPR, which integrates RDW levels and platelet count, can more comprehensively reflect the inflammatory and coagulation status of AIS patients and, to some extent, indicate the pathophysiological processes of short-term neurological injury and repair in these patients.
Additionally, after stratifying participants based on RPR quartiles, the results of the multivariable-adjusted model indicate that OR for RPR in the Q1, Q2, and Q3, compared to the Q1, were 1.186, 2.497, and 2.455, respectively. This suggests that there is an observable trend of increasing risk for unfavorable outcomes among AIS patients from the first quartile to the third, which stopped in the fourth quartile. This observation implies a potential non-linear association between RPR and unfavorable outcomes. To test this hypothesis, we employed logistic regression with cubic splines and discovered a non-linear link between them, identifying an inflection point at an RPR level of 0.33. The results of the two-piecewise linear regression analysis showed that on the left side of the inflection point, as the RPR value increased, the incidence of 90-day unfavorable outcomes in AIS patients tended to rise. However, on the right side of the inflection point, as RPR increased, the incidence of unfavorable outcomes showed a decreasing trend without statistical significance. Further analysis showed that participants with RPR levels greater than or equal to 0.33 exhibited higher values for age, Neu, BMI, HCY, and D-dimer compared to those with RPR levels less than 0.33. Additionally, a higher proportion of AIS patients with RPR values above 0.33 also had a history of CHD and AF (Supplementary Table S2). These factors are strongly associated with poor outcomes in AIS51,52,53,54,55. In the cohort with RPR values below 0.33, the levels of these risk factors were lower, and the effect of RPR on 90-day adverse outcomes was relatively enhanced. Conversely, when RPR surpasses 0.33, the effect of RPR on 90-day adverse outcomes was relatively attenuated due to the presence of these risk factors. This may help explain the nonlinear relationship between RPR and 90-day unfavorable outcomes in AIS patients. The discovery of this nonlinear relationship has certain clinical implications. As a simple and easily accessible hematological parameter, RPR exhibited an inflection point at 0.33, which provides a novel perspective for prognostic stratification in AIS patients. However, as an exploratory study, our results need to be externally validated in future multicenter, large-sample cohorts to confirm the general applicability and clinical reliability of this inflection point value, so as to ultimately provide valuable references for treatment strategies, rehabilitation, and reduction of complications in AIS patients.
This study offers several significant advantages. (i) It explores how RPR is associated with unfavorable outcomes in AIS patients by analyzing it as a continuous variable and as a categorical variable defined by quartiles. This dual method reduces and minimizes information loss, as well as accurately assesses the association between RPR and patient outcomes. (ii) We clarified the nonlinear link between RPR and the risk of adverse stroke outcomes and identified the inflection point, representing a significant advancement over previous research. (iii) The study utilizes imputation techniques to address missing data, thus increasing statistical power and minimizing potential biases that may result from missing covariate information. (iv) Multiple sensitivity analyses were conducted to reinforce the reliability of the results. These analyses included calculating E-values to assess the possible impact of unmeasured confounders, transforming independent variables, in addition to re-evaluating the association between RPR and short-term prognosis in AIS patients following the exclusion of individuals with hypertension, DM, and a BMI of 28 kg/m² or higher.
Several potential limitations must be considered. First, the study was a single-center study conducted solely with Chinese participants, which calls into question the applicability of the findings to other ethnic groups. In the future, we plan to collaborate with researchers both domestically and internationally to conduct further multicenter and multi-ethnic prospective studies for validation. Second, this research assessed RPR and other key parameters only at baseline, without examining how changes in RPR over time may impact the prognosis of patients with AIS. Addressing this limitation should be a vital focus for future investigations aimed at collecting more comprehensive longitudinal data on RPR fluctuations. Third, as with many observational studies, this research may be subject to uncontrolled or unmeasured confounding variables, despite having controlled for known potential confounders. However, the calculation of E-values indicates that such confounding factors are unlikely to significantly impact our findings. Finally, it is crucial to recognize that the observational nature of this study suggests an independent association between RPR and short-term outcomes in AIS patients, but it does not establish a causal relationship between them.
