Abstract
Organ transplantation extends life expectancy; however, tacrolimus, a key immunosuppressant, carries risks of nephrotoxicity. This study explores dexpanthenol’s potential to protect against tacrolimus-induced nephrotoxicity. This study was conducted using 30 male Wistar-Albino rats (aged 4 months, weighing 250–300 g), randomly divided into four groups. The control group received 0.5 cc of saline solution (SF) once daily, the TAC group received 0.4 mg/kg tacrolimus, the DEX group received 500 mg/kg dexpanthenol, and the TAC + DEX group received 0.4 mg/kg tacrolimus and 500 mg/kg dexpanthenol intraperitoneally. Biochemical parameters and kidney damage were assessed on day 14, with tissues examined by blinded pathologists using hematoxylin-eosin staining.The effects of tacrolimus on oxidative balance and the potential protective properties of dexpanthenol were investigated. Statistical analyses were performed using SPSS 20.0 software, with a p-value of < 0.05 considered significant. No significant differences were found between the groups for TAS (p = 0.827), TOS (p = 0.596), OSI (p = 0.575), SOD (p = 0.952), and NO (p = 0.365) values. However, significant differences were observed in IL-6 (p = 0.008), CRP (p = 0.034), TNF-alpha (p = 0.014), TGF-β (p = 0.012), MDA (p = 0.001), urea(p = 0.005), and creatinine (p = 0.002) levels. Pathological examination revealed significant findings in Bowman capsule expansion (p = 0.003), the presence of mitosis (p = 0.005), vasa recta leukocyte infiltration (VRLI) (p = 0.007), tubular regeneration (p = 0.004), tubular dilation (p = 0.003), tubular necrosis (p = 0.048), cast formation (p = 0.020), and loss of the brush border (p = 0.019). The only parameter without a significant difference among the groups was interstitial inflammation (p = 0.559). This study shows that dexpanthenol provides limited protection against tacrolimus-induced nephrotoxicity and requires further validation.
Similar content being viewed by others
Introduction
Solid organ transplantation holds critical significance as a life-saving intervention that substantially enhances the quality of life for patients suffering from end-stage organ failure1. In recent years, advancements in organ transplantation techniques have yielded remarkable outcomes, particularly among pediatric and young adult patients. Kidney transplantation, the cornerstone of solid organ transplantation, was first performed in the 1950s and has since become a vital treatment modality for patients with end-stage renal disease (ESRD), serving as an alternative to dialysis therapy. A successful kidney transplant frees patients from lifelong dependence on dialysis, significantly improving their quality of life2.
According to the World Health Organization (WHO), a total of 140,411 organ transplants were performed worldwide in 2021, including 92,532 kidney transplants. Of these, 38% were obtained from living donors, representing a 14.3% increase over the figures reported in 20203,4.
The concept of organ transplantation can be traced back to mythological accounts from prehistoric times but has developed substantially in the modern era. Scientific efforts in this field began in the early 20th century and culminated in the first successful kidney transplant between identical twins in 1954. In Turkey, this milestone was achieved in 1975, when Dr. Haberal and his team performed the nation’s first successful kidney transplant5,6.
The development of immunosuppressive drugs has represented a major milestone in improving graft survival after organ transplantation. These agents have markedly enhanced transplant outcomes by suppressing acute rejection; however, they have not fully eliminated the risk of chronic rejection7. “The mechanisms of action of immunosuppressive drugs involve processes such as the suppression of T-cell activation and cytokine production. Tacrolimus, a calcineurin inhibitor, acts by inhibiting IL-2 synthesis. Its mechanism of action is 10 to 100 times more potent than that of other immunosuppressive agents8,9,10. Tacrolimus has been widely used to prevent rejection after organ transplantation; however, its use has also been linked to several adverse effects, particularly nephrotoxicity9.
Dexpanthenol (DEX) is the biologically active alcohol analog of pantothenic acid (vitamin B5), which is readily converted to pantothenic acid in mammalian tissues. Pantothenic acid serves as a precursor of coenzyme A (CoA), a molecule that plays a pivotal role in glutathione synthesis, cellular metabolism, and fatty acid biosynthesis11,12. Through its antioxidant properties, DEX neutralizes free radicals, enhances endogenous antioxidant enzyme activity, and protects cells against oxidative damage by reducing lipid peroxidation13. Additionally, DEX supports wound healing by modulating key inflammatory mediators, including IL-6 and IL-1β.14 DEX exerts anti-inflammatory effects by promoting fibroblast proliferation and enhancing collagen synthesis15.
The protective effects of DEX on renal function have been demonstrated in various experimental models of nephrotoxicity induced by colistin16, amikacin17, cisplatin18,19 and contrast agents20. However, a review of the literature revealed no studies examining the protective effects of dexpanthenol against tacrolimus-induced nephrotoxicity19. In this study, we aim to evaluate the protective role of dexpanthenol on biochemical, histopathological, and oxidative stress mechanisms associated with tacrolimus-induced nephrotoxicity.
Materials and methods
This study was designed as an experimental, prospective, and randomized study and was approved by the Local Ethics Committee for Animal Experiments of Çukurova University during its meeting on September 18, 2023, under decision number 5 (Appendix-1). The research was conducted on 30 adult male Wistar-Albino rats, aged 4 months, raised at the Health Sciences Experimental Practice and Research Center. The study was conducted in accordance with the scientific principles outlined in Article 21 of the Declaration of Helsinki21, animal welfare principles, and ARRIVE 2.0 Guidelines22, with animals randomly divided into four groups. The groups were arranged with 6 rats in the control group and 8 rats in each of the other groups.
Experimental groups and drug administration
The tacrolimus used in the study was obtained from Molgentek® and prepared as a suspension by diluting 100 mg of the pure formulation with physiological saline (PS)23. The doses of the preparations used were determined as follows: PS 0.5 cc19, TAC 0.4 mg/kg/day24 and DEX 500 mg/kg19. Subsequently, the rats were divided into four groups:
-
1.
