Abstract
Metabolic syndrome (MetS) among adolescents with overweight/obesity (OW/OB) is rising rapidly in low- and middle-income countries (LMICs), yet evidence on theory-driven behavioral interventions remains limited. To estimate the prevalence and risk factors of MetS in Vietnamese adolescents aged 11–14 years with OW/OB, and to evaluate the effectiveness of a 12-month lifestyle intervention grounded in the COM-B model. We conducted a three-phase study: (1) a province-wide cross-sectional survey to determine MetS prevalence; (2) a matched case–control study to identify risk factors; and (3) a school-based COM-B intervention comprising health education, environmental modifications, and motivation enhancement. Adherence was quantified using a six-point scale and categorized as high (≥ 75%), moderate (40–<75%), or low (< 40%). The primary outcome was MetS resolution at 12 months according to International Diabetes Federation (IDF) pediatric criteria; secondary outcomes included anthropometric, biochemical, and behavioral changes. Of 6,009 students screened, 2,055 (34.2%) had OW/OB, among whom 628 (30.6%; 95% CI, 28.6–32.6) had MetS, with hypertriglyceridemia (87.9%) and low HDL-C (84.4%) being most prevalent. Waist-to-height ratio ≥ 0.46 was the strongest independent risk factor (OR = 2.96; 95% CI: 1.76–5.00), while exclusive breastfeeding for 6 months was protective (OR = 0.45; 95% CI: 0.35–0.58). A 12-month COM-B–guided lifestyle intervention in 300 OW/OB adolescents with MetS achieved a 71.7% resolution rate (95% CI: 66.2–76.7), with significant improvements in metabolic parameters (triglycerides − 0.41 mmol/L, HDL-C + 0.12 mmol/L, CAP − 29.6 dB/m; all p < 0.001) and health behaviors (sugar-sweetened beverage intake − 4.7 times/week, screen time − 3.6 h/day). High adherence was associated with the greatest cardiometabolic and behavioral gains, supporting a dose–response relationship consistent with the COM-B framework. A school-based COM-B intervention substantially improved MetS and health behaviors among adolescents with OW/OB, with effectiveness varying by adherence level. The model appears feasible and scalable in LMIC settings, although longer-term follow-up is needed to assess sustainability.
Similar content being viewed by others
Introduction
The prevalence of overweight and obesity (OW/OB) among children and adolescents is rising rapidly worldwide, with a particularly pronounced burden in low- and middle-income countries (LMICs)1,2. This trend is accompanied by early-onset metabolic disturbances, among which metabolic syndrome (MetS) is a strong risk factor for future cardiovascular disease and type 2 diabetes in adulthood3. Evidence indicates that youths with OW/OB have a substantially higher risk of MetS than their normal-weight peers, and that metabolic abnormalities often emerge during puberty4,5.
In Viet Nam, recent epidemiological data show that OW/OB among school-aged children has increased sharply—from 8.5% in 2010 to 19.0% in 2020 among those aged 5–19 years—particularly in urban areas6,7,8. However, studies on MetS prevalence, its risk factors, and theory-driven behavioral interventions in this age group remain limited. Most risk factors for MetS are behaviorally modifiable, including unhealthy dietary patterns, physical inactivity, insufficient sleep, and prolonged screen time9. We focused on adolescents aged 11–14 years because this corresponds to early-to-mid puberty—a period marked by rapid physical, hormonal, and behavioral changes—when dietary and activity habits begin to consolidate4,5.
The COM-B model (Capability, Opportunity, Motivation—Behaviour)10,11 is a widely used theoretical framework for designing health behavior change interventions. In adolescent settings, COM-B has been applied to co-design school-based physical activity interventions12, to develop programs enhancing physical activity among children in China13, and to guide nutrition/physical activity interventions across child and adolescent environments14,15. In LMIC contexts, COM-B has likewise informed formative research shaping child nutrition interventions (e.g., Kenya), supporting the generalizability of this framework beyond high-income settings16. Building on this evidence, we designed a COM-B–based intervention to concurrently enhance capability (education and skills), opportunity (school and family environments), and motivation (individual goal-setting and feedback) among adolescents aged 11–14 years with OW/OB.
This multi-phase study aimed to: (i) estimate the prevalence and identify risk factors of MetS among adolescents with OW/OB in a Mekong Delta province; (ii) evaluate the effectiveness of a 12-month COM-B–based lifestyle intervention on metabolic and behavioral outcomes; and examine the relationship between intervention adherence and clinical outcomes.