Conclusion
This study confirms a significant positive and nonlinear association between the RPR and 90-day unfavorable outcomes in AIS patients. Notably, when RPR is below 0.33, each 0.1-unit increase corresponds to a 70.8% higher risk of unfavorable outcomes. RPR emerges as a valuable prognostic biomarker for risk stratification in AIS patients. It provides a new perspective for improving the rehabilitation and management of stroke patients and ultimately improving their health status and quality of life, as well as providing a certain reference for future clinical studies in multicenter, large-sample cohorts.
Data availability
Due to the signing of a data security consent agreement, the provision of data is restricted, allowing only external researchers to access the data for research purposes. Researchers interested in these data can contact the corresponding author via email to obtain access. The email address is hanyong511023@163.com.
Abbreviations
- NEU:
-
neutrophil count
- CHD:
-
coronary heart disease
- HDL-c:
-
high-density lipoprotein cholesterol
- LYC:
-
lymphocyte count
- FPG:
-
fasting plasma glucose
- BMI:
-
body mass index
- FIB:
-
fibrinogen
- SVO:
-
small vessel occlusion
- HCY:
-
homocysteine
- TC:
-
total cholesterol
- ALB:
-
serum albumin
- HGB:
-
hemoglobin concentration
- TG:
-
triglycerides
- DM:
-
diabetes mellitus
- LAA:
-
large artery atherosclerosis
- LDL-C:
-
low-density lipoprotein cholesterol
- TIA:
-
transient ischemia attack
- NIHSS:
-
National Institute of Health stroke scale
- AF:
-
atrial fibrillation
- CE:
-
cardio embolism
References
Prabhakaran, S., Ruff, I. & Bernstein, R. A. Acute stroke intervention: A systematic review. Jama 313, 1451–1462 (2015).
Global, R., National Burden of Stroke and its & Risk Factors 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 20, 795–820 (2021).
Luengo-Fernandez, R. et al. Population-Based study of disability and institutionalization after transient ischemic attack and stroke: 10-Year results of the Oxford vascular study. Stroke 44, 2854–2861 (2013).
Han, Y. et al. Association between Triglyceride-to-High density lipoprotein cholesterol ratio and Three-Month outcome in patients with acute ischemic stroke: A second analysis based on a prospective cohort study. Bmc Neurol. 22, 263 (2022).
Powers, W. J. et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 50, e344-e418 (2019).
Desilles, J. P. et al. Diabetes Mellitus, admission Glucose, and outcomes after stroke thrombolysis: A registry and systematic review. Stroke 44, 1915–1923 (2013).
Echouffo-Tcheugui, J. B. et al. Diabetes and Long-Term outcomes of ischaemic stroke: findings from get with the Guidelines-Stroke. Eur. Heart J. 39, 2376–2386 (2018).
Shi, K. et al. Global brain inflammation in stroke. Lancet Neurol. 18, 1058–1066 (2019).
Jin, R., Liu, L., Zhang, S., Nanda, A. & Li, G. Role of inflammation and its mediators in acute ischemic stroke. J. Cardiovasc. Transl Res. 6, 834–851 (2013).
Bonaventura, A. et al. Update on inflammatory biomarkers and treatments in ischemic stroke. Int. J. Mol. Sci. 17, 1967 (2016).
Welsh, P. et al. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc. Dis. 27, 247–253 (2009).
Di Napoli, M., Papa, F. & Bocola, V. C-Reactive protein in ischemic stroke: an independent prognostic factor. Stroke 32, 917–924 (2001).
Wang, J. et al. Evaluation of red blood cell distribution Width-Platelet ratio as a predictor of adverse pregnancy outcomes and disease severity in systemic lupus erythematosus. Clin. Rheumatol. 41, 2987–2993 (2022).
Arora, S. et al. Red blood cell distribution Width-to-Platelet count ratio as a prognostic marker for predicting severity and various outcomes in acute pancreatitis. Cureus 17, e81747 (2025).
Zhou, Y. et al. Enhanced red blood cell distribution width to platelet ratio is a predictor of mortality in patients with sepsis: A propensity score matching analysis based on the Mimic-IV database. Bmj Open. 12, e62245 (2022).
Cai, Y. et al. Diagnostic accuracy of red blood cell distribution width to platelet ratio for predicting staging liver fibrosis in chronic liver disease patients: A systematic review and Meta-Analysis. Med. (Baltimore). 98, e15096 (2019).