CONTROL (PS): 0.5 cc of physiological saline (PS) was administered intraperitoneally (i.p.) daily for 14 days.
-
2.
TAC: Tacrolimus at a dose of 0.4 mg/kg was administered i.p. daily for 14 days.
-
3.
DEX: Dexpanthenol at a dose of 500 mg/kg was administered i.p. daily for 14 days.
-
4.
TAC + DEX: Tacrolimus (0.4 mg/kg) and Dexpanthenol (500 mg/kg) were administered i.p. daily for 14 days.
Anesthesia, analgesia, and euthanasia with blood sample collection
Twenty-four hours after the administration of tacrolimus and dexpanthenol, the weight of all rats was measured. Anesthesia was then induced by intraperitoneal administration of 20 mg/kg xylazine and 50 mg/kg ketamine25. For biochemical analysis, 5–7 cc of blood was collected intracardially from the rats and transferred into EDTA-containing purple-top tubes and gel-containing biochemistry tubes. All rats were euthanized using the cervical dislocation method. After coagulation, the blood samples were centrifuged, and the serum was transferred into Eppendorf tubes and stored at -20 °C.
Evaluation of blood samples
Whole blood
Blood samples collected in EDTA-containing purple-top tubes were analyzed within 1 h using the Exigo® Eos Veterinary Hematology Analyzer. This device is capable of measuring red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), leukocytes (WBC), and platelets (PLT). The samples were processed in accordance with the device protocol, and measurements were performed using the device’s automatic calibration. The analyses were based on optical methods and the impedance principle.
Whole blood samples were also utilized for GSH, GSSG, and total glutathione analyses using the MINDRAY-BS400 analyzer (Otto Scientific, Baran Medical, Ankara, Turkey), following the manufacturer’s procedures and kits with CAT.NO: Otto3053.
In samples treated with Trichloroacetic Acid (TCA), Oxidized Glutathione (GSSG) was reduced to Reduced Glutathione (GSH) using Sodium Borohydride (NaBH4) and Sodium Hydroxide (NaOH). The pH decrease caused by TCA was adjusted by adding Sodium Hydroxide (NaOH), and the excess Sodium Borohydride (NaBH4) was removed by treatment with Hydrochloric Acid (HCl).
GSH levels
GSH levels were measured using the Ellman method modified by Hu. In this method, the thiol groups of GSH reduce 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) to 2-nitro-5-benzoic acid, producing a spectrophotometric absorbance at 412 nm. The measured GSH content was subtracted from the total GSH and GSSG to calculate the amount of Oxidized Glutathione (GSSG). Based on these values, the GSH/GSSG ratio was determined26.
Glutathione reductase (GR)
GR levels were measured using a spectrophotometric method based on the reduction of oxidized glutathione (GSSG) to reduced glutathione (GSH) in the presence of NADPH. The unit of measurement is mmol/g, and the activity is typically reported as mmol/g Hb.27
Glucose-6-phosphate dehydrogenase (G6PDH)
G6PDH was measured in whole blood using a colorimetric method with a REL ASSAY MINDRAY-BS401 device (CAT.NO: RL0550). The activity was expressed in mmol/g and normalized to hemoglobin levels, with results reported as G6PDH-Hb (IU/g Hb).
ELISA (enzyme-linked immunosorbent assay) test
ELISA kits were used in accordance with the manufacturers’ instructions. The parameters analyzed from serum samples and the corresponding kit information are detailed in Table 1 below.
Histopathological Sample Collection
Following euthanasia, all rats underwent bilateral nephrectomy via abdominal incision. Kidney tissues were fixed in 10% formalin solution. The tissues were sectioned into 5 mm slices and processed with alcohol, xylene, and paraffin, followed by sectioning into 4 μm slices and staining with hematoxylin and eosin.
Histopathological evaluations were conducted blindly by two pathologists using a light microscope (Olympus, x10, x20, x40). Markers of tubular damage were quantitatively assessed by evaluating the percentage of tubules exhibiting cell necrosis, loss of the brush border, cast formation, Bowman capsule expansion, vasa recta leukocyte infiltration, tubular regeneration, interstitial inflammation, and tubular dilatation at the corticomedullary junction.
The scoring system was defined as follows
-
0, no damage;
-
1, ≤ 10%
-
2, 11–25%
-
3, 26–45%
-
4, 46–75%
-
5, > 76% of tubules affected28
Histopathological analysis was performed independently by two blinded observers. For each sample, a minimum of 10 high-power fields (HPFs) per section was examined under ×200 magnification.
Statistical analysis
Categorical data were summarized as numbers and percentages, while numerical data were presented as mean ± standard deviation or median (minimum - maximum). The Chi-square test was used for categorical variables. The normality of numerical data was assessed using the Shapiro-Wilk test.Data with normal distribution, including TAS, TOS, OSI, SOD, NO, RBC, HGB, and HCT, were analyzed using one-way ANOVA. Non-normally distributed data, such as IL-6, CRP, TNF-α, TGF-β, MDA, urea, and creatinine, were analyzed using the Kruskal-Wallis test.In cases where significant differences were detected, pairwise comparisons were performed using the Bonferroni test for normally distributed data and the Bonferroni-corrected Mann-Whitney U test for non-normally distributed data.All analyses were conducted using IBM SPSS Statistics 20.0, and a p-value of < 0.05 was considered statistically significant.
Results
Biochemical findings
Complete blood count results
Complete blood count (CBC) parameters were evaluated in the blood samples collected at the end of the experiment. RBC, HB, and HCT levels were significantly lower in the TAC and TAC + DEX groups compared to the control group (p < 0.001). However, WBC, PLT, LYM, and MONO levels did not show any significant differences between the groups (p > 0.05) (Table 2).
