Abstract
Early cancer diagnosis is crucial to improving disease prognosis. Although several studies have investigated the relationship between socioeconomic position (SEP) and stage at diagnosis, there is limited evidence from contexts with highly fragmented health systems and pronounced socioeconomic inequalities. This study analyzed the association between SEP and stage of cancer diagnosis. Data were obtained from the EquityCancer-LA baseline study. The sample included patients aged 18 or older with a confirmed cancer diagnosis within the 12 months prior to participation. Cancer stage was determined by the oncology committees of participating healthcare centers and logistic regression models were used to assess the association between SEP and cancer stage at diagnosis. A total of 343 individuals participated in the study, 39.1% of whom were diagnosed at a late stage. Two SEP indicators were associated with this outcome. After adjusting for covariates, participants without formal income had higher odds of late-stage diagnosis (OR = 2.14; 95% CI 1.02–4.53), and those who were non-head of household (OR = 1.83; 95% CI 1.11–3.02). When adjusting for all SEP variables, only non–head of household condition remained significantly associated (OR = 1.77; 95% CI 1.07–2.96). These results show that disadvantaged SEP was associated with higher odds of late-stage cancer diagnosis. The findings suggest the need for strategies that promote early diagnosis and address the socioeconomic inequities identified in this study.
Similar content being viewed by others
Background
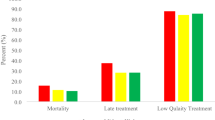
Cancer represents a major global health challenge; in Chile, it has become the leading cause of mortality1. According to epidemiological forecasts from the Global Cancer Observatory, cancer mortality is expected to increase by 62% worldwide by 20402, while in Chile, the increase could reach 96%3. To address this alarming situation, it is essential to detect cancer in early stages4 (commonly defined as stages 0, I, and II according to the TNM classification). This is because, in contrast, people who are diagnosed in late stages (stage III or IV according to the TNM classification) have a lower probability of survival4 and lower quality of life during the treatment phase5, which in turn generates higher costs for healthcare systems6. In Chile, the early stages of cancer diagnosis represent a significant challenge. On one hand, the proportion of people diagnosed at early stages is considerably lower compared to high-income countries. For example, in Chile, 69% of women with breast cancer are diagnosed at early stages7, whereas in the United Kingdom, this figure reaches 85%8.
The stage at which cancer is diagnosed depends on several factors. Some types of cancer, as is the case of lung or pancreatic cancer, present nonspecific symptomatology, which increases the probability of diagnosis in late stages9. On the other hand, patients with low awareness of cancer symptoms are more likely to present a late-stage of the pathology10. Likewise, the lack of oncology training of primary care physicians, difficulties in accessing exams, the lack of oncology specialists and the remoteness of health centers are factors related to late-stage cancer diagnosis11.
Patients with lower socioeconomic position (SEP) are more likely to be diagnosed with late-stage cancer12. SEP corresponds to the social and economic attributes that determine a person’s position within society13. Its main indicators include household income, housing conditions, type of occupation, and educational level, among other factors13. Each of these indicators highlights a specific aspect of social stratification14. In cancer-related studies, the most used indicators are household income, educational level, and area poverty level15. Other social determinants of health have also been used, such as lack of health insurance, social isolation and poor public health infrastructure16.
Several studies have analyzed the relationship between SEP and stage of cancer diagnosis. Riba et al.17 identified a positive association between higher SEP and early-stage breast cancer diagnosis in the U.S. population, a finding similar to those reported in studies conducted in the United Kingdom18 and Australia19. Boscoe et al.20 found a statistically significant association between high levels of area poverty and late-stage diagnosis in 14 of the 21 types of cancer studied in the United States. However, in the UK the effect of socioeconomic deprivation on late-stage diagnosis was observed in only four (melanoma, breast, prostate and endometrial) of the 10 most common cancers21.