Takeuchi, H. et al. Elevated red cell distribution width to platelet count ratio predicts poor prognosis in patients with breast cancer. Sci. Rep. 9, 3033 (2019).
Celık, T. et al. Predictive value of admission red cell distribution Width-Platelet ratio for No-Reflow phenomenon in acute St segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiol. J. 23, 84–92 (2016).
Feng, G. H., Li, H. P., Li, Q. L., Fu, Y. & Huang, R. B. Red blood cell distribution width and ischaemic stroke. Stroke Vasc Neurol. 2, 172–175 (2017).
Lippi, G. et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 133, 628–632 (2009).
Xu, T. et al. Platelet count and clinical outcomes among ischemic stroke patients with endovascular thrombectomy in Direct-Mt. Clin. Chem. Lab. Med. 60, 1675–1682 (2022).
Wang, L. X., Liu, R. L., Zhou, P., Hu, H. F. & Deng, Z. Nonlinear relationship between platelet count and 30-Day in-Hospital mortality in intensive care unit stroke patients: A multicenter retrospective cohort study. Front. Neurol. 15, 1374159 (2024).
Jiang, M., Shen, J., Muhammad, B. & Geng, D. Red blood cell distribution width to platelet ratio predicts early neurological deterioration in acute ischemic stroke patients receiving intravenous thrombolysis. J. Stroke Cerebrovasc. Dis. 32, 107146 (2023).
Li, X. et al. Novel peripheral blood cell ratios: effective 3-Month Post-Mechanical thrombectomy prognostic biomarkers for acute ischemic stroke patients. J. Clin. Neurosci. 89, 56–64 (2021).
Li, X., Xu, H. & Gao, P. Red blood cell distribution Width-to-Platelet ratio and other laboratory indices associated with severity of histological hepatic fibrosis in patients with autoimmune hepatitis: A retrospective study at a single center. Med. Sci. Monit. 26, e927946 (2020).
Banks, J. L. & Marotta, C. A. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: A literature review and synthesis. Stroke 38, 1091–1096 (2007).
Haggag, H. & Hodgson, C. Clinimetrics: modified Rankin scale (Mrs). J. Physiother. 68, 281 (2022).
Berkhemer, O. A. et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl. J. Med. 372, 11–20 (2015).
Lee, S. J., Heo, S. H., Ambrosius, W. T. & Bushnell, C. D. Factors mediating outcome after stroke: Gender, Thrombolysis, and their interaction. Transl Stroke Res. 9, 267–273 (2018).
Pantoja-Ruiz, C. et al. Risk Factors, Presentation, and outcome in acute stroke according to social position indicators in patients hospitalised in a referral centre in Bogotá 2011–2019. Neuroepidemiology 57, 170–175 (2023).
Song, T. J. et al. Characteristics and factors for Short-Term functional outcome in stroke patients with atrial Fibrillation, nationwide retrospective cohort study. Front. Neurol. 10, 1101 (2019).
Groenwold, R. H. et al. Missing covariate data in clinical research: when and when not to use the Missing-Indicator method for analysis. Can. Med. Assoc. J. 184, 1265–1269 (2012).
White, I. R., Royston, P. & Wood, A. M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 30, 377–399 (2011).
Lau, L. H., Lew, J., Borschmann, K., Thijs, V. & Ekinci, E. I. Prevalence of diabetes and its effects on stroke outcomes: A Meta-Analysis and literature review. J. Diabetes Investig. 10, 780–792 (2019).
Lu, Y. et al. Metabolic mediators of the effects of Body-Mass Index, Overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet 383, 970–983 (2014).
Losito, A., Pittavini, L., Ferri, C. & De Angelis, L. Reduced kidney function and outcome in acute ischaemic stroke: relationship to arterial hypertension and diabetes. Nephrol. Dial Transpl. 27, 1054–1058 (2012).
Haneuse, S., VanderWeele, T. J. & Arterburn, D. Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. Jama 321, 602–603 (2019).
White, J. V., Guenter, P., Jensen, G., Malone, A. & Schofield, M. Consensus statement: academy of nutrition and dietetics and American society for parenteral and enteral nutrition: characteristics recommended for the identification and Documentation of adult malnutrition (Undernutrition). Jpen J. Parenter. Enter. Nutr. 36, 275–283 (2012).