Methods
Study design
We conducted a three-phase sequential study in a province of the Mekong Delta, southern Viet Nam, from October 2023 to May 2025.
Phase 1: a school-based cross-sectional survey to estimate the prevalence of MetS among adolescents with OW/OB.
Phase 2: a matched case–control study to identify behavioral and clinical risk factors for MetS.
Phase 3: a 12-month school-based lifestyle intervention grounded in the COM-B model.
Reporting adhered to the STROBE and TIDieR guidelines17,18.
Participants
Inclusion criteria: students aged 11–14 years classified as OW/OB according to the WHO 2007 growth reference (body mass index [BMI]-for-age > + 1 SD for overweight; > +2 SD for obesity)19; without chronic endocrine, metabolic, or cardiovascular diseases; and free of acute illness during recruitment. For Phase 3, participants were additionally required to meet the 2007 International Diabetes Federation (IDF) consensus definition of MetS for ages 10–16 years (central obesity plus ≥ 2 additional criteria)20.
Exclusion criteria: refusal to participate, anticipated change of residence, or medical contraindications to physical activity. Written informed consent was obtained from parents/guardians and assent from students.
Sampling strategy
Phase 1: Sample size for prevalence estimation was calculated using a single-proportion formula, assuming an OW/OB prevalence of 27.8%21, design effect of 2, and a 6.1% margin of error. We used multistage cluster sampling stratified by urban/rural areas, randomly selected schools, and conducted school-wide anthropometric screening.
Phase 2: Each MetS case was matched 1:1 with a non-MetS control by age, sex, school, and nutritional status.
Phase 3: A single-group pre–post intervention required 300 participants, based on an expected improvement rate of 95%. We applied stratified cluster sampling with peer pairing to support group-based behavior change and followed participants for 12 months (Supplementary Methods Appendix, pp: 1–2).
Schools were notified one week in advance (principal and homeroom teachers). Students absent on the data-collection day were contacted again within one week.
Data collection and measurements
Data were collected on school premises by a trained research team. Anthropometry (weight, height, waist and hip circumferences) followed WHO procedures19. Blood pressure (BP) was measured three times at ≥ 1-minute intervals; the first reading was discarded and the mean of the second and third readings was used. If the second and third readings differed by > 5 mmHg, a fourth measurement was taken and the mean of the third and fourth readings was recorded.
Fasting venous blood was collected to quantify plasma glucose, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glycated hemoglobin (HbA1c) using internationally standardized methods. Non-invasive assessment of hepatic steatosis and fibrosis was performed with FibroScan® to obtain the controlled attenuation parameter (CAP) and liver stiffness measurement (LSM)22.
Diagnostic thresholds and cut-offs followed the IDF pediatric criteria (fasting glucose ≥ 100 mg/dL [5.6 mmol/L]; systolic BP ≥ 130 mmHg and/or diastolic BP ≥ 85 mmHg; HDL-C < 40 mg/dL [1.03 mmol/L]; TG ≥ 150 mg/dL [1.7 mmol/L])20; waist-to-hip ratio (WHR): elevated WHR > 0.8923; waist-to-height ratio (WHtR): at-risk WHtR ≥ 0.4624; American Diabetes Association (HbA1c)25; and CAP ≥ 248 dB/m and LSM ≥ 5.5 kPa on FibroScan®26,27.
Behavioral and lifestyle assessment
A structured questionnaire captured dietary habits, physical activity, screen time, and sleep. Students aged 11–12 years received assistance from teachers or parents to ensure comprehension; older students self-completed the questionnaire.
Intervention activities were coordinated across schools and families with collaboration among school staff, the research team, and parents.
-
Health education was delivered monthly through interactive classes, small-group discussions, and media videos. Content emphasized risks of obesity-related chronic diseases and the role of healthy lifestyles. Parent workshops were held quarterly to strengthen knowledge and home supervision.
-
Dietary guidance was provided via a nutrition handbook, sample menus, and evidence-based rules (reducing sugar, salt, and saturated fat, increasing vegetables and fiber) to improve diet quality while meeting developmental energy needs. No absolute calorie-restriction (weight-loss) diet was prescribed; the focus was on meal quality and eating behaviors. Parents were trained to support dietary adjustments at home.
-
Physical activity was encouraged for ≥ 60 min/day on ≥ 5 days/week, including school sports, walking, cycling, or home-based exercise. Students maintained activity logs and participated in peer-pair competitions to enhance motivation. Process data were collected periodically to assess behavior change.
