Abstract
Accurate risk stratification in community-acquired pneumonia (CAP) is essential for guiding timely and effective clinical management. This study evaluated whether the combined assessment of circulating T-lymphocyte subsets (T-cell subsets) and serum procalcitonin (PCT) enhances diagnostic accuracy and severity classification in CAP. T-cell subsets, as markers of immune response, may complement traditional inflammatory biomarkers. A retrospective analysis was conducted on 320 adult CAP patients admitted to a tertiary hospital in Zhejiang, China, between February 2020 and November 2021. Patients were stratified using the Clinical Pulmonary Infection Score (CPIS ≤ 6 vs. > 6) and platelet-to-lymphocyte ratio (PLR ≤ 163 vs. > 163). Laboratory assessments included serum PCT, white blood cell count (WBC), C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), interleukin-6 (IL-6), and T-cell subsets (CD3⁺, CD4⁺, CD8⁺ counts, and CD4/CD8 ratio). Pathogens were identified from 79 positive sputum or blood cultures. Patients with CPIS > 6 had significantly higher levels of PCT, PLR, WBC, CRP, NLR, and IL-6, along with lower CD3⁺ and CD4⁺ T-cell counts, a reduced CD4/CD8 ratio, and elevated CD8⁺ counts (all P < 0.05). Similar trends were observed in patients with PLR > 163. Acinetobacter baumannii was significantly more prevalent in patients classified as moderate-to-high risk or non-survivors (P < 0.05). T-cell subsets monitoring demonstrated a sensitivity of 93.2%, while PCT showed a specificity of 95.6% for predicting CAP severity. Combined evaluation of T-lymphocyte subsets and serum procalcitonin levels enhances the accuracy of CAP severity stratification. The elevated prevalence of Acinetobacter baumannii in severe cases highlights the importance of host–pathogen interactions. Integrated immune and inflammatory profiling may facilitate precision-based management strategies in CAP.
Similar content being viewed by others
Introduction
Community-acquired pneumonia (CAP) is a common lower respiratory tract infection and a leading cause of global morbidity and mortality, particularly outside hospital settings1. Its etiology is diverse, including bacteria, mycoplasma, chlamydia, and viruses. However, evolving microbial patterns, widespread antibiotic use, rising antimicrobial resistance, and an aging immunocompromised population have significantly altered CAP incidence and pathogen profiles2. T cells, especially thymus-derived subsets, play a central role in host immune responses and are critically involved in CAP pathogenesis3,4.
Accurate severity stratification is essential for guiding CAP treatment and clinical decision-making5. Common scoring systems such as the Pneumonia Severity Index (PSI) and CURB-65 are widely used to assess severity6. While CURB-65 helps predict 30-day mortality, its predictive power for broader clinical outcomes is limited7. As a result, there is growing interest in rapid and biologically relevant biomarkers to enhance risk assessment and inform treatment strategies8.
Inflammatory markers—including white blood cell (WBC) count, C-reactive protein (CRP), and procalcitonin (PCT)—are valuable for monitoring CAP progression9. In the UK, CAP accounts for over one million hospital admissions annually and is the seventh leading cause of death, with mortality rates between 8.9% and 15.7%10. In China, these rates are further driven by an aging population, pathogen shifts, and antibiotic overuse11. Individuals over 66 experience CAP at more than twice the rate of younger adults12, a trend linked to immunosenescence and nonspecific symptoms that delay diagnosis and treatment13,14. As CAP progresses, the risk of severe disease significantly increases15.
Severe CAP is associated with high healthcare resource use and increased mortality16. Its diagnosis requires more than clinical signs and includes laboratory, microbiological, and radiological assessments17. According to standard definitions, severe CAP may involve respiratory failure (respiratory rate ≥ 31/min, PaO₂ <61 mmHg), hemodynamic instability, altered consciousness, multi-lobar infiltrates, or renal dysfunction18. Although sputum and blood cultures are diagnostic gold standards, their low yield, slow turnaround, and high false-positive rates limit their use in acute settings19.
