Abstract
The composition of constituent elements in bioactive glasses is critical for achieving properties suitable for biological applications. Even minor compositional variations can significantly alter the system’s response. This study investigated silica-based bioactive glasses doped with 2, 4, and 6 wt% bismuth (Bi), utilizing an Analysis of Variance (ANOVA) model with a Central Composite Design (CCD) to examine the influence of compositional changes on structure and properties. Physical properties were characterized using Zeta potential measurements for stability, Scanning Electron Microscopy (SEM) for nanoparticle size, and Brunauer-Emmett-Teller (BET) analysis for porosity. Post-Simulated Body Fluid (SBF) investigations included transmission electron microscopy (TEM) with Selected Area Electron Diffraction (SAED) to observe hydroxyapatite (HA) formation, Energy-Dispersive X-ray (EDX) spectroscopy for compositional analysis, Fourier Transform Infrared (FTIR) spectroscopy to evaluate nanoparticle functional characteristics, and X-ray Photoelectron Spectroscopy (XPS) to determine the oxidation state of Bi. A correlation between physical properties and constituent composition was established through ANOVA. The predicted and actual values were consistent with the selected optimization model. In vitro assays confirmed the bioactivity of the synthesized samples, which were found to be biocompatible and non-cytotoxic for the intended applications.
Similar content being viewed by others
Introduction
Recently, Bi received significant inclination towards biological applications, specifically for tissue regeneration, theragnostic, therapeutic, anti-cancer, and as a sustained drug release agent due to its antimicrobial, biocompatibility, and regenerative capabilities. Nevertheless, Bi has been used ever since in medical applications for treating dyspepsia and other gastrointestinal disorders1, and is effective. Conversely, bioactive glasses have significantly contributed to various biological applications worldwide, including tissue regeneration in bones2, orthodontics, wound healing3, theragnostic, blood-brain barrier, drug delivery, and therapeutics4. Despite such versatility, there are still notable lacunae, such as unstable behavior of dopants in biological environments, induced secondary inflammation, undesired biodegradation, and uncontrollable ion interactions that cause collateral effects5. These may be due to various reasons, such as a lack of understanding of the coordination chemistry of materials with biological environments, constrained clinical trials, and technological challenges.
To surmount these challenges, it has been realized that, among the identified issues, the composition of the constituent materials of multicomponent systems, such as bioactive glasses, the processing method, and the influence of their parameters play a key role in deciding the desired characteristics of the developed system6,7. Hence, there is always a need to investigate a wide range of compositions of the constituents along with numerous processing parameters, which is of critical interest. Only a few works have investigated the influence of the composition of constituent elements in bioactive glass on their physical and biological characteristics, and these have been found to have a significant impact8,9,10. In light of this, recent studies have investigated the influence of these parameters using statistical optimization techniques, including response surface methodology (RSM), the Taguchi approach, and probabilistic methods11. Among these, RSM is widely recognized for its effectiveness in analysing and optimizing processes involving multiple input variables and responses. RSM models parametric relationships in three or more dimensions, with three-dimensional (3D) surfaces identifying regions of maximum or minimum response. By encompassing the full Design of Experiments (DOE) framework, RSM facilitates informed decision-making. This approach is particularly significant in the design of systems for biological applications. Accordingly, several studies have reported the application of RSM to assess the influence of processing variables12,13 and composition14,15.
Bioactive glasses are amenable to biological applications; among them, silica-based bioactive glass has the flexibility to host any dopant materials, which allows these glasses to display multifaceted versatility across different biological verticals16. The processing of bioactive glasses with dopant appears promising, but there remains a lack of understanding of the structure-property correlation due to compositional constraints. The composition of the constituents and dopant materials is of critical interest as they directly interact with the biological environment and may cause adverse effects17,18. Hence, it is essential to realize how composition affects the physical characteristics desired for biological activity. The use of structure-directing agents (SDAs) is a key factor influencing the shape and size of nanoparticles during synthesis. Several studies have highlighted the impact of these surfactants on nanoparticle morphology during sol-gel synthesis, as documented in the literature19,20,21. The critical micellization concentration (CMC) is particularly important because it determines the composition of the reaction constituents and, consequently, the resulting particle morphology. In this study, cetyltrimethylammonium bromide (CTAB) is employed as the SDA, with a CMC of 1.2.
In contrast, Bi-doped bioactive glass, with Bi having one of the largest coordinationchemistries, was found to be unstable at the nanoscale. The use of RSM studying to study a wide range of compositions whilst establishing a correlation between composition and properties such as porosity, size, and nanoparticle stability is reported for the first time, to the best of our knowledge. The results would enlighten and appraise the niche verticals of this area, potentially reducing material waste and time consumption. Also, currently, the focus of this work is limited to the physical characteristics, followed by biocompatibility and cytotoxicity testing of a few selected samples to ensure reproducibility and repeatability. Alongside, biocompatibility studies were carried out using Simulated body fluid (SBF), which remains a practical first screen for in-vitro apatite formation, though the kinetics are strongly influenced by composition, processing route, and testing medium. Recent studies highlight how network modifiers accelerate dissolution and nucleation, while comparisons between SBF and human serum demonstrate that choice of medium significantly alters layer crystallinity and growth rate22,23. Our observations of accelerated apatite-like layer formation align with these findings, with absolute timepoints remaining sensitive to SBF composition and sample surface area. Furthermore, biological studies indicate that certain bioactive glasses exert selective cytotoxicity against cancer cells, including breast carcinoma lines, while sparing non-neoplastic counterparts, through mechanisms such as ion release, local pH shifts, and oxidative stress. Consistent with these findings, our MCF-7 assays revealed reduced viability and morphological alterations24,25,26. Moreover, Bi doped bioactive glasses have been shown to enhance antiproliferative effects compared to pristine compositions, underscoring the importance of formulation, particle properties, and dosing conditions in modulating biological response. However, more detailed application-oriented biological investigations and analysis of the influence of parametric optimization are in progress for future work.