Intervention design (COM-B framework)
The 12-month intervention targeted three components10,11:
Capability: health education on nutrition and physical activity, with skills training for meal planning and exercise scheduling.
Opportunity: improvements to the school environment, provision of healthier food options, and active engagement of parents.
Motivation: goal setting, individualized feedback, peer support, and recognition of progress.
Intervention reporting followed the TIDieR checklist18.
Adherence assessment
Adherence was quantified on a six-point scale derived from WHO and CDC recommendations for pediatric obesity management9,28, encompassing three domains (maximum 2 points each):
Health education & family participation: attendance at ≥ 80% of sessions with ≥ 1 family member participating in counseling.
Dietary behavior: sugar-sweetened beverages < 1 time/day and ≤ 3 times/week; avoidance of eating out > 3 times/week or eating while using screens.
Physical activity & sedentary behavior: ≥60 min/day of moderate-to-vigorous activity on ≥ 5 days/week; recreational screen time < 2 h/day on ≥ 5 days/week.
Scores were recorded quarterly; the maximum cumulative score was 24 over 12 months. Categories were defined as low (< 40%; mean score < 2.5), moderate (40–<75%; mean 2.5–<4.5), and high (≥ 75%; mean ≥ 4.5). Scoring was based on student self-reports, corroborated by teachers/school health staff and parent logs; discrepancies were resolved by consensus.
The primary outcome was MetS remission at 12 months (no longer meeting IDF criteria). Secondary outcomes included changes in anthropometric, biochemical, hepatic, and behavioral indicators (diet, physical activity, screen time, sleep). The adherence score served both as an exposure (for subgroup analyses) and as a process measure (Supplementary Methods Appendix, pp: 3–66).
Statistical analysis
Descriptive statistics summarized baseline characteristics and pre–post changes. Paired t-tests or Wilcoxon signed-rank tests were used for continuous variables; McNemar’s test for categorical variables. Logistic regression identified baseline risk factors for MetS. Generalized estimating equations (GEE) evaluated time × adherence interactions for continuous outcomes. Receiver operating characteristic (ROC) analysis assessed predictive models. Missing data were handled by multiple imputation under a missing-at-random assumption24. Analyses were conducted in Stata, with two-sided p < 0.05 considered statistically significant (Supplementary Methods Appendix, pp: 67–76).
Ethics statement
This study was approved by the Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam (Approval No. 505/HĐĐ-ĐHYD, dated May 4, 2023; ID: 23387-DHYD). All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from all participants and their legal guardians prior to enrollment. All personal data were anonymized and kept strictly confidential.
Results
Between October 2023 and May 2025, a three-phase sequential study was conducted in a representative province of the Mekong Delta region in southern Vietnam, including a cross-sectional survey of 6,009 children to assess the prevalence of MetS among OWOB children (October 2023–February 2024); a case–control study involving 1,192 children (February–May 2024); and a 12-month community-based intervention for 300 children diagnosed with MetS (May 2024–May 2025), implemented across rural, peri-urban, and urban areas.
Prevalence of MetS and risk factors
Among 6,009 screened students aged 11–14 years, 2,055 (34.2%) were classified as OW/OB according to the WHO 2007 (Table 1). Within this subgroup, 628 adolescents (30.6%; 95% CI, 28.6–32.6) met the IDF criteria for MetS20 (Supplementary Table S1). The most prevalent MetS components were elevated triglycerides (87.9%) and low HDL-C (84.4%). Notably, 96.5% of those with MetS had a WHtR ≥ 0.46 (Table 2).
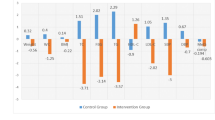
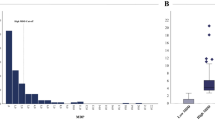
Based on univariable and multivariable logistic regression, several anthropometric, behavioral, and environmental factors independently predicted MetS among OW/OB adolescents. WHtR ≥ 0.46 emerged as the strongest risk factor (adjusted odds ratio [OR] 3.90; 95% CI, 2.29–6.65), followed by dislike of participating in physical activity (OR 2.28; 95% CI, 1.74–2.98) and sleep duration < 8 h/night (OR 2.43; 95% CI, 1.85–3.20) (Supplementary Tables S2, S4). Unhealthy eating behaviors were also contributory: eating out ≥ 3 times/week (OR 1.56; 95% CI, 1.23–1.99), eating while using screens (OR 1.71; 95% CI, 1.28–2.28), frequent availability of snack foods at home (OR 1.56; 95% CI, 1.22–2.01), frequent consumption of fried/stir-fried foods (OR 1.30; 95% CI, 1.01–1.67), and drinking bubble tea (OR 1.72; 95% CI, 1.34–2.21) (Supplementary Table S3). Sedentary behaviors—including watching TV > 2 h/day (OR 1.61; 95% CI, 1.17–2.21) and playing electronic games > 2 h/day (OR 1.41; 95% CI, 1.00–1.97)—were independently associated with MetS risk (Supplementary Table S4). Exclusive breastfeeding for the first six months was the only statistically significant protective factor (OR 0.58; 95% CI, 0.44–0.75) (Supplementary Table S2). A predictive model combining these 10 factors demonstrated good discrimination (AUC 0.77; optimal threshold 0.481), with sensitivity 70.3%, specificity 70.1%, positive predictive value 70.0%, and negative predictive value 70.1% (Fig. 1).