Results of antioxidant parameters
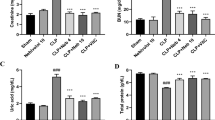
The antioxidant parameters analyzed from serum samples showed the following values: TAS, TOS, and OSI levels were measured as 1.5 ± 0.2 mmol/L, 10.7 ± 8.8 mmol/L, and 0.7 ± 0.5 in the TAC group, and 1.6 ± 0.5 mmol/L, 12.5 ± 15.0 mmol/L, and 0.8 ± 0.9 in the TAC + DEX group, respectively. However, these differences were not statistically significant (p = 0.827, p = 0.596, and p = 0.575, respectively). SOD levels were similar across all groups, and while slightly higher in the TAC group compared to the control group, this difference was not statistically significant (p = 0.952).
The mean values of GSH, GSH-Hb, GSSG, and GR showed no statistically significant differences between the groups (p = 0.321, p = 0.209, p = 0.262, and p = 0.820, respectively). However, G6PDH-Hb levels were measured as 23.3 ± 4.1 IU/g Hb in the TAC + DEX group and 19.9 ± 2.9 IU/g Hb in the TAC group, showing a statistically significant difference between groups (p < 0.001). Nonetheless, this difference was determined not to originate from the comparison between the TAC and TAC + DEX groups (Table 2).
Results of inflammatory parameters
The CRP level was measured as 0.3 ± 0.2 mg/L in the TAC and TAC + DEX groups, higher than in the Control (0.2 ± 0.1 mg/L) and DEX (0.1 ± 0.1 mg/L) groups. A statistically significant difference was detected between the groups, with the most notable difference observed between the TAC + DEX and DEX groups (p = 0.034) (Table 2).
MDA levels in the TAC (99.3 ± 5.4 nmol/ml) and TAC + DEX (98.5 ± 6.4 nmol/ml) groups were similar and lower than those in the DEX group (104.7 ± 4.1 nmol/ml). A statistically significant difference in MDA levels was identified among all groups; however, this difference was not attributable to the TAC and TAC + DEX groups (p = 0.008) (Table 2).
NO levels were highest in the TAC + DEX group (48.6 ± 4.6 µmol/L) and lowest in the Control group (42.8 ± 5.2 µmol/L); however, no significant differences were observed between the groups (p = 0.335) (Table 2).
TNF-α levels were measured as 139.0 (101.4-145.2) ng/L in the Control group, 173.9 (130.1-264.8) ng/L in the DEX group, 205.5 (108.6-286.8) ng/L in the TAC group, and 147.9 (83.4-234.3) ng/L in the TAC + DEX group. A statistically significant difference was detected among the TNF-α levels (p = 0.014); however, this difference was not observed between the TAC and TAC + DEX groups (Table 2).
IL-6 levels were determined as 4.5 (3.1–6.6) ng/L in the TAC group, 4.4 (3.4–7.3) ng/L in the TAC + DEX group, and 2.8 (1.2–4.2) ng/L in the Control group. A statistically significant difference was identified between the groups, with the difference being notable between the Control and TAC + DEX groups (p = 0.008) (Table 2).
TGF-Beta levels were measured as 164.0 (51.7–187.0) ng/L in the Control group, 171.5 (105.9-177.3) ng/L in the DEX group, 245.9 (163.3–320.0) ng/L in the TAC group, and 184.5 (127.8–270.0) ng/L in the TAC + DEX group. The differences among the groups were found to be statistically significant, with the variation originating from the differences between the Control and DEX groups and the TAC group (p = 0.012) (Table 2).
CREA levels were 0.7 (0.6–0.8) mg/dL in the Control group, 0.8 (0.7–0.9) mg/dL in the TAC group, and 0.8 (0.7-1.0) mg/dL in the TAC + DEX group. A statistically significant difference was observed among the groups, but no difference was found between the TAC and TAC + DEX groups (p = 0.002).
Urea levels were determined as 100.4 ± 25.6 mg/dL in the Control group, 121.9 ± 12.6 mg/dL in the TAC group, and 124.9 ± 21.0 mg/dL in the TAC + DEX group. A statistically significant difference was detected among the groups, although no significant difference was observed between the TAC and TAC + DEX groups (p = 0.005) (Table 2).
Histopathological findings
In the control group, renal structures were consistently within normal morphological limits across all histopathological examinations, with no evidence of inflammation, necrosis, or degeneration (Figs. 1, 2, 3, 4, 5, 6, 7, 8 and 9).
(A) Mild interstitial inflammation is observed. No marked signs of regeneration are present. The Bowman’s capsule space appears within normal limits. ×200. (B) Expansion of the Bowman’s capsule and foci of regeneration are evident within the area of interstitial inflammation, more prominently in the TAK + DEX group (black arrows). ×200.
Expansion of the Bowman capsule (Figs. 1 and 2) was observed in 87.5% of the TAC group and 100% of the TAC + DEX group. A statistically significant difference was detected between the groups (p = 0,003) (Table 3).
Tubular necrosis (Fig. 3) was not observed in the Control and DEX groups, but it was present in 50% of the TAC group and 12.5% of the TAC + DEX group. These findings were also found to be statistically significant (p = 0.048) (Table 3).
Vasa recta leukocyte infiltration (VRLI) (Fig. 4) was observed in 12.5% of the DEX group, 62.5% of the TAC group, and 75% of the TAC + DEX group. A statistically significant difference was detected among the groups (p = 0.007) (Table 3).
Tubular regeneration (Fig. 5) was identified in 87.5% of the DEX group and 100% of the TAC and TAC + DEX groups. A statistically significant difference was observed between the groups (p = 0.004) (Table 3).