Among biomarkers, PCT has shown high sensitivity and specificity for bacterial infections and systemic inflammation20,21. Serum PCT levels are typically < 0.06 ng/mL under non-infectious conditions, but can rise more than tenfold during bacterial infection, supporting early diagnosis. Similarly, profiling T-cell subsets (CD3⁺, CD4⁺, CD8⁺, and CD19⁺) via flow cytometry offers insight into immune function and cellular immunity22. T-cell subset analysis is useful for assessing immunologic competence and has diagnostic and prognostic relevance in infectious, autoimmune, and hematologic disorders23. Severe infections often involve T-cell dysfunction, with a disrupted CD4⁺/CD8⁺ ratio indicating immune dysregulation, commonly seen in severe CAP24,25.
This study aimed to evaluate the diagnostic and prognostic value of T-cell subset monitoring and PCT levels in CAP. Specifically, we examined their association with disease severity using Clinical Pulmonary Infection Score (CPIS) and platelet-to-lymphocyte ratio (PLR), and analyzed pathogen distribution across PSI risk groups. Our findings aim to support precision medicine approaches in CAP management.
Materials and methods
Study design and participants
We conducted a retrospective analysis of clinical data from 526 adult patients with community-acquired pneumonia (CAP) admitted to the Department of Respiratory and Critical Care Diseases at Zhejiang Rongjun Hospital between January 2020 and December 2021. After excluding patients with incomplete clinical data, 320 patients (218 males, 102 females; mean age 56 ± 16 years) were enrolled in the study. Inclusion criteria were as follows:
-
Age ≥ 18 years, and diagnosis of CAP according to Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) guidelines, including those meeting the British Thoracic Society/American Thoracic Society criteria for severe CAP.
Exclusion criteria were:
-
Hospital-acquired pneumonia;
-
Immunosuppressive conditions (e.g., AIDS, connective tissue diseases);
-
Recent use of immunosuppressive medications or glucocorticoids;
-
Diagnosis of malignancy;
-
Other respiratory infections;
-
Acute cardiovascular events (e.g., myocardial infarction, cerebral infarction).
In this study, Clinical Pulmonary Infection Score (CPIS) was designated as the primary severity classification for stratifying patients into non-severe and severe CAP groups due to its established utility in capturing early disease severity through clinical and laboratory indicators. CPIS was chosen as the reference standard for outcome comparisons involving biomarker levels, including T-cell subsets and procalcitonin. In addition to CPIS, we also examined disease severity using CURB-65 and Pneumonia Severity Index (PSI) to enable comparison with established prognostic scoring systems and enhance generalizability of findings. The platelet-to-lymphocyte ratio (PLR) was included as an exploratory inflammatory biomarker and not as a formal disease classification tool; PLR-based subgrouping served to identify immuno-inflammatory phenotypes rather than define clinical severity per se.
Group stratification and data collection
Patients were stratified by platelet-to-lymphocyte ratio (PLR) into two groups (PLR ≤ 163 and PLR > 163) and by Clinical Pulmonary Infection Score (CPIS) into two groups (CPIS ≤ 6 and CPIS > 6). Clinical data were recorded within 24 h of hospital admission and included demographics (age, sex, comorbidities), vital signs (body temperature, heart rate, respiratory rate, blood pressure, level of consciousness), and laboratory parameters. The laboratory parameters comprised T-lymphocyte subsets (T-cell subsets; including CD3⁺, CD4⁺, CD8⁺ T-cell counts and the CD4⁺/CD8⁺ ratio), procalcitonin (PCT), white blood cell count (WBC), C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), and interleukin-6 (IL-6).
Laboratory measurements
Six milliliters of fasting venous blood were collected from each patient in the morning. For T-cell subsets analysis, blood samples were collected into tubes containing sodium citrate as anticoagulant. T-cell subsets (CD3⁺, CD4⁺, and CD8⁺ T-cell counts, and the CD4⁺/CD8⁺ ratio) were quantified in whole blood using a flow cytometer (Attune NxT, Thermo Fisher Scientific, Waltham, MA, USA). For PCT measurement, 6 mL of fasting venous blood was centrifuged at 4,000 rpm for 9 min to obtain serum. Serum PCT levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Hubei Jinmei Bioengineering Co., Ltd., Wuhan, China) according to the manufacturer’s instructions. All assay kits and procedures were followed precisely as specified by the manufacturers. For culture-positive patients, fungal isolates were considered clinically significant infections only if supported by radiological evidence, systemic inflammatory markers, and clinical response to antifungal therapy. Otherwise, they were regarded as colonizers.