Adjusted risk estimates and model performance for predicting MetS among OW/OB adolescents. (A) Forest plot of adjusted odds ratios (ORs) with 95% confidence intervals (CIs) from the final multivariable logistic regression model predicting MetS among adolescents with OW/OB. The vertical dashed line denotes OR = 1; dots indicate point estimates and horizontal bars the 95% CIs. Variables retained in the final model included: WHtR ≥ 0.46, unwillingness to exercise, sleep < 8 h/night, CAP ≥ 248 dB/m, watching TV > 2 h/day, milk-tea consumption, HbA1c ≥ 5.7%, household snack availability, eating while watching TV/phone, and eating out ≥ 3 times/week; exclusive breastfeeding for 6 months was protective. (B) Receiver-operating-characteristic (ROC) curve for the 10-variable model. Model performance: AUC = 0.77; optimal probability threshold = 0.481, sensitivity = 70.3%, specificity = 70.1%, PPV = 70.0%, NPV = 70.1%. The ORs shown in the figure were derived from the adjusted ORs (aORs) reported in Supplementary Tables S2, S3, and S4; variables with statistically significant associations were entered into a final multivariable logistic regression model to predict MetS. All ORs were adjusted for covariates as described in the Methods section. See Methods for details on variable coding and multiple-imputation procedures. Two-sided tests were used for all analyses. ROC, receiver operating characteristic; AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; WHtR, waist-to-height ratio; CAP, controlled attenuation parameter; HbA1c, glycated hemoglobin.
Intervention adherence and behavior change by adherence level
The mean 12-month adherence score was 4.9 ± 0.9 points; 225 participants (75.0%) were classified as high adherence (≥ 4.5 points), 69 (23.0%) as moderate (2.5–<4.5), and 6 (2.0%) as low (< 2.5). High adherence was achieved across all three domains: health education/family participation (81.3%), dietary improvement (76.0%), and physical activity/reduction of sedentary behavior (78.7%). Compared with baseline, the high-adherence group showed marked improvements: eating out decreased by − 2.5 times/week (95% CI, − 2.8 to − 2.2), sugar-sweetened beverage intake by − 4.7 times/week (95% CI, − 5.0 to − 4.4), fried-food consumption by − 2.4 times/week (95% CI, − 2.7 to − 2.1), and recreational screen time by − 3.6 h/day (95% CI, − 3.9 to − 3.3). Sleep duration increased by + 1.5 h/night (95% CI, + 1.3 to + 1.7), and days/week with ≥ 60 min of activity increased by + 3.2 (95% CI, + 3.0 to + 3.4). All changes were significantly greater than in the moderate- and low-adherence groups (p < 0.001), reflecting behavior change aligned with COM-B target components (Table 3).
MetS resolution and clinical improvements
At 12 months, 215/300 participants (71.7%; 95% CI, 66.2–76.7) no longer met the IDF MetS criteria, alongside improvements in systolic blood pressure (− 3.67 mmHg), diastolic blood pressure (− 3.22 mmHg), fasting glucose (− 0.36 mmol/L), triglycerides (− 0.41 mmol/L), HDL-C (+ 0.12 mmol/L), HbA1c (− 0.15%), and hepatic steatosis (CAP: −29.6 dB/m); all p < 0.001. Mean WHtR decreased by 0.02 units (p < 0.001) (Supplementary Table S5). In GEE models, high adherence was associated with reductions in weight (β=−2.01), BMI (β=−1.04), waist circumference (β=−1.74), CAP (β=−7.65), and triglycerides (β=−0.42), all p < 0.001; HDL-C and height also improved (Table 4). Most associations remained significant after adjustment, indicating consistent intervention effects across cardiometabolic parameters. These findings highlight the comprehensive effectiveness of an intervention targeting capability, opportunity, and motivation—particularly in improving diet and physical activity in line with the COM-B model.