Materials and methodology
Materials
Tetraethyl orthosilicate (TEOS), Bismuth Nitrate Pentahydrate (BNP), Calcium nitrate tetrahydrate (CNTH), Ethanol, CTAB, Ammonia solution, Sodium Chloride (NaCl), Sodium bicarbonate (NaHCO3), Potassium chloride (KCl), dipotassium hydrogen phosphate (K2HPO4), magnesium chloride hexahydrate (MgCl2·6H2O), Hydrochloric acid (1.0 M) – HCl, calcium chloride dihydrate (CaCl2·2H2O), sodium sulfate (Na2SO4), Tris-hydroxymethyl aminomethane ((CH2OH)3CNH2) were purchased from SRL chemicals, Hyderabad, India. All the obtained chemicals are 98.9–99.1% pure and are ACS reagents.
Methodology
Sol-gel synthesis of nanoparticles
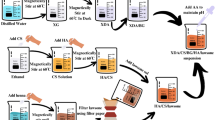
A gram batch preparation of all the samples was carried out using sol-gel synthesis. The sol-gel method was selected as it enables precise control over processing parameters, such as stirring speed, temperature, centrifuge speed, ultrasonication frequency, and drying temperature. Adjusting these parameters alters key nanoparticle characteristics, including morphology, network connectivity, and porosity18. These characteristics are particularly desirable for enhancing bioactivity in various biological applications. Initially, the desired amounts of deionized (DI) water, ethanol, and ammonia (added dropwise) were combined and stirred for 15 min to homogenize the mixture. Afterward, TEOS was added to the solution, which was stirred for approximately 2 h at 440 rpm. This hydrolysis step was followed by a condensation reaction that formed a three-dimensional sol network. The solution was centrifuged at 4000 rpm for 20 min to obtain uniform nanoparticles. The nanoparticles were then washed with DI water. A separate solution of ethanol and DI water was prepared, into which CNTH and BNP were added. While stirring for 15 min, surfactant CTAB (with a critical micellization concentration, CMC, of 1.2) is added to the solution to form a gel. The gel was aged to enhance structural integrity and allow further polycondensation.
The mixture was centrifuged again to ensure uniform particle morphology. Following this, ultrasonication was performed to separate the particles and prevent agglomeration. Afterward, the gel was heat-treated in a vacuum oven at 60 °C overnight to obtain the nanoparticles. Vacuum heating was preferred because it gently removed solvents and by-products, retained the amorphous structure, preserved nanoscale porosity, and prevented network distortion. This procedure was consistently applied to synthesize all nanoparticles. The compositions used to prepare these samples are listed in Table 1, and the synthesis process is illustrated in Fig. 1.
In-vitro evaluation of bioglass nanoparticles
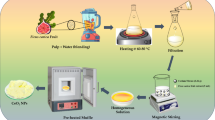
According to ISO 2331727, the SBF solution was prepared to monitor the of HA formation on nanoparticles over a defined time period. The preparation method for SBF is described in detail in this study. The specific steps and required quantities are provided in Table S2 in the Supplementary file, and the experimental setup is shown in Fig. 2.
A solution was prepared in 700 ml of distilled water using a polyethylene beaker and a magnetic stirrer. The beaker was covered with a watch glass or plastic wrap, and the temperature was maintained at 36.5 ± 1.5 °C. Reagents were added sequentially, up to the eighth step, ensuring each was fully dissolved before the next addition. CaCl2 was introduced granule by granule. The measuring cylinder was rinsed with HCl prior to measuring its volume. A calibrated pH meter was used to monitor the pH, maintained at 2.0 ± 1.0, while the temperature remained at 36.5 ± 1.5 °C. If the total solution volume was less than 900 ml, distilled water was added to compensate. TRIS was gradually added until fully dissolved and the pH stabilized. Additional TRIS was added to achieve a pH of 7.45 ± 0.01. HCl was introduced using a syringe to adjust the pH to 7.42 ± 0.01, ensuring it did not fall below 7.40. TRIS and HCl additions were alternated until the specified number of steps was completed, while maintaining 36.5 °C and pH 7.40. Throughout the procedure, the rate of dissolution, temperature, and solution volume were monitored, with temperature adjusted at a rate of 0.1 °C per minute. The pH meter was then removed and rinsed with distilled water. The solution was transferred to a one-litre volumetric flask, and the beaker was rinsed with distilled water, with the rinse also added to the flask. After cooling, distilled water was added to the flask up to the calibration line. The flask was sealed and secured with plastic film, and the solution was allowed to cool to 20 °C. Finally, distilled water was added to the marked line to complete the preparation.
Following the preparation of SBF, the pre synthesised samples were immersed in the solutions. Only nanoparticles with 5 wt% Bi variation (for model validation using a random composition) and 6 wt% Bi composition (as reported in Ref. [15] for validation and repeatability) was selected. These samples were soaked for 21 days to assess the formation of apatite. Subsequently, the three samples were analysed on the 21st day to evaluate their microstructure and confirm apatite formation using HRTEM, SEM-EDX, Fourier Transform Infrared Spectroscopy (FTIR), and X-ray diffraction (XRD). The characterized results informed further studies, including assay culture and cytotoxicity assessments on the same samples.