Interstitial inflammation (Fig. 6) was found in 87.5% of the TAC group and 100% of the TAC + DEX group. However, no statistically significant difference was observed between the groups (p = 0.559) (Table 3).
Tubular dilation (Fig. 7) was observed in 16.7% of the Control (SF) group, 50% of the DEX group, 87.5% of the TAC group, and 100% of the TAC + DEX group. A statistically significant difference was detected among the groups (p = 0.003) (Table 3).
The presence of mitosis (Fig. 6) was recorded as 0% in the Control (SF) group, 37.5% in the DEX group, 87.5% in the TAC group, and 75% in the TAC + DEX group. This difference was also found to be statistically significant (p = 0.005) (Table 3).
Loss of the brush border (Fig. 8) was observed in 87.5% of the TAC group and 100% of the TAC + DEX group. A statistically significant difference was detected between the groups (p = 0.019) (Table 3).
Cast formation (Fig. 9) was observed in 87.5% of the TAC group and 25.0% of the TAC + DEX group. A statistically significant difference was detected between the groups (p = 0.020) (Table 3).
Table 4 shows the distribution of rats based on damage scores across different histopathological parameters. The table categorizes the severity of damage using a scoring system ranging from 0 (no damage) to 5 (> 76% of tubules affected).
Discussion
In our study, we investigated the protective effect of dexpanthenol against tacrolimus-induced nephrotoxicity. Renal damage and inflammation were analyzed through biochemical parameters (IL-6, TAS, TOS, GSH, GSSG, GR, G6PDH, CRP, MDA, TNF-α, TGF-β, CREA, and urea) as well as histopathological evaluations.
Tacrolimus causes organ rejection at low blood levels, while at high levels, it induces nephrotoxicity9,29. The most common side effect observed with tacrolimus use is nephrotoxicity, which is primarily monitored through tacrolimus blood levels and creatinine measurements30. In our study, the elevated urea and creatinine levels observed in the TAC group compared to the control group are consistent with the data in the literature. However, the even higher urea and creatinine levels in the TAC + DEX group suggest that dexpanthenol was insufficient in mitigating these effects.
Oxidative stress and inflammation play a critical role in tacrolimus-induced nephrotoxicity31,32. Cueto-Manzano reported that CRP levels remain elevated for an extended period following kidney transplantation33. Aslan and Güler demonstrated that colistin treatment increases CRP levels, and this increase is mitigated by DEX or vitamin C administration16. In our study, while CRP levels increased in the TAC group, they remained similar in the TAC + DEX group. The low CRP levels observed in the DEX group suggest an anti-inflammatory effect. The statistically significant differences between the groups (p = 0.034) indicate the need to investigate the effects of DEX using different doses and protocols.
Studies have shown that MDA levels are higher in patients receiving tacrolimus treatment compared to the control group, indicating increased oxidative stress and lipid peroxidation34,35. When the protective effects of antioxidants like NAC against nephrotoxicity are examined, it has been reported that NAC improves kidney function by reducing MDA levels36. In our study, the elevated MDA levels in the TAC group indicate that tacrolimus increases oxidative stress. The observation of higher MDA levels in the DEX group compared to the control group suggests that DEX alone may have a potential oxidative stress-inducing effect. Although a slight decrease was observed in the TAC + DEX group, no statistically significant difference was detected between the groups. The significant difference among all groups (p = 0.008) was determined to result from the differences between the control and DEX groups.
It has been demonstrated that silymarin reduces tacrolimus-induced toxicity by lowering the levels of pro-inflammatory mediators such as TNF-α and IL-637. In our study, the significantly higher TNF-α levels in the TAC group compared to the control group (p = 0.014) indicate that tacrolimus enhances the inflammatory response. The decrease in TNF-α levels in the TAC + DEX group suggests that DEX may be effective in mitigating tacrolimus-induced inflammation.
In the study by Khalifa El-Dawy and colleagues, a reduction in creatinine, BUN, IL-6, and TGF-β levels was observed in the groups treated with DEX and ADMSCs19. Regateiro et al. (2011) emphasized that TGF-β plays a crucial role in preventing organ rejection following transplantation; however, elevated levels may increase the risk of fibrosis38. In our study, the higher TGF-β1 levels observed in the TAC group compared to the control group suggest that tacrolimus may trigger fibrosis. The reduction in TGF-β1 levels in the TAC + DEX group indicates that dexpanthenol may partially prevent fibrosis; however, this difference is not statistically significant. This finding could be supported by significant results in larger animal series.
Agents such as NAC and alpha-1 antitrypsin have been shown to exert protective effects against tacrolimus-induced nephrotoxicity by reducing IL-6 and TNF-α levels39,40. In our study, IL-6 levels were found to be higher in the TAC group compared to the control group (p = 0.008), indicating that TAC triggers inflammation. Although IL-6 levels were slightly lower in the TAC + DEX group, the similarity between the groups suggests that DEX may not sufficiently reduce inflammation. Furthermore, the mildly elevated IL-6 levels in the DEX group indicate that DEX could partially exacerbate inflammation.
A study has demonstrated that SOD is effective in reducing tacrolimus-induced oxidative stress and IL-6 levels41. However, in our study, the increase in SOD levels in the TAC group was not statistically significant (p = 0.952). Similarly, SOD levels in the TAC + DEX group remained comparable, indicating that DEX did not significantly affect this parameter.