Despite research advances, most of these studies have been conducted in high-income countries. In our region (Latin America), a few studies conducted in Brazil have found an association between SEP variables and late-stage cancer diagnosis22. In Chile this phenomenon has not been studied, and it is a challenge to address this knowledge gap. Although nearly 80% of the Chilean population is affiliated with the public healthcare system23, the widespread use of private healthcare services makes it difficult to ensure continuity of care within the public sector. This has led to Chile being considered a country with a highly fragmented healthcare system, a condition known to negatively impact population health and deepen disparities in access to care, particularly among socioeconomically vulnerable groups24. Considering the high levels of socioeconomic inequality in Chile (the average income of the richest 10% is 19 times higher than that of the poorest 10%)25, these findings suggest the need to examine whether SEP is associated with cancer stage at diagnosis. It is plausible that the association between SEP and stage at diagnosis could be even stronger than in higher-income countries with less fragmented public healthcare systems.
This study aims to analyze the association between SEP and stage of cancer diagnosis within two public healthcare networks in Chile. It contributes original and relevant evidence, addressing a research gap in Latin America. The findings could support policymakers and healthcare managers in developing or improving early cancer detection strategies for socioeconomically vulnerable populations, who are at greater risk of late-stage diagnosis. In the long term, such efforts may reduce mortality and improve healthcare system efficiency. As the cancer stage at diagnosis is a critical point in the cancer control continuum, our findings provide valuable insights within a highly fragmented healthcare system and are also relevant for countries with similar public–private healthcare service coexistence, such as Colombia26 and Mexico27, which face comparable equity challenges in early cancer detection.
Methods
Design, study population and sample
We conducted a cross-sectional study using data obtained from the EquityCancer-LA Project in Chile28. The EquityCancer-LA project, “Improving equity in access to early diagnosis of cancer: implementation research in different healthcare systems of Latin America” is a multicenter study carried out in Colombia, Ecuador, and Chile. The study aims to evaluate the contextual effectiveness of the implementation, with a participatory approach, of a multi-component integrated care intervention in improving early diagnosis of cancer in public health service networks28. The EquityCancer-LA project follows a dual design. On one hand, it employs a controlled quasi-experimental design, assessing conditions before and after the intervention. On the other hand, it incorporates a case study design using mixed methods to analyze in depth the barriers to early cancer diagnosis for the most common cancers28.
In Chile, the study is conducted in two healthcare networks in the Metropolitan Region of Santiago (North and South health networks). Both networks share similar population characteristics in terms of the number of people cared for, as well as the demographic, social, and economic features of their populations29,30. For the purposes of this study, we used data from the baseline quantitative study of the EquityCancer-LA Project in Chile.
The study population consisted of individuals aged 18 or older with a primary diagnosis of breast, gastric, lung, colorectal, prostate, kidney, bladder, or testicular cancer, confirmed with staging up to 12 months before participation in the study. The sampling frame was obtained from the oncology registry databases of each health service, and the sample was randomly selected and proportionally stratified by cancer type. The first contact was made by the professionals of each healthcare network. Subsequently, the participants were contacted by the research team of the EquityCancer-LA Project in Chile to coordinate the application of the survey. The survey was applied in the participants’ homes or in the health centers.
A total of 643 patients were considered between August 2022 and December 2023. Of these, 1.2% did not respond to the call to participate (8 patients), 8.3% were deceased (54 patients), 8.9% were excluded because these patients entered the public health network only at the treatment phase (57 patients), 12.6% did not meet the inclusion criteria (81 patients), 14.5% declined to participate (93 patients) and 1.1% without information about cancer stage (7 patients). The final sample consisted of 343 patients.
Data collection
The information was collected through a questionnaire specifically designed for the EquityCancer-LA study28,31. This questionnaire was developed to analyze patients’ pathways during the cancer diagnostic process. It was designed within the conceptual framework of the EquityCancer-LA study, drawing on a review of the literature, relevant existing instruments, and findings from the qualitative component of the project31. Specifically, it was based on the Equity-LA questionnaire on access to care for patients with chronic diseases and Aarhus statement guidelines to ensure the systematic collection of data on essential time points and intervals during the cancer diagnostic pathway31. The instrument was adapted to the Chilean context, its content was rigorously validated by a panel of experts, and it was subsequently tested through cognitive interviews (pretest) and pilot test31. Data collection was conducted between August 2022 and December 2023 by previously trained interviewers. Other variables of interest, such as cancer stage and public health insurance category (FONASA), were obtained from oncology committee records and the FONASA database, respectively.