Preparation of assay culture
The cytotoxicity of Bioglass nanoparticles was assessed by dissolving the nanoparticles in dimethyl sulfoxide (DMSO) and water, followed by evaluation on MCF-7 human breast cancer cells. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to measure cell proliferation. MCF-7 cells were seeded at a density of 5 × 103 cells per well in a 96-well plate and allowed to adhere for 24 h. Subsequently, various concentrations of the bioactive glass material were added, with untreated cells serving as controls. Each experimental condition was performed in triplicate. After the addition of Bi-doped bioactive glass nanoparticles, the plates were incubated for 48 h. Cell viability and proliferation were assessed using the MTT assay, which produces blue formazan crystals in viable cells. At the end of the incubation, the supernatant was removed, and 100 µL of MTT dye (0.45 mg/mL in 1x DPBS) was added to each well. The plate was incubated at 37 °C for 4 h to allow the formation of formazan crystals. After incubation, the supernatant was aspirated, and 100 µL of DMSO was added to dissolve the formazan crystals. Optical density was measured at 595 nm using a Bio-Rad microplate reader. The percentage of viable cells was calculated using following:
Blank absorbance was determined using media alone, and control absorbance was measured from cells cultured with media only. Cells without extracts served as the reference for 100% cell survival. Data from three independent experiments were averaged. Statistical analysis was conducted using the t-test and Spearman’s correlation coefficient. Statistical significance was defined as a p-value less than 0.05. Graphs were generated using GraphPad Prism version 9.0.0.
In this study, DMSO was used in minimal amount i.e., 0.05% (v/v) solely as a dispersion aid to facilitate the uniform distribution of Bioglass nanoparticles prior to their dilution in water and cell culture medium for direct exposure to MCF-7 cells. This approach differs from indirect extract-based cytotoxicity assays described in ISO 10993-5, which assess leachables obtained by incubating materials in an aqueous medium. Because the final DMSO concentration in the culture wells was very low (well below levels known to cause cytotoxicity, typically ≥ 0.5–1%), and vehicle-only controls were included, any observed effects can be attributed primarily to the nanoparticles themselves rather than the solvent. Furthermore, DMSO was only used at the end of the assay to solubilize the Formazan crystals during the MTT assay, a standard and widely accepted procedure that does not influence cell viability measurements. This controlled use of DMSO ensured reliable assessment of nanoparticle-induced cytotoxicity without introducing solvent-related artifacts.
Antibacterial studies
The Minimum Inhibitory Concentration (MIC) was determined using the microdilution method. A 0.5 McFarland standard bacterial culture was prepared by suspending the target bacteria in sterile Luria-Bertani broth (LB) medium, and the inoculum density was adjusted to match the standard turbidity. The test compound was serially diluted two-fold in a sterile microtiter plate. Each well received the standardized bacterial suspension. A positive control well contained culture medium only, while wells with the compound served as blanks. Ampicillin dilutions were included as an additional control. Plates were incubated at 37 °C for 24 h. After incubation, absorbance at 600 nm was measured using a plate reader. The MIC was defined as the lowest compound concentration that completely inhibited visible microbial growth. All experiments were conducted in triplicate to ensure reproducibility.
Design of experiments (DOE)
The DOE for this study employs the RSM and utilizes a central composite design (CCD). RSM is an efficient optimization technique that can be represented by a lower-order polynomial within a defined experimental region. Additionally, response surfaces identify optimal regions, interactions, and curvature by analyzing the maxima and minima28. The CCD model directs experimental procedures more systematically than trial-and-error methods, providing visual tools such as three-dimensional surfaces and contour plots to support informed decision-making. The input factors include two numeric factors: Bi composition, ranging from 0 to 6 wt%, and Ca composition, varying from 34 to 40 wt%. Si is held constant at 60 wt%, forming 100% bioactive glass composition. The desired responses are the nanoparticles’ porosity, size, and stability. Table 1 presents the suggested DOE for the current study, and the nanoparticle was synthesized through the sol-gel route (Fig. 1). Experiment runs 1, 2, 4, 6, and 10 are color-coded as they represent the same central space type. Runs 3, 5, 7, and 9 correspond to factorial designs along the diagonal, while runs 8, 11, 12, and 13 are categorized as axial-type points on the cube shown in Fig. 3.
Characterization
The bioactive glass nanoparticle samples were characterized for their physical properties. SEM was used to examine the shape and size of the bioactive glass nanoparticles. EDX was performed to identify the composition of the constituent elements, specifically, to confirm the retention of the Bi in the synthesized nanoparticles. Additionally, zeta potential measurements were conducted to assess the stability of the nanoparticles. BET analysis was performed to estimate the porosity and surface area of the developed nanoparticles. The specifications of the instrumentation used in the current work are shown in Table 2.
Statistical analysis
In RSM, ANOVA is applied to the CCD model generated by RSM to assess the significance of both the model and the interaction between individual factors. This analysis allows identification of the statistical relationship between inputs and responses. The resulting mathematical correlations help optimize experiments, reducing material waste and time consumption.
Validation studies
To reproduce the optimized model equations, a random Bi composition (5wt.%) is fed to the model, which was not suggested by the RSM composition in Table 1. For validation, a Bi composition of 6wt.% mentioned in Ref. [15] was considered. Further to verify the presence of Bi, EDX investigations were reported for compositions of Bi with 5 wt% and 6 wt%, shown in Fig. S1 (Supplementary file).