Cook et al. (2009) reported that tacrolimus induces hypertension by inhibiting endothelial nitric oxide synthase (eNOS)42. Yoshihiko Watarai and colleagues demonstrated that tacrolimus negatively affects renal blood flow by reducing NO production43. In our study, NO levels were compared among the four groups, with the highest value observed in the TAC + DEX group (48.6 ± 4.6 µmol/L). However, these differences were not statistically significant (p = 0.365) and did not align with previous reports. The lack of significance in serum antioxidant composites (TAS, TOS, OSI) and in SOD/NO (see Results) limits the interpretation that protection is solely based on systemic antioxidant enhancement. In contrast, significant differences in CRP, IL-6, TNF-α, TGF-β, urea, creatinine, and multiple histopathological endpoints suggest that the protective effect is mediated primarily through modulation of inflammation and tubular-level tissue responses. For future research, targeted oxidative stress measurements in kidney tissue (e.g., SOD/CAT/GPx, GSH/GSSG) and time-series sampling (days 3–7–14–21) are recommended.
In a study investigating the effect of dexpanthenol on amikacin-induced nephrotoxicity, it was reported that TOS and OSI levels decreased, while TAS levels increased in the groups treated with dexpanthenol17. In our study, when TAS, TOS, and OSI levels were compared, TAS was slightly increased in the TAC group (p = 0.827), while TOS and OSI levels were higher compared to the control group (p = 0.596 and p = 0.575, respectively). In the TAC + DEX group, TAS and TOS levels were similar to those in the TAC group, indicating that DEX was ineffective in mitigating oxidative stress. These findings were not consistent with the literature.
Studies by Ammar et al. (2024) and Gao et al. (2021) have demonstrated that tacrolimus increases oxidative stress, while agents such as Astragalus membranaceus (AS-IV) mitigate this effect by enhancing glutathione peroxidase (GPx) activity44,45. In our study, the higher GSH levels observed in the TAC group compared to the control group were interpreted as an adaptive response to oxidative stress. However, the decrease in GSH levels in the TAC + DEX group suggests that dexpanthenol did not fully achieve its expected protective effect. The reduction in GR levels in the TAC group indicates that tacrolimus suppresses this enzyme under oxidative stress, while the increase in GR levels in the TAC + DEX group suggests that dexpanthenol partially supports this enzyme. However, these differences were not statistically significant.
Protective effects of agents that enhance glucose-6-phosphate dehydrogenase (G6PDH) enzyme activity have been reported in cisplatin-induced nephrotoxicity46,47. In our study, the increased G6PDH levels observed in the TAC and TAC + DEX groups compared to the control group suggest that tacrolimus enhances this enzyme as an adaptive response to oxidative stress. The highest G6PDH levels observed in the TAC + DEX group indicate that dexpanthenol supports this adaptive mechanism. The difference between the groups is statistically significant (p = 0.001).
The adverse effects of tacrolimus on hematological parameters have been reported by Vicari-Christensen et al.48. In our study, a marked decrease in RBC, HB, and HCT levels was observed in the TAC group, supporting the adverse effects of tacrolimus on hematological parameters. However, the expected improvement in these parameters was not achieved in the TAC + DEX group. A statistically significant difference was found between the groups (p < 0.001). These results suggest that the protective effect of dexpanthenol on hematological parameters was insufficient.
Nikolić et al. reported that antioxidants improve decreases in hematological parameters caused by toxic elements49. Nevertheless, in our study, dexpanthenol did not demonstrate a comparable effect in mitigating tacrolimus-induced hematological disturbances. Further comprehensive studies are required to elucidate the efficacy of dexpanthenol in this context.
Aslan et al. (2021) observed marked inflammation, dilatation, and necrosis in the proximal tubules in kidney injury induced by colistin treatment. However, these pathological changes were significantly reduced in the groups treated with dexpanthenol and vitamin C16. In our study, the lower rate of tubular necrosis observed in the TAC + DEX group compared to the TAC group demonstrates the protective effect of dexpanthenol.
Pınar et al. (2021) reported that dexpanthenol reduced renal tubular damage and inflammation and improved histopathological scores in cisplatin-induced nephrotoxicity18. In our study, the high rates of Bowman capsule expansion and tubular damage observed in the TAC group support the nephrotoxic effects of tacrolimus. However, the significantly high rate of expansion in the TAC + DEX group suggests that dexpanthenol was ineffective in preventing these effects.
Doğan et al. (2016) demonstrated the protective effect of dexpanthenol against amikacin-induced nephrotoxicity and its ability to reduce inflammation in the proximal tubules17. In our study, the higher vasa recta leukocyte infiltration observed in the TAC + DEX group compared to the TAC group suggests that dexpanthenol was insufficient in preventing inflammation.
Sütçüoğlu et al. (2019) reported that dexpanthenol reduced acute tubular necrosis and acute tubular injury in contrast-induced nephropathy20. In our study, the high rates of tubular regeneration observed in the TAC and TAC + DEX groups suggest that tacrolimus may stimulate regeneration and that dexpanthenol may support these processes.
El-Dawy et al. (2023) reported that dexpanthenol and mesenchymal stem cells reduced cisplatin-induced tubular necrosis, inflammation, and fibrosis19. In our study, the rate of interstitial inflammation was found to be 87.5% in the TAC group and 100% in the TAC + DEX group. These findings suggest that dexpanthenol was insufficient in reducing the inflammatory response.
Lessio et al. (2005) demonstrated that cyclosporine A suppresses inducible nitric oxide synthase (iNOS) expression, leading to a reduction in nitric oxide synthesis and contributing to its vasoconstrictive effects. However, N-acetylcysteine prevented this reduction, suggesting a potential protective effect against cyclosporine-induced vascular dysfunction50. In our study, the high rate of tubular dilatation observed in the TAC group confirms the toxic effects of tacrolimus on the kidney. However, similar findings in the TAC + DEX group suggest that the protective efficacy of dexpanthenol is limited.
Tubular dilatation was detected in 87.5% of the TAC group and 100% of the TAC + DEX group, indicating that tacrolimus causes tubular damage and that dexpanthenol is ineffective in preventing this damage. Tubular dilatation was observed in 50.0% of the DEX group, suggesting that the protective effect of dexpanthenol is limited.