Discussion
This multi-phase study provides new evidence on the prevalence, risk factors, and modifiable behavioral correlates of MetS among adolescents with OW/OB in a LMIC and evaluates the effectiveness of a COM-B–based lifestyle intervention in improving both biomarkers and behaviors. We found that 30.6% of OW/OB adolescents aged 11–14 years met the IDF criteria for MetS20, indicating early metabolic risk in preadolescents—an age group that is seldom prioritized in school screening programs. This prevalence is comparable to reports from other LMICs29,30. Dyslipidemia—particularly hypertriglyceridemia and low HDL-C—was the most frequent abnormality, consistent with pediatric metabolic profiles described previously29,31.
The COM-B framework guided an intervention targeting (1) capability (nutrition education and physical-activity skills), (2) opportunity (supportive school and family environments), and (3) motivation (feedback, group goals, positive reinforcement). The intervention achieved a 12-month MetS remission rate of 71.7%, with the greatest improvements among participants with high adherence. Stratification by adherence revealed a clear dose–response pattern: higher adherence was associated with larger gains in health outcomes. Compared with lower-adherence peers, highly adherent students showed clinically meaningful improvements in BMI, blood pressure, hepatic steatosis indices, fasting glucose, and linear growth, together with behavioral changes—less eating out, reduced sugar-sweetened beverage intake, more physical activity, less screen time, and longer sleep duration. These findings align with prior work indicating that full engagement with health-education and lifestyle components is a prerequisite for reducing MetS risk32,33.
Our results are broadly consistent with COM-B–informed interventions among adolescents in LMIC settings. In China, Wang et al. (2021) applied the Behaviour Change Wheel/Theoretical Domains Framework to enhance capability for physical activity—mirroring our emphasis on skills for healthy meal planning and activity, and accompanying reductions in sugar-sweetened beverages and eating out34. In Kenya, formative research by McClintic et al. underscored the importance of opportunity in designing child-nutrition programs, including mobilizing family and environmental support—consonant with our integration of school setting and parental engagement to sustain positive behaviors16. Furthermore, as tested by Willmott et al. (2021), motivation can mediate the effects of capability and opportunity on behavior change, supporting our observation that goal-setting, feedback, and peer recognition helped sustain change15.
Generalized estimating equation (GEE) models using change scores (Δ) highlighted adherence as a central driver of anthropometric and metabolic improvements among OW/OB students. After adjusting for age, sex, grade, site, and household income, each one-unit increase in adherence (0–6 scale) was associated with a mean decrease of 1.04 BMI units (β = −1.04; 95% CI, − 1.19 to − 0.90; p < 0.001), corresponding to − 2.01 kg of body weight and − 1.74 cm in waist circumference over 12 months. These statistically significant effects are also practically meaningful and meet WHO definitions of “useful response” in community interventions35,36. Notably, mean height increased by 0.96 cm in the high-adherence group, indicating that the program did not impede physical growth and may support holistic development. This is salient given persistent concerns about growth compromise in pediatric weight-management programs, particularly in high-income settings where stringent energy-restriction models have sometimes been associated with unintended effects on stature37,38. In adjusted analyses, some indices—such as fasting glucose, ALT, and diastolic blood pressure—did not reach statistical significance, possibly reflecting the limited intervention duration or the need for more intensive family-based components, consistent with Meng (2025) on NAFLD risk stratification in pediatric obesity39. Similar patterns have been reported in long-term European programs (e.g., IDEFICS, STOPP), where deeper metabolic changes often require ≥ 18 months of exposure and adherence > 75% to yield stable improvements40,41.
The sample was drawn from a single province in southern Viet Nam, which may limit generalizability to regions with different ethnic compositions or socioeconomic contexts. Dietary habits and family engagement characteristic of the Mekong Delta could have enhanced responsiveness. The 12-month follow-up may be insufficient to assess long-term maintenance of behavior and metabolic improvements; future research should extend follow-up duration in line with adolescent intervention literature. Early-life covariates—including pubertal stage, birthweight, and maternal gestational diabetes—were unavailable, raising the possibility of residual confounding.