Results and discussion
Microstructure analysis (as-synthesized nanoparticles)
Sample morphology was examined using SEM. Particle size and shape were quantified using ImageJ software to determine the average particle size from SEM micrographs. Table S1 (Supplementary file) and Fig. 4 present the particle size, standard deviation, and pore radius for the different compositions. The SEM micrographs indicate that the median size was determined for all samples. In each micrograph, the particle closest to the median particle size for each sample was marked. One particle was selected based on its size relative to the other particles in the sample. The particles exhibited a uniform shape with only minor variations in size, which may be attributed to several factors, including composition, type of surfactant, polarizer, solvents used, andprocessing parameters. In light of current work, the CMC of CTAB was considered. Further EDX scan was performed on the samples to verify the retention of Bi in the processed bioactive glass nanoparticles, and it was found that Bi is retained and is reported in Fig. S1 (supplementary file) for particular samples, i.e., 5 wt%, 6wt.% of Bi and Ref. [15].
Porosity
The porosity and surface area of the as-prepared nanoparticles were analyzed using BET analysis. The BET isotherms exhibited an IUPAC type IV adsorption-desorption hysteresis loop, confirming the presence of mesoporosity. These isotherms are illustrated in Fig. 5a and summarized in Table S1. The results indicate that the porosity is located within the particles rather than between them, making the nanoparticles suitable for biomineralization in bone tissue regeneration applications. This characteristic is crucial for developing scaffolds with uniform porosity, which facilitates consistent cell proliferation and the osteogenesis. The pre-diameter measurements for all runs are provided in Table S1 (Supplementary file). Furthermore, the porosity of the particles can be visualized in the SEM micrographs shown in Fig. 4. To assess the stability of the as-prepared nanoparticles, zeta potential investigations were conducted and are discussed in the following section.
Stability
The stability of nanoparticles is crucial because they transport drugs or dopants needed for regenerative processes in biological environments. Therefore, the developed nanoparticles need to exhibit excellent stability. The Zeta potential curves shown in Fig. 5b provide insight into the stability range of the nanoparticles and their suitability for applications as a regenerative envelope, as detailed in Table S1 (Supplementary file). The peak in Fig. 5b demonstrates the highest stability for run 13 and the lowest stability for run 1. These results suggest that a lower Bi concentration in the bioactive glass composition leads to a strong electrostatic repulsion. Whereas, a higher concentration enhances the coordination chemistry of Bi. Table S1 (Supplementary file) summarizes the stability values for all samples across their respective runs. The stability measurements indicate that the as-prepared samples are not highly stable; however, they fall within a range described as less stable to moderately stable, making them suitable for regenerative applications. The stability of the as-prepared nanoparticles is influenced not only by their functional and morphological characteristics but also by the oxidation state of Bi. XPS analysis confirmed a stable oxidation state of + 3 for Bi, with an ionic radius of 1.03 Å (Fig. S2).
Microstructural analysis (Post SBF immersion of nanoparticles)
HRTEM was conducted on samples at days 1, 7, 14, and 21. Maximum HA {Ca5(PO4)3(OH)} formation was observed at 21 days. Figure 6a–c presents micrographs following immersion of nanoparticles in SBF. All samples exhibited minimal or no apatite formation at day 1, indicating negligible changes in particle morphology or crystal phase. By day 7, a slight HA layer was detected, though its intensity remained low and resembled porous silicon. At day 21, all compositions demonstrated substantial HA layer formation, with calcium phosphate as the primary phase, as confirmed by XRD, as shown in Fig. S3. The XRD patterns for the 5 wt% and 6 wt% Bi-doped samples, as well as the reference sample, confirm the formation of Hydroxyapatite (HAP) following immersion in Simulated Body Fluid (SBF). Sharp and well-defined diffraction peaks at characteristic 2θ positions, including 25.9°, 31.7°, 32.9°, 34.0°, 39.8°, 46.7°, 49.4°, and 53.1°, indicate the nucleation and growth of crystalline HAP on the glass surface.
The observed phases correspond to standard JCPDS card references (00-001-100829 and 00-009-043230) for HAP, with diffraction peaks indexed to planes such as (002), (211), (300), (202), (310), and (222). Increasing peak intensity over time demonstrates the transformation from an initially amorphous calcium phosphate-rich layer to a more crystalline apatite structure. This transformation is attributed to ion exchange reactions between the glass surface and SBF, where the release of calcium and phosphate ions from the glass results in supersaturation and subsequent precipitation of apatite. Incorporation of Bi enhances the bioactivity of the glass by accelerating HAP layer formation, as indicated by the stronger and sharper HAP peaks in Bi-containing samples compared to the reference. SAED further verified the hexagonal structure for 5 wt% and 6 wt% Bi, as well as the reference sample [15], as shown in Fig. 7a–c. Additionally, HA formation within silicon pores, where bismuth is entrapped, suggests that increased Bi content enhances the effectiveness of layer formation.
The SAED pattern displays the presence of both semi-amorphous and crystalline nature, corresponding to the mesoporosity within the particle and crystallite formation on the surface of the nanoparticles, with excellent network connectivity. The SAED pattern in Fig. 7a–c clearly infers the formation of HA crystals on the 21st day for all bioactive glass samples. Further corroboration of the confirmation of HA can be realized from the EDX analysis, and it can be inferred from Fig. S4 that Si, Ca, and P are present after 21 days. The sample also discloses the formation of crystals with excellent network connectivity, indicating suitability for biomineralization.
Fourier transform infrared (FTIR) spectroscopy
Figure 8a shows the FTIR spectra of the nanoparticle samples before immersion in SBF. The peaks corresponding to 440 cm−1 reflect the presence of Bi-O-Bi bending vibrations, the band appearing near 1100 cm−1 corresponds to the Si–O–Si asymmetric stretching vibrations of the silicate network in the glass. Whereas, after immersion, new PO42− bands appear in the same region (1030–1100 cm−1), overlapping and partially replacing the Si–O–Si contribution, which is why this region becomes a key indicator for the onset of Hydroxyapatite formation, and it can be inferred in Fig. 8b.