Mitotic rates were detected as 87.5% in the TAC group and 75% in the TAC + DEX group, suggesting that dexpanthenol may slightly reduce the mitotic rate. While no mitosis was observed in the control group (0%), a mitotic rate of 37.5% was detected in the DEX group, indicating that dexpanthenol may partially stimulate proliferation.
Wu et al. (2007) demonstrated that ischemia/reperfusion injury in mice leads to significant tubular necrosis, brush border loss, sloughed cellular debris (cast formation), tubular dilatation, inflammation, apoptosis, and fibrosis. However, they found that Toll-like receptor 4 (TLR4) inhibition mitigates this damage and inflammatory response, thereby preserving renal function28. In our study, the highest incidence of cast formation in the TAC group indicates that tacrolimus induces significant tubular damage. However, the similar rate of cast formation in the TAC + DEX and DEX groups suggests that dexpanthenol may exert a protective effect against this type of injury. On the other hand, the loss of the brush border was most prominent in the TAC + DEX group and was observed across all groups, indicating that dexpanthenol did not provide sufficient protection against this specific alteration. While our findings regarding cast formation are consistent with the literature, the results concerning brush border loss do not align with previous reports.
While dexpanthenol may partially mitigate the adverse effects of tacrolimus by reducing inflammation and tubular damage, its limited impact on renal function markers necessitates further studies on dose optimization and long-term administration. Additionally, larger-scale investigations monitoring IL-6 and TGF-β levels may provide valuable insights into the early detection of kidney graft rejection and the prevention of fibrosis.
Integrated interpretation
The absence of statistically significant differences in systemic antioxidant indices (TAS, TOS, OSI), SOD, and NO does not, by itself, negate a protective effect; rather, the overall pattern supports a multimodal mechanism. As shown in the Results, there were significant between-group differences in inflammatory and renal-injury markers (CRP, IL-6, TNF-α, TGF-β, urea, creatinine) together with clear separation across multiple histopathological endpoints (e.g., Bowman’s capsule expansion, tubular necrosis, vasa recta leukocyte infiltration, tubular regeneration, brush-border loss, cast formation). These findings suggest that DEX may act predominantly through inflammation modulation and tubular-level tissue responses, while serum redox panels may lack sensitivity to tissue-restricted effects or time-dependent fluctuations (e.g., early peaks with partial normalization by day 14). Given the sample size and multiple comparisons, a Type II error for serum redox readouts also remains plausible.
Future directions
To clarify the mechanism, future studies should prioritize: (i) kidney tissue–level oxidative-stress assessments (enzymatic SOD/CAT/GPx, GSH/GSSG, 4-HNE, 8-OHdG) rather than relying solely on serum composites; (ii) time-course sampling (e.g., days 3–7–14–21) to capture transient redox signals; and (iii) targeted interrogation of the renal NO axis (eNOS/iNOS by immunohistochemistry or activity assays).
Limitations and strengths of the study
Key limitations include the modest sample size, single dosing regimen, and absence of long-term follow-up, which preclude conclusions about sustained effects and dose- or duration-response. In addition, as discussed above, serum antioxidant panels may not fully reflect tissue- or time-specific redox dynamics, constraining mechanistic inference. Despite these constraints, the study offers notable strengths: a comprehensive evaluation integrating oxidative stress, inflammatory mediators, and blinded histopathology, enabling a multidimensional appraisal of dexpanthenol’s putative renoprotective effects. To our knowledge, this is the first investigation of dexpanthenol in tacrolimus-induced nephrotoxicity, providing novel, clinically relevant insights to inform subsequent work.
Conclusİon
This study demonstrates that in the tacrolimus-induced nephrotoxicity model, dexpanthenol does not exert a general nephroprotective effect but rather provides partial and selective protection. Although improvements were observed in certain histopathological endpoints, these findings were not consistently reflected in renal function markers, nor were significant changes detected in systemic antioxidant indices. Under the present dose and duration conditions, the protective effect of dexpanthenol appears to be limited. Further studies employing alternative dose–duration protocols and incorporating tissue-level, target-specific assessments are needed to more accurately determine its therapeutic potential.
Data availability
The data that support the findings of this study are available from the corresponding author, [Mehmet Zubaroglu], upon reasonable request.
References
Grinyó, J. M. Why is organ transplantation clinically important? Cold Spring Harb Perspect. Med. 3 (6), a014985. https://doi.org/10.1101/cshperspect.a014985 (2013).
Moradi, H., Kalantar-Zadeh, K. & Introduction Precision medicine in end-stage kidney disease and personalized renal replacement therapy: Challenges and unmet need. Semin Nephrol. 38 (4), 315–316. https://doi.org/10.1016/j.semnephrol.2018.05.001 (2018).
Global Report. n.d. (2021). Available from: https://www.transplant-observatory.org/2021-global-report-5/ [Last accessed: 12/9/2023].
Anonymous Reports. n.d. Available from: https://www.transplant-observatory.org/2020-internationctivities-report-%e2%80%8e/ [Last accessed: 12/9/2023].
Tolou-Ghamari, Z. Nephro and neurotoxicity of calcineurin inhibitors and mechanisms of rejections: A review on tacrolimus and cyclosporin in organ transplantation. J. Nephropathol. 1 (1), 23–30. https://doi.org/10.5812/jnp.6 (2012).
Haberal, M. Transplantation in Turkey. Clin. Transpl. 175–180 (2013).
Claeys, E. & Vermeire, K. Immunosuppressive drugs in organ transplantation to prevent allograft rejection: Mode of action and side effects. J. Immunol. Sci. 3, 14–21. https://doi.org/10.29245/2578-3009/2019/4.1178 (2019).