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Continuous variables were tested for normality. Normally distributed data are presented as mean ± standard deviation (SD) and compared between two groups using Student’s t-test. Non-normally distributed data are presented as median (interquartile range) and compared using the Mann–Whitney U test. Categorical variables are expressed as counts (percentages) and compared using the chi-square (χ²) test. Spearman’s rank correlation coefficient was used to assess relationships between continuous variables. ROC analysis was conducted to assess the diagnostic accuracy of biochemical and immune markers in distinguishing severe vs. non-severe CAP as defined by CPIS. Prognostic endpoints such as mortality or ICU admission were recorded but not modeled in this analysis. The PLR cutoff value of 163 was determined by ROC curve analysis using the Youden Index to identify the optimal discrimination point for severe CAP (CPIS > 6). A two-tailed P value < 0.05 was considered statistically significant.
Results
General patient characteristics
A total of 320 adult patients with CAP were enrolled (218 men and 102 women; mean age 56 ± 16 years). According to the Pneumonia Severity Index (PSI), 219 patients were classified as low risk (LR) and 101 as moderate-to-high risk (MHR). By the CURB-65 criteria, 261 patients were considered low risk and 59 as high risk. Based on standard criteria for severe CAP, 285 patients had non-severe disease and 35 had severe disease. Seventeen patients died during hospitalization.
Comparison of biochemical marker levels by CPIS groups
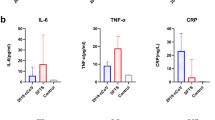
Patients were stratified by CPIS into two groups (≤ 6 vs. > 6). Serum levels of platelet-to-lymphocyte ratio (PLR), procalcitonin (PCT), white blood cell count (WBC), C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), and interleukin-6 (IL-6) were compared between the groups. As shown in Fig. 1, the CPIS > 6 group had significantly higher levels of all these markers than the CPIS ≤ 6 group (all P < 0.05).
T-cell subsets level discrepancies among patients with varying CPIS scores
Patients were stratified by CPIS into two groups (≤ 6 vs. > 6) and serum levels of T-cell subsets were compared. As shown in Fig. 2, the CPIS > 6 group had significantly lower CD3⁺ and CD4⁺ T cell counts and a lower CD4⁺/CD8⁺ ratio compared to the CPIS ≤ 6 group (all P < 0.05). In contrast, CD8⁺ T cell counts were significantly higher in the CPIS > 6 group (P < 0.05).
Biochemical marker levels by PLR groups
Patients were divided into two PLR groups (≤ 163 vs. > 163). Serum levels of PCT, WBC, CRP, NLR, and IL-6 were compared between the groups. As shown in Fig. 3, the PLR > 163 group had significantly higher levels of these markers compared to the PLR ≤ 163 group (all P < 0.05).
Comparative analysis of T-cell subsets levels across CAP patients with divergent PLR levels
Patients were stratified into two groups based on PLR (≤ 163 vs. > 163), and serum levels of T-cell subsets were compared. As shown in Fig. 4, the PLR > 163 group had significantly lower CD3⁺ and CD4⁺ T cell counts and a lower CD4⁺/CD8⁺ ratio than the PLR ≤ 163 group (all P < 0.05). In contrast, CD8⁺ T cell counts were significantly higher in the PLR > 163 group (P < 0.05).
The significance of serum biochemical markers and T-cell subsets levels in assessing CAP severity
Receiver operating characteristic (ROC) curves were constructed to evaluate the diagnostic utility of serum biochemical markers and lymphocyte subsets in determining CAP severity. As shown in Fig. 5A; Table 1, among the biochemical markers, PLR exhibited the largest area under the curve (AUC), while the WBC count had the smallest AUC. Similarly, in Fig. 5B; Table 1, the CD4⁺/CD8⁺ ratio showed the largest AUC and the CD3⁺ T cell count had the smallest AUC for predicting CAP severity.
Pathogenic bacteria distribution among CAP patients with identical PSI scores
In total, 70 Gram-negative bacterial strains (57.7%), 16 Gram-positive strains (11.7%), and 33 fungal strains (30.5%) were isolated from the 320 CAP patients (Table 2). Seven patients had fungal infections. According to PSI classification, the detection rate of Acinetobacter baumannii was significantly higher in the moderate-to-high risk (MHR) group than in the low-risk group (P < 0.05). In contrast, the detection rates of Pseudomonas aeruginosa and Staphylococcus aureus did not differ significantly between these groups (both P > 0.05).