After 21 days of immersion in SBF, FTIR spectra indicate the progressive formation of HA on the surface of Bi-doped bioactive glass mesoporous nanoparticles. After 21 days, the HA layer is fully developed, as indicated by well-defined phosphate peaks at 1000–1100 cm−1, and sharper carbonate peaks at 1400–1500 cm−1. Silanol groups (Si–OH) at approximately 950 cm−1 serve as nucleation sites for HA, whereas the increased intensity of the hydroxyl peak at 3400–3700 cm−1 reflects the hydration of the mature HA layer. The presence of Bi–O–Bi bending vibrations near 440 cm−1 confirms the retention of the Bi network, which contributes to the bioactive properties of the material. The gradual transformation observed in the FTIR spectra demonstrates the successful nucleation and growth of a biologically relevant HA layer, facilitated by the interaction of silanol groups, phosphate, and calcium ions in simulated body fluid (SBF), with Bi supporting the process. These findings are further illustrated in Fig. 8b.
In vitro cell culture investigations
In vitro studies were conducted on selected samples (5 wt%, 6 wt%, and Ref. [15]) to evaluate hydroxyapatite HA formation, and assess their suitability for bone tissue engineering applications. The presence of the HA layer was previously confirmed through SEM, TEM, XRD, and FTIR analyses, as discussed above. Following this confirmation, the same HA-coated samples underwent cell culture investigations using MCF-7 cell lines for MTT assay evaluation. Additionally, antibacterial studies were conducted using the S. aureus bacterial strain, which is relevant for soft tissue applications. The results are presented in Fig. 9a-b. All samples exhibit moderate cytotoxicity, which becomes more pronounced at higher concentrations. This effect may result from the release of ions or chemical interactions with the cell membrane. The 6 wt% Bi sample and the reference sample [15], OD600 decreases sharply as drug concentration increases, indicating substantial bacterial inhibition. Near-complete growth inhibition is observed at approximately 6 mg/mL. The inhibition shows a slight decline at lower drug concentrations and plateaus beyond 6 mg/mL. Incubation with these samples at 80 µg/mL for 24 h results in a significant increase in the proportion of dead cells. These results corroborate the findings of the MTT assay and confirm the cytotoxicity of the synthesized bismuth-doped bioactive glass mesoporous nanoparticles against the MCF-7 cell line. Further studies on cell inhibition and proliferation are ongoing, including live staining and confocal microscopy, as well as investigations into sustained drug release.
Statistical significance was assessed using the t-test and Spearman’s correlation coefficient, with a threshold of p < 0.05. The cytotoxicity data in Fig. 9a indicate a dose-dependent reduction in % cell viability for both 5 wt% Bi and 6 wt% Bi nanoparticles compared to Ref. [15]. Student’s t-test analysis confirmed that viability reductions at concentrations of 1.0 mg/mL or higher were statistically significant (p < 0.05) relative to untreated controls. Additionally, comparison of 5 wt% and 6 wt% Bi formulations revealed significant differences at concentrations of 1.5 mg/mL and above, with 6 wt% Bi consistently resulted in higher cell viability than both 5 wt% Bi and Ref. [15]. Spearman’s correlation analysis demonstrated a strong negative correlation (p < 0.05) between nanoparticle concentration and MCF-7 cell viability, supporting the conclusion that cytotoxicity is concentration-dependent.
Figure 9b presents antibacterial activity as OD600 measurements for bacterial growth in the presence of Bi-doped samples. The t-test indicated that both 5 wt% and 6 wt% Bi significantly inhibited bacterial growth compared to Ref. [15] at concentrations of 2.5 mg/mL or higher (p < 0.05). At concentrations of 5 mg/mL and above, the difference between 5 wt% and 6 wt% Bi was also significant, with 6 wt% Bi exhibiting greater antibacterial efficacy as evidenced by lower OD600 values. Spearman’s correlation analysis identified a strong negative correlation (p < 0.05) between drug concentration and bacterial growth across all groups, confirming dose-dependent antibacterial activity. Agar plate images in Fig. 9b qualitatively corroborate these quantitative findings.
Additionally, antibacterial studies with S. aureus demonstrated similar responses when exposed to native bioglass 45S531, bioglass S53P432 with an altered constituent composition, and bioactive glasses doped with silver33, as previously reported. The observed trends in the current study are consistent with these prior findings and align with established scientific understanding.
Analysis of variance (ANOVA)
Fit summary
ANOVA is performed to realize the influence of the composition of the constituent elements, mainly dopant, which in the current work is Bi, on the porosity, size, and stability of the as-prepared nanoparticles. The following Table 3 will emphasize the significance of the models chosen based on the F and p scores. High F and low p-values are always desired, providing significant evidence of the factor’s importance.
The ANOVA results presented in Table 4 for the linear model indicate that the model is statistically significant, with a p-score of 0.0464. This suggests that the included factors can reliably explain the variation in porosity. Among the input variables, the Bi composition was found to significantly influence porosity, with a p-score of 0.0155, underscoring its critical role in the studied system. Furthermore, the adequate precision value calculated from the optimization tool was 6.0620, which exceeds the recommended threshold of 4, indicating a satisfactory signal-to-noise ratio for the selected optimization method. This confirms that the model effectively discriminates within the design space. These findings emphasize the importance of Bi composition as a key parameter affecting porosity and support using the current model framework for exploratory analysis within the design space.