These findings support integrating theory-driven behavioral interventions—particularly COM-B—into school-based obesity prevention and management. A combined emphasis on capability, opportunity, and motivation can concurrently improve cardiometabolic and behavioral outcomes, especially when adherence is high. Scaling this model to other provinces in Viet Nam and to comparable LMICs contexts may represent a cost-effective strategy to reduce early MetS risk and its long-term sequelae.
Conclusion
In this multi-phase study, we show that MetS affects a substantial proportion of adolescents with OW/OB in southern Viet Nam, and that a school-based lifestyle intervention designed using the COM-B framework can significantly improve both cardiometabolic risk and health behaviors within 12 months. Intervention effectiveness was strongly adherence-dependent, with the most favorable outcomes observed among participants achieving high adherence.
These findings highlight the importance of embedding theory-driven behavior-change frameworks, such as COM-B, into adolescent obesity prevention and management programs—particularly in low- and middle-income countries where resources must be optimized. Concurrently targeting capability, opportunity, and motivation provides a feasible, scalable, and locally adaptable model to mitigate early cardiometabolic risk.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and ethical restrictions but are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CAP:
-
Controlled attenuation parameter
- CI:
-
Confidence interval
- COM-B:
-
Capability, opportunity, motivation – behavior
- DBP:
-
Diastolic blood pressure
- GEE:
-
Generalized estimating equation
- HbA1c:
-
Hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- IDF:
-
International Diabetes Federation
- LSM:
-
Liver stiffness measurement
- MetS:
-
Metabolic syndrome
- NPV:
-
Negative predictive value
- OR:
-
Odds ratio
- OWOB:
-
Overweight and obesity
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- SBP:
-
Systolic blood pressure
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- TIDieR:
-
Template for Intervention Description and Replication
- WHR:
-
Waist-to-hip ratio
- WHtR:
-
Waist-to-height ratio
- WHO:
-
World Health Organization
References
Global Burden of Disease Study. Global, regional, and National prevalence of child and adolescent overweight and obesity, 1990–2021, with forecasts to 2050: a forecasting study for the global burden of disease study 2021. Lancet. 405(10481), 785–812. https://doi.org/10.1016/s0140-6736(25)00397-6 (2025) (Epub 2025/03/07).
Zhang, X. et al. Global prevalence of overweight and obesity in children and adolescents: A systematic review and meta-analysis. JAMA Pediatr. 178(8), 800–813. https://doi.org/10.1001/jamapediatrics.2024.1576 (2024) (Epub 2024/06/10).
Chung, Y. L. & Rhie, Y-J. Metabolic syndrome in children and adolescents. Ewha Med. J. 45(4), e13. https://doi.org/10.12771/emj.2022.e13 (2022).
de Lamas, C. et al. Progression of metabolic syndrome and associated cardiometabolic risk factors from prepuberty to puberty in children: the PUBMEP study. 13–2022 https://doi.org/10.3389/fendo.2022.1082684 (2022).
Sun, Y. et al. Early puberty: a review on its role as a risk factor for metabolic and mental disorders. 12–2024 https://doi.org/10.3389/fped.2024.1326864 (2024).
Tran, T. V. A., Vu, T. Q. C., Tran, Q. D., Nguyen, D. T. & Phan, N. Q. The prevalence of obesity among school-aged children in vietnam: A systematic review and meta-analysis. Hum. Nutr. Metabolism. 31, 200184. https://doi.org/10.1016/j.hnm.2023.200184 (2023).
Pham, T. T. P. et al. Reducing the incidence of overweight and obesity by a healthy lifestyle intervention program for schoolchildren in Hanoi, Vietnam: a randomized controlled trial. BMC Public. Health. 24(1), 2579. https://doi.org/10.1186/s12889-024-20120-9 (2024).
Ministry of Health (Viet Nam). The Ministry of Health announces the results of the 2019–2020 National Nutrition Survey. Hanoi: Ministry of Health. Updated 15 Apr 2021. https://moh.gov.vn/hoat-dong-cua-lanh-dao-bo/-/asset_publisher/TW6LTp1ZtwaN/content/bo-y-te-cong-bo-ket-qua-tong-ieu-tra-dinh-duong-nam-2019-2020? (2021) (Accessed 25 May 2025).
World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour. https://www.ncbi.nlm.nih.gov/books/NBK566046/ (World Health Organization, 2020) (Accessed 27 May 2024).
Michie, S., van Stralen, M. M. & West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 6(1), 42. https://doi.org/10.1186/1748-5908-6-42 (2011).
Cane, J., O’Connor, D. & Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 7(1), 37. https://doi.org/10.1186/1748-5908-7-37 (2012).