As shown in Figs. 6 and 17 patients died during hospitalization. Among these non-survivors, the isolated pathogens included 7 strains of A. baumannii, 2 of S. aureus, 2 of P. aeruginosa, and 1 fungal strain. The detection rate of A. baumannii was significantly higher in the deceased patients compared to survivors (P < 0.05).
Utility of T-cell subsets monitoring in conjunction with PCT detection for severe CAP diagnosis
For diagnosing severe CAP, T-cell subsets monitoring had a sensitivity of 93.2% and a specificity of 83.1%. In comparison, PCT detection had a sensitivity of 87.5% and a specificity of 95.6%.
Discussion
The rising incidence and mortality of community-acquired pneumonia (CAP) are closely linked to evolving pathogen profiles and an increasing number of immunocompromised individuals. This has intensified efforts to identify reliable biomarkers and microbial indicators for early diagnosis and risk stratification. Traditional inflammatory markers such as white blood cell (WBC) count and C-reactive protein (CRP) have long been used in clinical practice. More recently, the platelet-to-lymphocyte ratio (PLR) and procalcitonin (PCT) have enhanced diagnostic accuracy. As a calcitonin precursor, PCT is highly specific for bacterial infections and is now widely utilized to guide antibiotic therapy. Systemic inflammation in CAP can also trigger coagulation pathways, contributing to increased capillary permeability and thrombosis26. Nonetheless, the relationship between these biomarkers and CAP severity remains under investigation, and pathogen distributions vary geographically.
PCT is typically produced at low levels by thyroid parafollicular cells under non-infectious conditions. During bacterial infection, however, its synthesis is markedly upregulated by peripheral mononuclear cells and the liver. Multiple studies have confirmed PCT’s superior sensitivity over WBC and CRP in detecting bacterial CAP27,28. Its association with disease severity has also been demonstrated29,30, and when combined with tools like PSI and CURB-65, it provides improved prognostic insight compared to PCT alone31.
In our study, CPIS-based stratification revealed that patients with CPIS > 6 had significantly elevated levels of PLR, PCT, WBC, CRP, neutrophil-to-lymphocyte ratio (NLR), and interleukin-6 (IL-6). They also exhibited lower CD3⁺ and CD4⁺ T-cell counts, a reduced CD4⁺/CD8⁺ ratio, and elevated CD8⁺ T-cell counts (all P < 0.05), suggesting that increased severity is accompanied by both inflammatory amplification and immune dysregulation. We further analyzed pathogen distribution across 320 CAP patients stratified by PSI scores. Gram-negative bacteria accounted for 75.8% of isolates, with Pseudomonas aeruginosa being the most frequently detected (25/95). Among Gram-positive organisms, Staphylococcus aureus was the most common (10/95), and nine cases involved fungal pathogens. In the low-risk (LR) group, P. aeruginosa predominated (17/43), while traditional CAP pathogens like Streptococcus pneumoniae and Haemophilus influenzae were detected in only a few cases. In contrast, A. baumannii was more frequent in the moderate-to-high-risk (MHR) group (14/37) and absent in low-risk survivors. Among the 17 non-survivors, A. baumannii was isolated in seven cases, suggesting a possible link to poor outcomes.
This study incorporated multiple severity scoring systems (CPIS, CURB-65, PSI) to allow cross-comparison of diagnostic markers within different clinical frameworks. CPIS was selected as the primary reference for statistical analysis due to its real-time applicability and emphasis on infection-related parameters. The inclusion of PSI and CURB-65 provided additional context on patient prognosis, particularly mortality risk, while PLR stratification was used to explore immune-inflammatory trends rather than to define severity. While multivariate logistic regression across all severity indices was beyond the scope of this retrospective study, future prospective work should aim to integrate biomarker levels within multivariable severity prediction models.
Our pathogen distribution findings align with prior literature indicating increased pathogen detection rates in patients with higher PSI scores. However, some studies report no clear differences in specific pathogen types (e.g., S. pneumoniae, Mycoplasma, H. influenzae) across severity levels. Notably, few Chinese studies have systematically evaluated pathogen profiles by severity grade. In our cohort, detection of classic CAP pathogens such as S. pneumoniae and H. influenzae was low, while multidrug-resistant organisms were more prevalent. Several factors likely contributed: (1) inconsistent sputum quality, with nearly half of samples deemed inadequate; (2) widespread empirical antibiotic use prior to hospitalization, particularly fluoroquinolones, which suppress typical bacteria and promote resistance; and (3) suboptimal sampling timing. These factors may have led to underdiagnosis of conventional CAP pathogens. Improved patient education and sampling protocols are needed to enhance diagnostic accuracy.