The ANOVA results for the linear model assessing particle size indicate that the model is statistically significant, with a p-score of 0.0230 and an F-score of 5.64. This suggests that the observed variation is not merely due to random noise. Among the model terms, the Bi composition (A) has a statistically significant effect on particle size, underscoring its importance in influencing the response variable. The adequate precision value of 6.6351 was found to be effective, as discussed earlier. Although the Predicted R2 (0.2252) is lower than the Adjusted R2 (0.4359), the overall model accounts for approximately 52.99% of the total variance (R2 = 0.5299). Furthermore, the lack of fit is insignificant (p = 0.5655), indicating that the model provides a satisfactory fit for the data, as confirmed by the software. These findings validate the significance of Bi composition as a determining factor for particle size and suggest that, although the model could be improved, it provides a reliable basis for interpretation and further experimentation.
The ANOVA results for the cubic model applied to the response variable, stability, indicate that the model is statistically significant. It has a p-score of 0.0028 and an F-score of 18.37, suggesting that the observed effects are implausible due to random variation. Among the factors in the model, the Bi composition (A), its squared term (A2), the squared term of Ca composition (B2), and the interaction term (AB2) are significant, all with p-score less than 0.05 which is standard limit. This establishes that these factors meaningfully influence the stability of the system. Additionally, the model demonstrates excellent predictive capability, as shown by a high R2 value of -1.3952 and an Adjusted R2 of 0.9102. These values indicate that the model explains over 91% of the variability in the response. The Adequate Precision value of 14.846 confirms the model’s reliability for navigating the design space, as discussed earlier. Overall, the results strongly support the use of the cubic model for capturing key effects and optimizing the system’s stability.
Model equation
The coded equations from Eqs. 1–3 represents the model regarding standardized factor levels, where each factor’s high and low levels are coded as + 1 and − 1, respectively. It can be used to predict the response (porosity, size, and stability) based on the relative levels of the input factors. Additionally, the magnitude and sign of the coefficients indicate the strength and direction of each factor’s effect on the response, making this form of the equation particularly useful for assessing the relative influence of each variable:
Equation for the porosity estimation:
Equation for the size (S):
Equation for the Stability (ST)
Residual studies
The residual analysis plots provide valuable insights into the adequacy of the model and the assumptions underlying the regression. In the residual vs. predicted plot shown in Fig. 10a-b, the residuals are randomly scattered around the zero line without any discernible pattern. This indicates homoscedasticity (constant variance) and suggests that the model fits the data well across the range of predicted values. The lack of a systematic structure in this plot supports the assumptions of linearity and independence of errors. Furthermore, the color gradient representing porosity, stability, and size values does not show any clustering that could indicate a correlation between the residuals and the responses, further validating the model’s adequacy.
Overall, the residual plots confirm that the assumptions of the model are reasonably met, and the regression results can be considered reliable for predicting the stability response variable. Additionally, all points representing the runs remain close to the diagonal line for the predicted vs. actual plot. This indicates that the chosen model is appropriate for the given runs and can be optimized within the design space. The normal probability plot of residuals, shown in Fig. 5b, indicates that most residuals are relatively close to the reference line. This suggests that the residuals are approximately normally distributed, supporting the normality assumption of regression. However, some deviations observed at the tails may indicate minor departures from normality. These deviations are not severe enough to undermine the validity of the model.
Optimization
The variation in Bi composition significantly impacts porosity, stability, and particle size, as illustrated in Fig. 11. As the Bi content increases from 0 to 6wt.%, porosity gradually rises from approximately 23 nm to 27 nm (Fig. 11a). This suggests that higher Bi levels contribute to a more porous structure, likely due to microstructural changes during the material’s formation. Stability, measured in millivolts, shows a nonlinear trend with Bi composition. Stability (Fig. 11b) is relatively higher (more negative) at intermediate Bi levels (around 2–4 wt%) but decreases at lower and higher extremes. This implies that moderate concentrations of Bi enhance the surface charge and structural uniformity, thereby improving stability. In contrast, the average particle size (Fig. 11c) increases significantly with rising Bi content, growing from about 100 nm to over 140 nm. This indicates that higher Bi levels promote particle growth or agglomeration. Overall, while increasing Bi composition favors porosity and particle size, optimal stability is achieved at mid-range Bi levels. Thus, a balanced Bi concentration in the range of 2–4wt.% appears to be ideal for achieving desirable material properties. The standard error for all the three responses, i.e., porosity, size and stability are also in the acceptable range and hence the chosen model is in the favor of optimization.
Validation studies
The point prediction for the optimization model chosen in the current work for a two-sided interval confidence yielded a 95% certainty level, with a population level of 99%. To confirm the reproducibility, validation, incorporation and concentration of Bi, two case studies were performed. The reproducibility and validation results, in terms of porosity, stability, and particle size, are presented in Table 5. Case-I was for reproducibility, considering a random composition (5wt.% Bi and 35wt.% Ca), which was not suggested by RSM. From Case-I, at a random composition of 5wt.% Bi, the predicted model in the present study (Table 5) confirmed the incorporation and concentration of Bi with a reasonable percentage difference, i.e., porosity (1.51%), stability (6.30%), and size (6.30%). Case-II was for validation, where 6 wt% Bi and 34 wt% Ca was considered as per Ref. [15]. In Case-II, the validation study results showed that the physical properties, including porosity, stability, and particle size, were in close agreement with percentage differences of 1.6%, 3.17%, and 2.31%, respectively, which fell within the tolerance limits of ± 5%. These results are also closely aligned with the correlation equation. It was found that the experimental results were in good agreement with the model developed in the present study. The stability of at 5 wt. Bi was reported as 10.32mV, which is very low indicating poor stability due to aggregation and agglomeration, thus influencing the desired characteristics for biological environments, such as the biodegradation rate. Whereas the higher value is moderately stable at higher composition of Bi i.e., 6wt.%.