Variables
Dependent: stage of cancer diagnosis (early/late)
We used the cancer stage to determine early-or late-stage, following the frameworks of previous studies22. Stage was determined by specialists in the oncology committee of each health center. Patients with stage I and II were classified as early-stage, while those with stage III and IV were considered late-stage cancer32.
Independents: SEP
We considered a set of SEP variables. According to Galobardes et al.14, using multiple SEP indicators allows for a better understanding of its effect on the phenomenon under study. This approach helps explain how social stratifiers relate to stage of cancer diagnosis. For this study, we used educational level, income per month and head of household status as SEP variables.
Educational level: According to Beebe-Dimmer et al.33, average educational attainment has increased across birth cohorts. For this reason, we classified educational level according to participants’ birth cohorts, using the average educational attainment by age group reported in the Chilean National Socioeconomic Characterization Survey (CASEN, for its acronym in Spanish) as a reference34. Participants aged 18 to 44 who reported having completed secondary education or less, and those aged 45 or older with only primary education or less, were classified as having a low educational level. Participants with technical or university-level education were classified as having a high educational level.
Income per month: As a proxy for income, we used the FONASA classification system. FONASA affiliates are categorized into four groups according to formal income of the last 12 months, with Group A representing individuals without formal income and Group D those with the highest income levels35. For our study, income was converted from Chilean pesos to United States dollar (USD). We used the average exchange rate reported by the Chilean Internal Revenue Service between August 1, 2022, and December 31, 2023, which was 1 USD = 860 Chilean pesos36. Based on this, the following categories were defined: without formal income, ≤ 510 USD per month, and > 510 USD per month.
Head of household status: We used head of household status as a contextual variable of social stratification. Participants who reported being the main economic provider were classified as head of household. This variable has been proposed by Conway et al.15 as an individual-level indicator of SEP. We considered it relevant in our context, as it may reflect household dynamics (such as gender roles, decision-making power over household resources, etc.) that could be associated with the outcome variable.
Covariates
The following covariates were considered as potential confounders regarding previous studies: gender (male or female), age37, family size (1, 2, and 3 or more persons)37, comorbidities (none and yes (1 or more))37, and type of cancer (breast, colorectal, gastric, prostate, lung, kidney/bladder, and testicle)21.
Statistical analysis
A descriptive analysis of the variables under study was conducted. For categorical variables, frequencies and proportions were reported, while for continuous variables, the median and interquartile range were provided. The distribution of continuous variables was assessed using the Shapiro-Wilk test. The relationship between independent and dependent variables was analyzed through a bivariate analysis. For categorical variables, chi-square test was used, and for continuous variables, the Mann-Whitney U test or Kruskal–Wallis test were applied.
Additionally, we assessed the potential multicollinearity of the independent variables using variance inflation factor (VIF) threshold of 10 and a tolerance of 0.1. To estimate the association of SEP and stage of cancer diagnosis, we used both crude and adjusted logistic regression models. The odds ratios (OR) for late-stage cancer diagnosis were reported with a 95% confidence interval for the crude model (with each SEP variables unadjusted by covariables), Model 1 (with each SEP variables adjusted for gender and age), Model 2 (with each SEP variables adjusted for all covariates), and Model 3 (all SEP variables adjusted for all covariates). Confounders were selected by previous evidence. In addition, we evaluated interaction with covariables (gender, age and type of cancer). The Hosmer-Lemeshow test was used to assess the goodness of fit. All statistical analyses were two-sided, and a p-value < 0.05 was considered statistically significant. Statistical analyses were performed using Stata SE 18 software (StataCorp, CollegeStation, TX).
Ethical aspects
This study adhered to the ethical principles established in the Declaration of Helsinki and the Council for International Organizations of Medical Sciences (CIOMS). The Ethics Committees of the Metropolitan North Health Service (No. 062-2021) and the Metropolitan South Health Service (No. 39-16052022) approved the study protocol. The study was conducted in accordance with the guidelines and regulations of national legislation.
All participants were provided with information regarding the study’s objectives, the voluntary nature of participation, the risks and benefits of the research, and their rights, including anonymity, confidentiality, data protection, and the right to decline participation. This information was communicated through the informed consent process. Participation was confirmed by signing the informed consent document.