While several fungal species were isolated, we recognize that some may represent colonization, particularly in patients with chronic pulmonary conditions or prior broad-spectrum antibiotic use. Only eight fungal cases were classified as true infections based on clinical, radiologic, and inflammatory criteria. This distinction is critical, as misclassification could influence perceived associations with disease severity or outcomes.
Notably, the proportion of S. pneumoniae in our cohort (3 out of 95 identified pathogens) was considerably lower than the 7.43% reported in a recent national survey of adult CAP in China32. Several factors may account for this discrepancy. First, our study population included a substantial number of elderly patients and individuals with underlying comorbidities, particularly COPD, which is associated with increased colonization or infection by A. baumannii and P. aeruginosa rather than S. pneumoniae. Second, pre-hospital antibiotic use was common among the patients referred to our hospital, which may have disproportionately suppressed typical pathogens like S. pneumoniae, while allowing multidrug-resistant organisms to predominate in culture. Third, the quality and timing of specimen collection, especially in patients with limited sputum production, may have influenced the recovery of fastidious organisms such as S. pneumoniae. Taken together, these variables underscore the importance of considering patient background and institutional context when interpreting pathogen distribution patterns in CAP.
Interestingly, our data revealed elevated CD8⁺ T-cell counts in patients with higher CPIS scores, in contrast to previous reports of CD8⁺ depletion in severe pneumonia33. This discrepancy may be due to population differences; many of our patients had chronic inflammatory conditions such as COPD, which can alter T-cell dynamics and induce compensatory CD8⁺ proliferation. Moreover, the timing of immune profiling may play a role—early activation can precede immune exhaustion. These observations highlight the need for longitudinal immunophenotyping in CAP.
Despite these limitations, our findings have practical implications. In the LR group, P. aeruginosa was frequently isolated in elderly patients, underscoring the need for vigilance in older individuals. In the MHR group, A. baumannii was strongly associated with mortality. Clinicians should maintain heightened suspicion for this pathogen in high-risk CAP cases. Given our study’s single-center, retrospective nature, future multicenter prospective studies are warranted to validate these associations and further clarify the role of immune biomarkers and pathogen profiles in CAP severity.
Limitations
This study’s retrospective design presents several limitations. First, a subset of patients was transferred from external hospitals or care facilities after receiving broad-spectrum antibiotics (e.g., fluoroquinolones, third-generation cephalosporins), introducing the potential for misclassification bias. Some cases presumed to represent community-acquired pneumonia (CAP) may have instead reflected healthcare-associated (HCAP) or early hospital-acquired pneumonia (HAP). The relatively high detection rates of Acinetobacter baumannii and Pseudomonas aeruginosa—organisms more typically associated with nosocomial infections—support this concern. Although patients with documented HAP were excluded, aspiration events or early nosocomial infections in referred patients may have been inadvertently included, potentially inflating the proportion of multidrug-resistant pathogens and underrepresenting traditional CAP organisms such as Streptococcus pneumoniae.
Second, fungal isolates may not always indicate true infection. Only eight fungal-positive cases in our cohort were deemed clinically significant based on radiologic findings, systemic inflammatory markers, and response to antifungal therapy. The possibility of colonization, particularly in patients with chronic pulmonary disease or prior antibiotic exposure, cannot be excluded. Future studies should consider excluding transferred patients and those with likely nosocomial pathogens to more accurately delineate the immune profile of true CAP.
Third, although this study emphasized the diagnostic utility of T-cell subsets and procalcitonin (PCT) in assessing CAP severity, their prognostic value—such as predicting ICU admission or in-hospital mortality—was not directly analyzed. While mortality data and pathogen distributions by survival status were collected, formal survival analysis was not performed. Prospective studies incorporating time-to-event models are warranted to validate these biomarkers as independent prognostic tools.
Additionally, due to the retrospective nature and limited event rates, multivariable regression analyses were not conducted. Therefore, we could not control for potential confounding factors such as age, comorbidities, or baseline disease severity. As a result, the independent predictive value of T-cell subsets and PCT remains uncertain. Future prospective studies should incorporate logistic or Cox regression models to improve the robustness and generalizability of the findings.