McQuinn, S., Belton, S., Staines, A. & Sweeney, M. R. Co-design of a school-based physical activity intervention for adolescent females in a disadvantaged community: insights from the girls active project (GAP). BMC Public. Health. 22(1), 615. https://doi.org/10.1186/s12889-022-12635-w (2022).
Khambalia, A. Z., Dickinson, S., Hardy, L. L., Gill, T. & Baur, L. A. A synthesis of existing systematic reviews and meta-analyses of school-based behavioural interventions for controlling and preventing obesity. Obes. Reviews: Official J. Int. Association Study Obes. 13(3), 214–233. https://doi.org/10.1111/j.1467-789X.2011.00947.x (2012) (Epub 2011/11/11).
Rosenkranz, R. R., Ridley, K., Guagliano, J. M. & Rosenkranz, S. K. Physical activity capability, opportunity, motivation and behavior in youth settings: theoretical framework to guide physical activity leader interventions. Int. Rev. Sport Exerc. Psychol. 16(1), 529–553. https://doi.org/10.1080/1750984X.2021.1904434 (2023).
Willmott, T. J., Pang, B. & Rundle-Thiele, S. Capability, opportunity, and motivation: an across contexts empirical examination of the COM-B model. BMC Public. Health. 21(1), 1014. https://doi.org/10.1186/s12889-021-11019-w (2021).
McClintic, E. E. et al. Application of the capabilities, opportunities, motivations, and behavior (COM-B) change model to formative research for child nutrition in Western Kenya. Curr. Developments Nutr. 6(7), nzac104. https://doi.org/10.1093/cdn/nzac104 (2022).
von Elm, E. et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ (Clinical Res. ed). 335(7624), 806–808. https://doi.org/10.1136/bmj.39335.541782.AD (2007) (Epub 2007/10/20).
Hoffmann, T. C. et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. Br. Med. J. 348, g1687 https://doi.org/10.1136/bmj.g (2014).
de Onis, M. et al. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 85(9), 660–667. https://doi.org/10.2471/blt.07.043497 (2007) (Epub 2007/11/21).
Zimmet, P. et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr. Diabetes. 8(5), 299–306. https://doi.org/10.1111/j.1399-5448.2007.00271.x (2007) (Epub 2007/09/14).
Phan, H. D. et al. National survey of impaired blood glucose and risk factors for type 2 diabetes among Vietnamese children aged 11–14 years, 2018. Vietnam J. Diabetes Endocrinol. (42), 72–79. https://doi.org/10.47122/vjde.2020.42.10 (2021).
European Association for the Study of the Liver. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J. Hepatol. 75(3), 659–689. https://doi.org/10.1016/j.jhep.2021.05.025 (2021) (Epub 2021/06/25).
Widjaja, N. A., Arifani, R. & Irawan, R. Value of waist-to-hip ratio as a predictor of metabolic syndrome in adolescents with obesity. Acta bio-medica: Atenei Parmensis. 94(3), e2023076. https://doi.org/10.23750/abm.v94i3.13755 (2023) (Epub 2023/06/16).
Zong Xn, Kelishadi, R. et al. Establishing international optimal cut-offs of waist-to-height ratio for predicting cardiometabolic risk in children and adolescents aged 6–18 years. BMC Med. 21(1), 442. https://doi.org/10.1186/s12916-023-03169-y (2023).
Committee, ADAPP. 2. Diagnosis and classification of diabetes: standards of care in diabetes—2025. Diabetes Care. 48(Supplement_1), S27-S49. https://doi.org/10.2337/dc25-S002 (2025).
Fajrudheen, M., Mahapatro, S., Panigrahi, M. K., Naik, S. & Satapathy, A. K. Noninvasive assessment of nonalcoholic fatty liver disease in children with overweight and obesity by transient elastography. 29(2), 230–236. https://doi.org/10.4103/ijem.ijem_150_24 (2025).
Sirli, R. & Sporea, I. Controlled attenuation parameter for quantification of steatosis: which cut-offs to use? Can. J. Gastroenterol. Hepatol. 2021, 6662760. https://doi.org/10.1155/2021/6662760 (2021) (Epub 2021/04/10).
Centers for Disease Control Prevention. Physical Activity Guidelines for Children and Adolescents. Atlanta. (2022).
Bitew, Z. W. et al. Metabolic syndrome among children and adolescents in low and middle income countries: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 12(1), 93. https://doi.org/10.1186/s13098-020-00601-8 (2020).