Results
343 participants were included in the study. Median age was 63 years (IQR = 54–71). Of the participants, 59.2% were women, 88.6% lived with at least one other person. 59.2% of the participants reported not having a chronic disease, while 37.9% were diagnosed with breast cancer (Table 1). In relation to SEP, 50.1% of the participants had low educational level, 13.1% had no formal income per month, and 65.6% were head of household (Table 1). No multicollinearity was detected among the SEP variables (Supplementary Table S1). Figure 1 shows the proportions according to I, II, III, and IV stage of TNM system. 39.1% of the participants presented late-stage of cancer diagnosis (Fig. 1).
Table 2 shows the description of the participants and the bivariate analysis between the independents and dependent variables. Statistically significant differences were found between early-and late-stage groups in age, type of cancer, income per month and head of household status. The bivariate analysis between covariates and SEP variables is shown in Supplementary Table S2. Statistically significant differences were observed between age and all SEP variables, while for gender and family size only for the head of household variable.
Table 3 presents the association between SEP variables and stage of cancer diagnosis (early/late). Both the crude model and Model 1 (adjusted for gender and age) independently showed that having no formal income per month and non-head of household were associated with a late-stage of cancer diagnosis. Model 2 (adjusted for all covariates and including each SEP indicator separately) revealed that individuals without formal income had 2.14 times higher odds of late-stage of cancer diagnosis compared to those with formal income greater than 510 USD (95%CI = 1.02–4.53). Similarly, non-head of households had higher odds of late-stage of cancer diagnosis compared to those who were head of household (OR = 1.83; 95%CI = 1.11–3.02). Although low educational level was associated with late-stage in the crude model, Model 1, and Model 2, this association was not statistically significant. Finally, Model 3 (adjusted for all SEP variables and all covariates) showed a similar pattern of associations as in the previous models; however, head of household was only variable with statistical significance. Non-heads of households had 1.77 times higher odds of late-stage diagnosis compared to heads of household (95%CI = 1.07–2.96). No interactions were detected with gender, age (as a categorical variable), or cancer type. Model 3 indicated a good fit (p-value = 0.379).
Discussion
Our study analyzed the association between SEP and stage of cancer diagnosis. We found that lower income per month and non-head of household were significantly associated with a late-stage of cancer diagnosis. Although a lower level of education showed a similar trend, this association was not statistically significant.
Firstly, in our study, the prevalence of late-stage cancer diagnosis was 39.1%, which is similar to findings from other studies conducted in Latin America and the Caribbean. For example, de Lemos et al.38 reported that the proportion of late-stage breast cancer diagnoses (stages III and IV) in South America was 37.7%. This similarity may be explained by the comparable socioeconomic conditions across populations in the region. South America is characterized by pronounced socioeconomic inequalities and limited healthcare availability, which contribute to a higher proportion of late-stage cancer diagnoses compared to regions with better living conditions and healthcare systems39.
Regarding SEP variables, we found a significant association of two indicators with late-stage of cancer diagnosis. Individuals with lower income per month had higher odds of late-stage diagnosis compared to those with higher income. This finding is consistent with previous studies employing different methodologies and data levels. For example, Clegg et al.40 reported that lower annual family income was associated with distant-stage breast and prostate cancer in the United States, while Ruan et al.41 found an association between lower neighborhood-level income and late-stage diagnosis across all cancer sites in Alberta, Canada.
As a possible interpretation of this finding, we may hypothesize that individuals with lower income have fewer material resources and face greater difficulties in accessing services. In this context, our study may provide evidence of the cumulative effect of income throughout the cancer control continuum in Chile. Several studies have shown that people with low income are more exposed to cancer-related etiological factors and face greater barriers to accessing early detection strategies42, which may help explain why this group has a higher chance of receiving a late-stage cancer diagnosis.
Additionally, we observed an association between non-head of household and late-stage cancer diagnosis. Similar findings have been reported in other studies but based on the income of the head of household43. The observed association between head of household status and stage at diagnosis may be understood from several perspectives. First, people who are heads of household are more likely to have formal employment44. In Chile, the workplace plays an important role in providing access to preventive strategies (e.g., occupational health screenings) and health information (e.g., awareness campaigns)45. Therefore, individuals non-head of household may have reduced exposure to cancer prevention and early detection strategies due to lower participation in the labor market.