Lastly, the PLR threshold of 163 was determined via internal ROC curve analysis. While statistically valid for this cohort, its applicability in other populations or clinical contexts requires external validation.
Conclusion
This study demonstrates that monitoring T-cell subsets and serum procalcitonin levels provides valuable insight into the severity of community-acquired pneumonia (CAP), correlating well with established indices such as PSI and CURB-65. Both biomarkers offer rapid and accessible tools for early risk stratification, aiding in timely clinical decision-making. Notably, patients in the low-risk group—particularly elderly individuals—should be monitored for Pseudomonas aeruginosa, while those in the moderate-to-high-risk group showed a higher prevalence of Acinetobacter baumannii, warranting empiric antibiotic coverage. The high treatment success rate observed supports the effectiveness of this stratified approach. Importantly, T-cell subsets monitoring exhibited greater sensitivity, while PCT offered higher specificity in identifying severe CAP. Therefore, the combined use of T-cell subsets and PCT enhances diagnostic accuracy and supports personalized management strategies in CAP.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Bian, L. Q. et al. T cell responses in senior patients with community-acquired pneumonia related to disease severity. Exp. Cell. Res. 361 (1), 56–62 (2017).
Hannaway, R. F. et al. Mucosal-associated invariant T cells and T cells in community acquired pneumonia: association of abundance in sputum with clinical severity and outcome. Clin. Exp. Immunol. 199 (12), 201–215 (2020).
Lu, Y. et al. Expression and clinical correlations of costimulatory molecules on peripheral t lymphocyte subsets of early-stage severe sepsis: a prospective observational study. Shock Vol. 49 (6), 631–640 (2018).
Iturra, P. A. et al. Progression of type 2 helper T cell-type inflammation and airway remodeling in a rodent model of naturally acquired subclinical primary Pneumocystis infection. Am. J. Pathol. 188 (2), 417–431 (2018).
Trisolini, R. et al. Bronchoalveolar lavage findings in severe community-acquired pneumonia due to Legionella Pneumophila serogroup 1. Respir Med. 98 (22), 1222–1226 (2004).
Fan, G. et al. Comparison of severity scores for COVID-19 patients with pneumonia: a retrospective study, Eur Respir. vol. 56, no. 3, pp. ArticleID 2002113. (2020).
Zhao, B., Chen, Y. Y. & Tan, M. Q. Value of lymphocyte count in assessing cellular immune function in patients with community-acquired pneumonia. Nan Fang Yi Ke Da Xue Xue Bao. 36 (2), 273–276 (2016).
Grayson, K. M. et al. Activation-dependent modulation of Streptococcus pneumoniae-mediated death in human lymphocytes. Pathog Dis. 75 (2), ftx008 (2017).
Lu, B. et al. IL-17 production by tissue-resident Mait cells is locally induced in children with pneumonia. Mucosal Immunol. 13 (5), 824–835 (2020).
Small, C. B. et al. Community-acquired opportunistic infections and defective cellular immunity in heterosexual drug abusers and homosexual men. Am. J. Med. 74 (3), 433–441 (1983).
Zhu, W. et al. Serum levels of SIRT3 and other inflammatory factors are associated with clinical outcomes and prognosis in severe community- acquired pneumonia in adults: a prospective study. Med. (Baltim). 100 (32), e26721 (2021).
Rachina, S. et al. Biomarker value in the diagnosis of community-acquired pneumonia with concomitant chronic heart failure. J. Clin. Med. 10 (19), 4570 (2021).
Keramat, F. et al. Association of serum procalcitonin and c-reactive protein levels with curb-65 criteria among patients with community-acquired pneumonia. Int. J. Gen. Med. Vol. 13 (11), 217–223 (2018).
Lee, J. Y. et al. Clinical significance of serum procalcitonin in patients with community-acquired Lobar pneumonia. Korean J. Lab. Med. 30 (4), 406–413 (2010).
Stockmann, C. et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J. Pediatr. Infect. Dis. Soc. Vol. 7 (1), 46–53 (2018).
Quah, J. et al. Impact of microbial aetiology on mortality in severe community-acquired pneumonia. BMC Infect. Dis. Vol. 18 (1), 451 (2018).
Póvoa, P. Serum markers in community-acquired pneumonia and ventilator-associated pneumonia. Curr. Opin. Infect. Dis. Vol. 21 (2), 157–162 (2008).