Wentzel, A. et al. Prevalence of metabolic syndrome in children and adolescents with obesity: a systematic review and meta-analysis. Obes. (Silver Spring Md). 33(1), 12–32. https://doi.org/10.1002/oby.24159 (2025) (Epub 2024/12/03).
Noubiap, J. J. et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: a systematic review and modelling analysis. Lancet Child. Adolesc. Health. 6(3), 158–170. https://doi.org/10.1016/S2352-4642(21)00374-6 (2022).
Baumgartner, P. C., Haynes, R. B., Hersberger, K. E. & Arnet, I. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front. Pharmacol. 9, 1290. https://doi.org/10.3389/fphar.2018.01290 (2018) (Epub 2018/12/14).
Giovanazzi, A. et al. Current practice in the measurement and interpretation of intervention adherence in randomised controlled trials: A systematic review. Contemp. Clin. Trials. 118, 106788. https://doi.org/10.1016/j.cct.2022.106788 (2022) (Epub 2022/05/14).
Wang, H., Blake, H. & Chattopadhyay, K. Development of a school-based intervention to increase physical activity levels among Chinese children: A systematic iterative process based on behavior change wheel and theoretical domains framework. 9–2021. https://doi.org/10.3389/fpubh.2021.610245 (2021).
World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World (World Health Organization, 2018).
Mitsinikos, T. et al. FISPGHAN statement on the global public health impact of metabolic dysfunction-associated steatotic liver disease. J. Pediatr. Gastroenterol. Nutr. 80(3), 397–407. https://doi.org/10.1002/jpn3.12399 (2025) (Epub 2024/12/27).
Brown, T. et al. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 7(7), Cd001871. https://doi.org/10.1002/14651858.CD001871.pub4 (2019) (Epub 2019/07/25).
Colquitt, J. L. et al. Diet, physical activity, and behavioural interventions for the treatment of overweight or obesity in preschool children up to the age of 6 years. Cochrane Database Syst. Rev. 3(3), Cd012105. https://doi.org/10.1002/14651858.Cd012105 (2016) (Epub 2016/03/11).
Meng, F. et al. Ultrasound-guided attenuation parameter: a liver fat quantification technique for forecasting the progression of metabolic dysfunction-associated steatotic liver disease in overweight/obese patients. Clin. Radiol. 84, 106854. https://doi.org/10.1016/j.crad.2025.106854 (2025) (Epub 2025/04/09).
Sobko, T. et al. A randomised controlled trial for overweight and obese parents to prevent childhood obesity–Early STOPP (STockholm obesity prevention Program). BMC Public. Health. 11, 336. https://doi.org/10.1186/1471-2458-11-336 (2011) (Epub 2011/05/20).
Smit, M. S., Boelens, M., Mölenberg, F. J. M., Raat, H. & Jansen, W. The long-term effects of primary school-based obesity prevention interventions in children: A systematic review and meta-analysis. Pediatr. Obes. 18(3), e12997. https://doi.org/10.1111/ijpo.12997 (2023) (Epub 2022/12/23) .
Acknowledgements
The authors would like to express their sincere gratitude to the Department of Science and Technology, Department of Health, and Department of Education and Training of the province, as well as the District Education Offices, for their strong support during the implementation of this study. We also deeply appreciate the cooperation of school principals, teachers, parents, and students who participated in data collection. Finally, we acknowledge the valuable assistance of staff from provincial general hospitals and local health units in facilitating data collection and processing.
Funding
This study was funded by the Department of Science and Technology under Contract No. 1490/HĐ-SKH&CN dated October 10, 2023. The funder had no role in the design of the study; data collection, analysis, or interpretation; or the decision to publish the results.
Author information
Authors and Affiliations
Contributions
Nguyen Thanh Nam: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Visualization, Writing – original draft. Ta Van Tram: Methodology, Validation, Resources, Writing – review & editing. Phung Nguyen The Nguyen: Conceptualization, methodology, supervision, project administration, funding acquisition, writing – review & editingAll the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam (Approval No. 505/HĐĐĐ-ĐHYD, dated May 4, 2023). Written informed consent was obtained from all participants and their legal guardians prior to data collection.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nam, N.T., Tram, T.V. & Nguyen, P.N.T. A COM-B-guided lifestyle intervention reduces metabolic syndrome in overweight and obese adolescents: a multiphase study in Vietnam. Sci Rep 15, 40296 (2025). https://doi.org/10.1038/s41598-025-24085-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-24085-6