From a gender perspective, we observed that a high proportion of non-heads of household were women. It is widely recognized that women often take on caregiving responsibilities and unpaid domestic labor46, frequently prioritizing the care of family members over their own health47. This may result in lower awareness of preventive measures or early signs and symptoms of cancer. Furthermore, persons non-head of household may experience economic dependency on those who are. This situation could create power dynamics and decision-making patterns regarding the use of family resources, potentially hindering timely access to healthcare services.
Regarding educational level, we observed an association between lower educational level and late-stage cancer diagnosis. However, this association was not statistically significant. Our findings differ from previous studies. De Almeida et al.48 found that educational level is an important predictor of late-stage cancer diagnosis in Sao Paulo-Brazil. In light of our results, we believe that future studies, with greater statistical power, may identify statistically significant associations between educational level and stage of cancer diagnosis (early vs late).
Finally, we highlight the association of two SEP indicators, with a stronger effect observed for the head of household status. These findings warrant further studies that explore these indicators in greater depth using diverse methodological approaches. Such research efforts would contribute to a more comprehensive understanding of the social and economic inequities identified in this study.
Strength and limitations
Our study has several strengths. The data collected are of high quality, given the rigorous survey application process and the use of official databases. In this context, the exposures and outcome variables were obtained directly from primary official databases of the Health Services included in the study, which helped minimize potential information bias.
Another strength of this study was the use of multiple SEP indicators. This approach provides new insights into three distinct SEP measures in relation to the phenomenon under study, within the context of a highly fragmented healthcare system. An additional strength was the contextual treatment of the educational level variable. To address potential cohort effects on educational attainment, we followed the approach proposed by Beebe-Dimmer et al.33.
This study has several limitations. First, its cross-sectional design does not allow causal inference between SEP and stage at cancer diagnosis. Accordingly, our findings should be interpreted as associations measured at diagnosis, rather than as evidence of causal effects over time. Second, TNM stages were collapsed into a binary variable (I–II vs III–IV), which may mask heterogeneity within stages and limit the granularity of the results. A potential limitation of this study was the exclusion of patients who accessed the public health networks only at the treatment phase, and whose entire cancer diagnostic process was therefore conducted in private healthcare services. However, given that individuals who use private healthcare generally have greater socioeconomic resources (higher SEP), we hypothesize that including this group would likely have resulted in an even stronger association, thereby reinforcing our findings.
One potential limitation of our study is the use of certain SEP variables that may be susceptible to reverse causality. Specifically, Galobardes et al.14 have reported this potential issue with the income variable, noting that poor health can lead to reduced income. However, we used FONASA affiliation as a proxy for income, which reflects earnings over the previous 12 months. This approach substantially reduced the likelihood of reverse causality in our analysis.
Another limitation was the lack of data on informal income at both the individual and household levels. Future studies could consider assessing total household income—including both formal and informal sources—as well as income fluctuations over time. This approach may provide a more comprehensive understanding of the phenomenon under study. Moreover, this study did not include other social stratification variables such as ethnicity or type of occupation. Future research should incorporate these indicators to explore potential associations with the stage of cancer diagnosis. Likewise, it would be relevant to study the relationship between SEP and outcomes of previous points to diagnosis in the cancer control continuum (etiology, prevention and detection), which would provide information on the potential cumulative effects at the stage of cancer diagnosis.
Although data on diagnostic intervals were available, it was not included in this analysis because our primary objective was to examine socioeconomic differences in stage at diagnosis. Similarly, screening participation was not considered as a variable because our sample included several cancer types, some of which lack organized screening programs (e.g., gastric or prostate cancer), which would have introduced heterogeneity and reduced comparability across cancers. Future research could examine diagnostic intervals and screening participation as complementary outcomes or mediators to further elucidate pathways leading to late-stage diagnosis.
Finally, we reported odds ratios in this study. Given the high prevalence of late-stage diagnosis in our sample (39.1%), the interpretation of the odds ratios may overestimate the strength of associations and should therefore be interpreted with caution.