Zhao, W. et al. [Epidemiologic characteristics of dyslipidemia in people aged 18 years and over in China]. Zhonghua Yu Fang Yi Xue Za Zhi. 39, 306–310 (2005).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (Strobe) statement: guidelines for reporting observational studies. Int. J. Surg. 12, 1495–1499 (2014).
He, K., Xie, X., Duan, X., Zhou, Q. & Wu, J. Red cell distribution Width-to-Platelet count ratio: A promising predictor of in-Hospital All-Cause mortality in critically ill patients with acute ischemic stroke. Cerebrovasc. Dis. 52, 692–699 (2023).
Feske, S. K. Ischemic stroke. Am. J. Med. 134, 1457–1464 (2021).
Tracey, K. J. et al. Cachectin/Tumor necrosis factor induces Cachexia, Anemia, and inflammation. J. Exp. Med. 167, 1211–1227 (1988).
Hong, R. et al. Red blood cell distribution width is associated with neuronal damage in acute ischemic stroke. Aging (Albany Ny). 12, 9855–9867 (2020).
Rondanelli, M. et al. A structural equation model to assess the pathways of body adiposity and inflammation status on dysmetabolic biomarkers via red cell distribution width and mean corpuscular volume: A Cross-Sectional study in overweight and obese subjects. Lipids Health Dis. 19, 154 (2020).
Cines, D. B. et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 123, 1596–1603 (2014).
Fan, L., Gui, L., Chai, E. Q. & Wei, C. J. Routine hematological parameters are associated with Short- and Long-Term prognosis of patients with ischemic stroke. J. Clin. Lab. Anal. 32, e22244 (2018).
Kannan, M., Ahmad, F. & Saxena, R. Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood Rev. 37, 100583 (2019).
Fuentes, E., Moore-Carrasco, R., de Andrade Paes, A. M. & Trostchansky, A. Role of platelet activation and oxidative stress in the evolution of myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 24, 509–520 (2019).
D’Erasmo, E. et al. Platelet Count, mean platelet volume and their relation to prognosis in cerebral infarction. J. Intern. Med. 227, 11–14 (1990).
Fan, R. et al. Risk factors for stroke outcomes in adults: stroke in China. Med. (Baltimore). 102, e36606 (2023).
Matsuo, R. et al. Smoking status and functional outcomes after acute ischemic stroke. Stroke 51, 846–852 (2020).
Cho, H. et al. Untreated hypertension and prognosis paradox in acute ischemic stroke. Neurol. Sci. 44, 2087–2095 (2023).
Shrestha, S., Poudel, R. S., Khatiwada, D. & Thapa, L. Stroke Subtype, Age, and baseline Nihss score predict ischemic stroke outcomes at 3 months: A preliminary study from central Nepal. J. Multidiscip Healthc. 8, 443–448 (2015).
Ming, C. et al. Impact of traditional and Non-Traditional lipid parameters on outcomes after intravenous thrombolysis in acute ischemic stroke. J. Clin. Med. 11, 7148 (2022).
Acknowledgements
The Emergency Department and data collectors from Shenzhen Second People’s Hospital contributed clinical support and facilitated data collection for this research.
Funding
This study was supported by the Stroke Neurorehabilitation Research Project (Xu Gelin’s Research Start-up Fund), Grant No. KJ08000037.
Author information
Authors and Affiliations
Contributions
Jiaqian Zhu and Yong Han initiated this research and were responsible for drafting the initial manuscript and carrying out the statistical analyses. Yong Han and liming Cao also played a significant role in designing the study and contributed to the manuscript revisions. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and participant consent
This study received approval from the Ethics Review Committee at Shenzhen Second People’s Hospital (Approval No. 2023-305-01PJ). Informed consent was obtained from all participants prior to their enrollment in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, J., Han, Y. & Cao, L. Nonlinear dose-response relationship between red blood cell distribution width to platelet ratio and 90-day unfavorable outcomes in acute ischemic stroke: a prospective cohort study. Sci Rep 15, 39791 (2025). https://doi.org/10.1038/s41598-025-23428-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-23428-7