RASYONEL TEDAVİ YÖNÜNDEN TIBBİ FARMAKOLOJİ 10. Baskı - OĞUZ KAYAALP | Nadir Kitap. n.d. Available from: https://www.nadirkitap.com/rasyonel-tedavi-yonunden-tibbi-farmakoloji-10-baski-oguz-kayaalp-kitap7125955.html [Last accessed: 11/1/2023].
Khonsary, S. A. Goodman and gilman’s the Pharmacological basis of therapeutics. Surg. Neurol. Int. 14, 91 (2023). : 10.25259/SNI_184_2023.
Liu, J. et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66 (4), 807–815. https://doi.org/10.1016/0092-8674(91)90124-h (1991).
Etensel, B. et al. Dexpanthenol attenuates lipid peroxidation and testicular damage at experimental ischemia and reperfusion injury. Pediatr. Surg. Int. 23 (2), 177–181. https://doi.org/10.1007/s00383-006-1781-x (2007).
Semenovich, D. et al. Effects of panthenol and N-Acetylcysteine on changes in the redox state of brain mitochondria under oxidative stress in vitro. Antioxidants 10, 1699. https://doi.org/10.3390/antiox10111699 (2021).
Shin, J. Y. et al. Dexpanthenol promotes cell growth by preventing cell senescence and apoptosis in cultured human hair follicle cells. Curr. Issues Mol. Biol. 43 (3), 1361–1373. https://doi.org/10.3390/cimb43030097 (2021).
Heise, R. et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin. Pharmacol. Physiol. 25 (5), 241–248. https://doi.org/10.1159/000341144 (2012).
Dell’Acqua, G. & Schweikert, K. Panthenyl triacetate transformation, stimulation of metabolic pathways, and wound-healing properties in the human skin. J. Cosmet. Sci. 63 (1), 1–13 (2012).
Aslan, T. et al. Dexpanthenol and ascorbic acid ameliorate colistin-induced nephrotoxicity in rats. Eur. Rev. Med. Pharmacol. Sci. 25 (2), 1016–1023. https://doi.org/10.26355/eurrev_202101_24671 (2021).
Doğan, E. E. et al. Protective effect of dexpanthenol against nephrotoxic effect of amikacin: An experimental study. Biomed. Pharmacother. Biomed. Pharmacother.. 89, 1409–1414. https://doi.org/10.1016/j.biopha.2017.03.019 (2017).
Pınar, N. et al. Protective effect of dexpanthenol on cisplatin induced nephrotoxicity in rats. Biotech. Histochem. Off. Publ. Biol. Stain. Commun. 97 (1), 39–43. https://doi.org/10.1080/10520295.2021.1890215 (2022).
El-Dawy, K. et al. Dexpanthenol improved stem cells against cisplatin-induced kidney injury by Inhibition of TNF-α, TGFβ-1, β-catenin, and fibronectin pathways. Saudi J. Biol. Sci. 30 (9), 103773. https://doi.org/10.1016/j.sjbs.2023.103773 (2023).
Sutcuoglu, O. et al. Is it possible to prevent contrast-induced nephropathy with dexpanthenol? Int. Urol. Nephrol. 51 (8), 1387–1394. https://doi.org/10.1007/s11255-019-02194-2 (2019).
WMA - The World Medical Association-WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Participants. n.d. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki/ [Last accessed: 1/10/2025].
Sert, N. P. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18 (7), e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Tacrolimus | Fujimycin | Macrolide Antibiotic | TargetMol. n.d. Available from: https://www.targetmol.com/compound/tacrolimus [Last accessed: 7/21/2024].
Nielsen, F. T., Leyssac, P. P., Kemp, E., Starklint, H. & Dieperink, H. Nephrotoxicity of FK-506 in the rat. Studies on glomerular and tubular function, and on the relationship between efficacy and toxicity. Nephrol Dial Transplant. 10(3), 334–340 (1995).
Dholakia, U. et al. Anesthetic effects and body weight changes associated with ketamine-xylazine-lidocaine administered to CD-1 mice. PLoS ONE. 12 https://doi.org/10.1371/journal.pone.0184911 (2017).
Zhang, Y. et al. Short-term exposure to dimethyl fumarate (DMF) inhibits LPS-induced IκBζ expression in macrophages. Front. Pharmacol. 14, 1114897. https://doi.org/10.3389/fphar.2023.1114897 (2023).
Jiang, C. et al. Cytidine-gold nanoclusters as peroxidase mimetic for colorimetric detection of glutathione (GSH), glutathione disulfide (GSSG) and glutathione reductase (GR). Spectrochim Acta Mol. Biomol. Spectrosc. 250, 119316. https://doi.org/10.1016/j.saa.2020.119316 (2021).
Wu, H. et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Invest. 117 (10), 2847–2859. https://doi.org/10.1172/JCI31008 (2007).
Abu-Elmagd, K. et al. The effect of graft function on FK506 plasma levels, dosages, and renal function, with particular reference to the liver. Transplantation 52 (1), 71–77. https://doi.org/10.1097/00007890-199107000-00015 (1991).
Venkataramanan, R. et al. Clinical pharmacokinetics of tacrolimus. Clin. Pharmacokinet. 29 (6), 404–430. https://doi.org/10.2165/00003088-199529060-00003 (1995).
Bowman, L. J. & Brennan, D. C. The role of tacrolimus in renal transplantation. Expert Opin. Pharmacother.. 9 (4), 635–643. https://doi.org/10.1517/14656566.9.4.635 (2008).
Zhou, X. et al. Hydrogen peroxide mediates FK506-induced cytotoxicity in renal cells. Kidney Int. 65 (1), 139–147. https://doi.org/10.1111/j.1523-1755.2004.00380.x (2004).
Cueto-Manzano, A. M. et al. Markers of inflammation before and after renal transplantation. Transplantation 80 (1), 47–51. https://doi.org/10.1097/01.tp.0000164348.16689.03 (2005).