Conclusions
In conclusion, our study found an association between SEP variables and stage at cancer diagnosis. Although the Chilean public health system guarantees free care for its population, substantial social and economic inequities persist in the stage of cancer diagnosis. Individuals facing greater socioeconomic vulnerability have higher odds of late-stage cancer diagnosis compared to those with greater socioeconomic privilege. These findings are relevant for policymakers and health system managers, as they highlight the need for more targeted and equitable early detection strategies. In light of recent advances in cancer control in Chile, these results may inform future reflections and updates to the National Cancer Plan.
Finally, future research should investigate the relationship between different social stratifiers and stage at cancer diagnosis using longitudinal designs in the Chilean context. Such studies would help identify potential explanatory pathways and provide a more comprehensive understanding of socioeconomic inequities in cancer stage at diagnosis.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality policies but are available from the corresponding author on reasonable request.
Abbreviations
- SEP:
-
Socioeconomic position
- OR:
-
Odds ratio
- TNM:
-
Tumor, node, metastasis
- FONASA:
-
National Health Fund (Fondo Nacional de Salud by its acronym in spanish)
- CASEN:
-
Socioeconomic Characterization Survey (Encuesta de Caracterización Socioeconómica by its acronym in spanish)
- USD:
-
United States Dollar
References
Instituto Nacional de Estadísticas (INE). Anuario de Estadísticas Vitales 2019. https://www.ine.cl/docs/default-source/nacimientos-matrimonios-y-defunciones/publicaciones-y-anuarios/anuarios-de-estad%C3%ADsticas-vitales/anuario-de-estad%C3%ADsticas-vitales-2019.pdf?sfvrsn=97729b7b_5 (2020).
International Agency for Research on Cancer. World Cancer Report: Cancer Research for Cancer Prevention. https://publications.iarc.fr/586 (2020).
International Agency for Research on Cancer. Chile Fact Sheet. https://gco.iarc.fr/today/data/factsheets/populations/152-chile-fact-sheets.pdf (2020).
Crosby, D. et al. Early detection of cancer. Science 375, eaay9040. https://doi.org/10.1126/science.aay9040 (2022).
Richards, M. A. The size of the prize for earlier diagnosis of cancer in England. Br. J. Cancer 101, S125–S129. https://doi.org/10.1038/sj.bjc.6605402 (2009).
Hamilton, W., Walter, F. M., Rubin, G. & Neal, R. D. Improving early diagnosis of symptomatic cancer. Nat. Rev. Clin. Oncol. 13, 740–749. https://doi.org/10.1038/nrclinonc.2016.109 (2016).
Prieto, M. Epidemiología Del cáncer de Mama En Chile. Rev. Méd Clín Las Condes. 22, 428–435. https://doi.org/10.1016/S0716-8640(11)70447-3 (2011).
National Health Services (UK). Cancer registrations statistic, England 2021. https://digital.nhs.uk/data-and-information/publications/statistical/cancer-registration-statistics/england-2021---summary-counts-only/cancer-incidence-by-stage (2023).
American Cancer Society. Understanding advanced and metastatic cancer. https://www.cancer.org/treatment/understanding-your-diagnosis/advanced-cancer/what-is.html (2020).
World Health Organization (WHO). Guide to Cancer Early Diagnosis. https://apps.who.int/iris/bitstream/handle/10665/254500/9789241511940-eng.pdf (2017).
Cancer Research, U. K. Why is early cancer diagnosis important? (2023). https://www.cancerresearchuk.org/about-cancer/cancer-symptoms/why-is-early-diagnosis-important
Ward, E. et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J. Clin. 54, 78–93. https://doi.org/10.3322/canjclin.54.2.78 (2004).
Krieger, N. A glossary for social epidemiology. J. Epidemiol. Commun. Health 55, 693–700 (2001).
Galobardes, B., Shaw, M., Lawlor, D. A., Lynch, J. W. & Smith, G. D. Indicators of socioeconomic position (part 1). J. Epidemiol. Commun. Health 60, 7–12. https://doi.org/10.1136/jech.2004.023531 (2006).
Conway, D. I. et al. Measuring socioeconomic status and inequalities in Reducing Social Inequalities in Cancer: Evidence and Priorities for Research (ed Vaccarella, S.) 29–40 (IARC, 2019).
Pinheiro, L. C. et al. Social determinants of health and cancer mortality in the reasons for geographic and Racial differences in stroke (REGARDS) cohort study. Cancer 128, 122–130. https://doi.org/10.1002/cncr.33894 (2022).