Pilotto, A. et al. Combined use of the multidimensional prognostic index (MPI) and procalcitonin serum levels in predicting 1-month mortality risk in older patients hospitalized with community-acquired pneumonia (CAP): A prospective study. Aging Clin. Exp. Res. Vol. 30 (2), 193–197 (2018).
Crisafulli, E. et al. Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest 143 (4), 1009–1017 (2013).
Taşbakan, M. S. et al. Serum procalcitonin and C-reactive protein kinetics as indicators of treatmentoutcome in hospitalized patients withcommunity-acquired pneumonia. Turk. J. Med. Sci. 46 (5), 1422–1427 (2016).
Chen, L. et al. Incidence and risk factors for cardiovascular events in patients hospitalized with community-acquired pneumonia. Zhonghua Xin Xue Guan Bing Za Zhi. 48 (3), 228–235 (2020).
Schuetz, P. et al. ProHOSP study group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the prohosp randomized controlled trial. JAMA Vol. 302 (10), 1059–1066 (2009).
Zhou, Q. et al. Clinical efficacy of nutrition support therapy combined with antibiotics in the patients of community-acquired pneumonia and its influence on serum PCT and CRP. Pak J. Pharm. Sci. Vol. 32 (5), 2477–2480 (2019).
Wang, Y. et al. The usefulness of serum procalcitonin, c-reactive protein, soluble triggering receptor expressed on myeloid cells 1 and clinical pulmonary infection score for evaluation of severity and prognosis of community-acquired pneumonia in elderly patients. Arch. Gerontol. Geriatr. no. 80, 53–57 (2019).
Çelikhisar, H., Daşdemir, G., Ilkhan, Ç. & Arabaci Prognostic factors in elderly patients admitted to the intensive care unit with community-acquired pneumonia. Aging Male. 23 (5), 1425–1431 (2020).
Zhang, L. et al. Predictive value of arterial blood lactate/serum albumin ratio for myocardial injury in elderly patients with severe community-acquired pneumonia. Med. (Baltim). 101 (4), e28739 (2022).
Horie, M. et al. Diagnostic and prognostic value of procalcitonin in community-acquired pneumonia. Am. J. Med. Sci. 343 (1), 30–35 (2012).
Chen, L. et al. Lipid profiles and differential lipids in serum related to severity of community-acquired pneumonia: a pilot study. PLoS One Vol. 16 (3), e0245770 (2021).
Sartori, L. F. et al. Pneumonia severity in children: utility of procalcitonin in risk stratification. Hosp. Pediatr. 11 (3), 215–222 (2021).
Kim, J. H. et al. Usefulness of plasma procalcitonin to predict severity in elderly patients with community-acquired pneumonia. Tuberc Respir Dis. (Seoul). 74 (5), 207–214 (2013).
Christ-Crain, M. et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am. J. Respir Crit. Care Med. 174 (1), 84–93 (2006).
He, L. X. [Pay attention to new evolution trends in the etiology of community-acquired pneumonia in adults]. Zhonghua Jie He He Hu Xi Za Zhi. 47 (6), 576–581. https://doi.org/10.3760/cma.j.cn112147-20231024-00264 (2024). Chinese.
Wang, J. et al. CD4⁺ T cells related to disease severity in elderly and frailty community-acquired pneumonia patients: A retrospective cohort study. Immun. Inflamm. Dis. 11 (9), e1009. https://doi.org/10.1002/iid3.1009 (2023).
Author information
Authors and Affiliations
Contributions
Fuquan Qi contributed to data collection, statistical analysis, and manuscript drafting. Yaoyao Zheng conceptualized the study, supervised the project, and revised the manuscript critically for intellectual content. Shuxian Zhang assisted with laboratory testing and data interpretation. Hua Yang contributed to clinical data acquisition. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Zhejiang Rongjun Hospital (Approval No. 881/ZRH). All methods were performed in accordance with relevant guidelines and regulations, including the Declaration of Helsinki. Informed consent was obtained from all participants or their legal guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qi, F., Zheng, Y., Zhang, S. et al. Assessment of T-cell subsets and procalcitonin for diagnosing and stratifying severity in community acquired pneumonia. Sci Rep 15, 40130 (2025). https://doi.org/10.1038/s41598-025-23926-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-23926-8