To corroborate the relationship between compositional variation and nanoparticle properties such as porosity, stability, and morphology, zeta potential, TEM, and BET analyses were conducted for both Case-I and Case-II. Figure 12a presents the zeta potential measurements for nanoparticles containing 6 wt% Bi, comparing the present study with data from Ref. [15]. The observed deviations in stability are minimal and within acceptable limits. The BET isotherm in Fig. 12b indicates mesoporosity, with nanoparticle diameters of 24.70 nm for Ref. [15] and 25.00 nm for the current work, demonstrating only a minor difference. TEM micrographs confirm that the nanoparticles are uniformly spherical and consistent in size. The presence of Bi was further assessed to determine its influence on biological behavior. EDX verified the elemental composition, as shown in Fig. S1. Additional studies, including biocompatibility, cytotoxicity, and antibacterial assays, demonstrated that the nanoparticles are suitable for biological applications. Notably, nanoparticles with 6wt% Bi exhibited lower porosity, higher cell viability, reduced cytotoxicity, and improved biocompatibility compared to those with 5wt% Bi, which showed moderate cytotoxicity. This suggests that reduced porosity, likely resulting from the encapsulation of Bi by Si and subsequent network distortion, contributes to increased cytotoxicity.
Conclusions
The current study utilized a bioactive glass system with varying compositions of bismuth (0–6 wt%, Bi) through the sol-gel process. To assess the influence of Bi composition on the physical characteristics of the bioactive glass for biological applications, a CCD based RSM optimization technique was employed. The following conclusions were drawn:
-
Regarding porosity, the ANOVA results for the linear model indicated a p-value of 0.0155, suggesting that the chosen model terms are significant.
-
For stability, the cubic model’s ANOVA results showed a p-value of 0.0004, indicating that the model is significant. Both Bi and calcium (Ca) compositions significantly influenced stability, as observed from Eq. 3, which includes the combination of factors A and B.
-
In terms of nanoparticle size, the linear model’s ANOVA analysis yielded a p-value of 0.0004, indicating that the selected model parameters have a significant impact on the results.
-
Reproducibility and validation studies suggested the robustness of the optimization study carried out in the present study.
-
The biocompatibility, cytotoxicity, and antibacterial investigations confirmed that the as-prepared samples displayed moderate toxicity, i.e., within the acceptable range, indicating that an increase in Bi wt% may induce cytotoxicity, as expected. While biocompatibility, i.e., HA formation, has been found to be suitable for regeneration applications in bone tissues, further enhanced studies are in progress. Furthermore, antibacterial investigations suggested excellent antibacterial activity against the chosen bacterial strain. However, more detailed and various bacterial strains are under investigation for future work.
Based on these conclusions, it can be confirmed that the selected model for the current system configuration is suitable and that the optimization method employed is feasible. Future work aims to enhance the biocompatible characteristics of this model, thereby realizing its full potential.
Data availability
The raw data has been provided as a supplementary file.
References
Griffith, D. M., Li, H., Werrett, M. V., Andrews, P. C. & Sun, H. Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles. Chem. Soc. Rev. 50 (21), 12037–12069 (2021).
Zhang, X. et al. Functionalized mesoporous bioactive glass scaffolds for enhanced bone tissue regeneration. Sci. Rep. 6 (1), 19361 (2016).
Mehrabi, T., Mesgar, A. S. & Mohammadi, Z. Bioactive glasses: A promising therapeutic ion release strategy for enhancing wound healing. ACS Biomater. Sci. Eng. 6 (10), 5399–5430 (2020).
Moeini, A. et al. A critical review of bioactive glasses and glass-ceramics in cancer therapy. Int. J. Appl. Glass Sci. 14 (1), 69–87 (2023).
Hench, L. L. & Jones, J. R. Bioactive glasses: Frontiers and challenges. Front. Bioeng. Biotechnol. 3, 194 (2015).
Adam, S. N. F. S., Zainuddin, F. & Osman, A. F. Effect of varying phosphate content on the structure and properties of sol-gel derived SiO2-CaO-P2O5 bio-glass. J. Phys.: Conf. Ser. 2080 (1), 012018 (2021).
Flores-Jacobo, A., Aguilar-Reyes, E. A. & León-Patiño, C. A. Effect of dopants on the physical, mechanical, and biological properties of porous scaffolds for bone tissue engineering. Biomed. Mater. Devices. 1 (1), 234–255 (2023).
Abd Aladel, B., Sabree, I. K. & Edrees, S. J. Effects of Mgo wt% on the structure, mechanical, and biological properties of bioactive glass-ceramics in the SiO2, Na2O, CaO, P2O5, Mgo system. Int. J. Mech. Eng. Technol. 10, 97–106 (2019).
Taherian, M., Rojaee, R., Fathi, M. & Tamizifar, M. Effect of different sol-gel synthesis processes on microstructural and morphological characteristics of hydroxyapatite-bioactive glass composite nanopowders. J. Adv. Ceram. 3, 207–214 (2014).
Lepry, W. C., Naseri, S. & Nazhat, S. N. Effect of processing parameters on textural and bioactive properties of sol–gel-derived Borate glasses. J. Mater. Sci. 52, 8973–8985 (2017).
Khuri, A. I. & Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev.: Comput. Stat 2 (2), 128–149 (2010).
Akturk, A., Erol Taygun, M. & Goller, G. Optimization of the electrospinning process variables for gelatin/silver nanoparticles/bioactive glass nanocomposites for bone tissue engineering. Polym. Compos. 41 (6), 2411–2425 (2020).