Aslankoç, R. et al. Oksidatif Stres durumunda Antioksidan Enzimlerin rolü - Süperoksit dismutaz (SOD), Katalaz (CAT) ve glutatyon Peroksidaz (GPX). Med. J. Süleyman Demirel Univ. 26 (3), 362–369. https://doi.org/10.17343/sdutfd.566969 (2019).
Al-Harbi, N. O. et al. Olmesartan attenuates tacrolimus-induced biochemical and ultrastructural changes in rat kidney tissue. BioMed. Res. Int. 2014, 607246. https://doi.org/10.1155/2014/607246 (2014).
Duru, M. et al. Protective effects of N-acetylcysteine on cyclosporine-A-induced nephrotoxicity. Ren. Fail. 30 (4), 453–459. https://doi.org/10.1080/08860220801985942 (2008).
Terzi, F. & Ciftci, M. K. Protective effect of Silymarin on tacrolimus-induced kidney and liver toxicity. BMC Complement. Med. Ther. 22 (1), 331. https://doi.org/10.1186/s12906-022-03803-x (2022).
Regateiro, F. S. et al. TGF-β in transplantation tolerance. Curr. Opin. Immunol. 23 (5), 660–669. https://doi.org/10.1016/j.coi.2011.07.003 (2011).
Lim, J-H. et al. Renoprotective effects of Alpha-1 antitrypsin against Tacrolimus-Induced renal injury. Int. J. Mol. Sci. 21 (22), 8628. https://doi.org/10.3390/ijms21228628 (2020).
Wazny, L. D. Oral N-Acetylcysteine (NAC) to prevent Contrast-Induced nephrotoxicity: Is NAC the answer? Can. Pharm. J. Rev. Pharm. Can. 137 (8), 31–36. https://doi.org/10.1177/171516350413700806 (2004).
Fatima, N. et al. Effect of Short-Term tacrolimus exposure on rat liver: An insight into serum antioxidant Status, liver lipid Peroxidation, and inflammation. Mediators Inflamm. 2021, e6613786. https://doi.org/10.1155/2021/6613786 (2021).
Cook, L. G. et al. Kidney Int. ;75(7):719–726 ; doi: https://doi.org/10.1038/ki.2008.697. (2009).
Watarai, Y. et al. Effect of tacrolimus and cyclosporine on renal microcirculation and nitric oxide production. Transpl. Proc. 36 (7), 2130–2132. https://doi.org/10.1016/j.transproceed.2004.08.109 (2004).
Ammar, M. et al. Tacrolimus trough level and oxidative stress in Tunisian kidney transplanted patients. Ren. Fail. 46 (1), 2313863. https://doi.org/10.1080/0886022X.2024.2313863 (2024).
Gao, P. et al. Astragaloside IV alleviates Tacrolimus-Induced chronic nephrotoxicity via p62-Keap1-Nrf2 pathway. Front. Pharmacol. 11 https://doi.org/10.3389/fphar.2020.610102 (2021).
Aggarwal, S. K. Calcium modulation of toxicities due to cisplatin. Met-Based Drugs. 5 (2), 77–81. https://doi.org/10.1155/MBD.1998.77 (1998).
C, Y. & X, Z. N-Acetylcysteine protects against Cobalt chloride-induced endothelial dysfunction by enhancing glucose-6-phosphate dehydrogenase activity. FEBS Open. Bio. 12 (8). https://doi.org/10.1002/2211-5463.13449 (2022).
Vicari-Christensen, M. et al. Tacrolimus: Review of pharmacokinetics, pharmacodynamics, and pharmacogenetics to facilitate practitioners’ Understanding and offer strategies for educating patients and promoting adherence. Prog Transpl. Aliso Viejo Calif. 19 (3), 277–284. https://doi.org/10.1177/152692480901900315 (2009).
Nikolić, R. et al. Monitoring the toxic effects of Pb, cd and Cu on hematological parameters of Wistar rats and potential protective role of lipoic acid and glutathione. Toxicol. Ind. Health. 31 (3), 239–246. https://doi.org/10.1177/0748233712469652 (2015).
Lessio, C. et al. Cyclosporine A and NAC on the inducible nitric oxide synthase expression and nitric oxide synthesis in rat renal artery cultured cells. Kidney Int. 68 (6), 2508–2516. https://doi.org/10.1111/j.1523-1755.2005.00726.x (2005).
Acknowledgements
The authors gratefully acknowledge the support of the Çukurova University Scientific Research Projects Coordination Unit in the conduct of this study.
Funding
Supported by Çukurova University Scientific Research Projects with project number TTU-2023-16297.
Author information
Authors and Affiliations
Contributions
Mehmet Zubaroğlu: Conceptualization, study design, data collection, manuscript writing, critical revision, and final approval.Nebil Akdoğan: Study supervision, methodology, data interpretation, and manuscript review.Sinan Kandır: Experimental procedures, biochemical analyses, and manuscript editing.Tuğba Toyran: Histopathological evaluations, data analysis, and figure preparation.Sevinç Püren Yücel: Statistical analysis, validation of data, and manuscript revision.Erkan Demir: Experimental execution, data acquisition, and technical support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent requirement
This experimental, prospective, and randomized study was approved by the Çukurova University Animal Experiments Local Ethics Committee with decision number 5 on 18.09.2023 (Appendix-1). The study was conducted at the Health Sciences Experimental Application and Research Center (SABİDAM) in accordance with the scientific principles outlined in Article of the Declaration of Helsinki and animal welfare principles.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zubaroglu, M., Akdogan, N., Kandır, S. et al. Protective effect of dexpanthenol in tacrolimus-induced nephrotoxicity in rats. Sci Rep 15, 40635 (2025). https://doi.org/10.1038/s41598-025-24432-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-24432-7