Fernandez, C., Verné, E., Vogel, J. & Carl, G. Optimisation of the synthesis of glass-ceramic matrix biocomposites by the response surface methodology. J. Eur. Ceram. Soc. 23 (7), 1031–1038 (2003).
Filgueiras, M. R. T., La Torre, G. & Hench, L. L. Solution effects on the surface reactions of three bioactive glass compositions. J. Biomed. Mater. Res. 27 (12), 1485–1493 (1993).
Gara, D. K., Gujjala, R., SP, R. & Madaboosi. Sol–gel synthesis and characterization of silica intertwined bismuth-based bioactive glass coalescence possibility towards biological applications. J. Nanoparticle Res., 26 (5), 85 (2024).
Turner, J. et al. The effect of Si species released from bioactive glasses on cell behaviour: A quantitative review. Acta Biomater. 170, 39–52 (2023).
Aklilu, T. C. et al. Citric acid catalyst-assisted bioactive glass with hydrogen peroxide for in vitro bioactivity and biodegradability using sol‐gel method. Int. J. Biomater. 2023 (1), 9911205 (2023).
Bakare, F. F., Ewente, B. G., Akililu, T. C., Jara, A. D. & Gonfa, G. M. Synthesis and characterization of bioactive glass via CTAB modified Sol-Gel method for in vitro biological activities. J. Biomed. Mater. Res. Part. B: Appl. Biomater. 113 (3), e35548 (2025).
Chuni, T., Dachasa, K., Gochole, F., Hunde, T. & Bakare, F. F. Effect of morphology on the in vitro bioactivity and biocompatibility of spray pyrolyzed bioactive glass. Adv. Mater. Sci. Eng. 2023 (1), 5858858 (2023).
Zambon, A., Malavasi, G., Pallini, A., Fraulini, F. & Lusvardi, G. Cerium containing bioactive glasses: A review. ACS Biomater. Sci. Eng. 7 (9), 4388–4401 (2021).
Baino, F., Montazerian, M. & Verné, E. Cobalt-doped bioactive glasses for biomedical applications: A review. Materials 16 (14), 4994. (2023).
Kaou, M. H., Furkó, M., Balázsi, K. & Balázsi, C. Advanced bioactive glasses: The newest achievements and breakthroughs in the area. Nanomaterials. 13 (16), 2287 (2023).
Szewczyk, A., Skwira-Rucińska, A., Osińska, M. & Prokopowicz, M. The apatite-forming ability of bioactive glasses–A comparative study in human serum and kokubo’s simulated body fluid. Ceram. Int. 50 (23), 51030–51042 (2024).
Fellenberg, J. et al. Bioactive glass selectively promotes cytotoxicity towards giant cell tumor of bone derived neoplastic stromal cells and induces MAPK signalling dependent autophagy. Bioact. Mater. 15, 456 (2022).
Bakar, S. A. A. et al. Combination of goniothalamin and sol-gel‐derived bioactive glass 45S5 enhances growth inhibitory activity via apoptosis induction and cell cycle arrest in breast cancer cells MCF‐7. BioMed Res. Int. 2022 (1), 5653136 (2022).
Dziadek, M., Dziadek, K., Checinska, K., Zagrajczuk, B. & Cholewa-Kowalska, K. Bioactive glasses modulate anticancer activity and other polyphenol-related properties of polyphenol-loaded PCL/bioactive glass composites. ACS Appl. Mater. Interfaces. 16 (19), 24261–24273 (2024).
Kokubo, T. & Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27 (15), 2907–2915 (2006).
Güven, G., Perendeci, A. & Tanyolaç, A. Electrochemical treatment of deproteinated Whey wastewater and optimization of treatment conditions with response surface methodology. J. Hazard. Mater. 157 (1), 69–78 (2008).
Al-Shahrabalee, S. Q. & Jaber, H. A. Bioinorganic preparation of hydroxyapatite and rare earth substituted hydroxyapatite for biomaterials applications. Bioinorg. Chem. Appl. 2023 (1), 7856300 (2023).
Karampour, H., Parsa, M. A., Moghadam, A. H., Pourhasan, B. & Ashiri, R. Facile solution-based synthesis of impurity-free hydroxyapatite nanocrystals at ambient conditions. J. Mater. Res. Technol. 16, 656–674 (2022).
Hu, S., Chang, J., Liu, M. & Ning, C. Study on antibacterial effect of 45S5 Bioglass®. J. Mater. Science: Mater. Med. 20 (1), 281–286 (2009).
Zhou, P., Garcia, B. L. & Kotsakis, G. A. Comparison of antibacterial and antibiofilm activity of bioactive glass compounds S53P4 and 45S5. BMC Microbiol., 22 (1), 212 (2022).
Pajares-Chamorro, N. et al. Silver-doped bioactive glass particles for in vivo bone tissue regeneration and enhanced methicillin-resistant Staphylococcus aureus (MRSA) inhibition. Mater. Sci. Eng.: C, 120, 111693 (2021).
Author information
Authors and Affiliations
Contributions
D.K.G, R.G, and S.P.P: investigation, methodology, and wrote the main manuscriptN.S.M, and S.O.: investigation, methodology, formal analysis, and data curationA.K.B Writing – review & editing, data curation, and visualisation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gara, D.K., Gujjala, R., P., S.P. et al. Advanced optimization of dopant profiles in bismuth-enhanced bioactive glass through response surface methodology. Sci Rep 15, 39060 (2025). https://doi.org/10.1038/s41598-025-23580-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-23580-0