Riba, L. A., Gruner, R. A., Alapati, A. & James, T. A. Association between socioeconomic factors and outcomes in breast cancer. Breast J. 25, 488–492. https://doi.org/10.1111/tbj.13250 (2019).
Barclay, M. E., Abel, G. A., Greenberg, D. C., Rous, B. & Lyratzopoulos, G. Socio-demographic variation in stage at diagnosis of breast, bladder, colon, endometrial, lung, melanoma, prostate, rectal, renal and ovarian cancer in England and its population impact. Br. J. Cancer 124, 1320–1329. https://doi.org/10.1038/s41416-021-01279-z (2021).
MacDermid, E. et al. The effect of socioeconomic deprivation on presentation stage and long-term outcomes in patients undergoing colorectal cancer resection in Western Sydney. ANZ J. Surg. 91, 1563–1568. https://doi.org/10.1111/ans.17048 (2021).
Boscoe, F. P., Henry, K. A., Sherman, R. L. & Johnson, C. J. The relationship between cancer incidence, stage and poverty in the united States. Int. J. Cancer 139, 607–612. https://doi.org/10.1002/ijc.30087 (2016).
Lyratzopoulos, G. et al. Socio-demographic inequalities in stage of cancer diagnosis: Evidence from patients with female breast, lung, colon, rectal, prostate, renal, bladder, melanoma, ovarian and endometrial cancer. Ann. Oncol. 24, 843–850. https://doi.org/10.1093/annonc/mds526 (2013).
de Lima, K. Y. N., de Cancela, M. C. & de Souza, D. L. B. Spatial assessment of advanced-stage diagnosis and lung cancer mortality in Brazil. PLoS One. 17, e0265321. https://doi.org/10.1371/journal.pone.0265321 (2022).
Ministerio de Desarrollo Social y Familia. Acceso a salud en la población chilena. Encuesta CASEN 2022 (2024). https://observatorio.ministeriodesarrollosocial.gob.cl/storage/docs/casen/2022/Resultados_Salud_Casen2022.pdf
Juárez, L. A. et al. Reformas de atención primaria En América latina: Avances En Brasil, Chile, Colombia, México y Perú. Gac Sanit. 38, 102430. https://doi.org/10.1016/j.gaceta.2024.102430 (2024).
Organization for Economic Cooperation and Development (OECD). Society at a glance 2019: OECD social indicators 10.1787/soc_glance-2019-en. (2019).
Vargas, I. et al. Barriers to healthcare coordination in market-based and decentralized public health systems: A qualitative study in healthcare networks of Colombia and Brazil. Health Policy Plan. 31, 736–748. https://doi.org/10.1093/heapol/czv126 (2016).
Huesca Reynoso, L., Martínez-Martínez, O. & Velázquez Leyer, R. Reconfiguring social policy from the left in mexico: So Far, not so good? Lat Am. Policy 11, 345–353. https://doi.org/10.1111/lamp.12198 (2020).
Vázquez, M. L. et al. Improving equity in access to early diagnosis of cancer in different healthcare systems of Latin america: Protocol for the EquityCancer-LA implementation-effectiveness hybrid study. BMJ Open 12, e067439. https://doi.org/10.1136/bmjopen-2022-067439 (2022).
Gattini, C. Servicio de Salud Metropolitano Norte. Perfil institucional. https://www.ochisap.cl/wp-content/uploads/2022/05/9-SS-Metropolitano-Norte.pdf (2015).
Gattini, C. Servicio de Salud Metropolitano Sur. Perfil institucional. https://www.ochisap.cl/wp-content/uploads/2022/05/13-SS-Metropolitano-Sur.pdf (2015).
Espinel-Flores, V. et al. A questionnaire for the measurement of access to cancer diagnosis from the patients’ perspective. Gac Sanit. 37 (S1), S326–S374 (2023). https://www.gacetasanitaria.org/es-pdf-X0213911123036420?local=true
Brierley, J. D., Gospodarowicz, M. K. & Wittekind, C. (eds). TNM classification of malignant tumours, 8th edition. (Wiley-Blackwell, 2016).
Beebe-Dimmer, J. et al. Childhood and adult socioeconomic conditions and 31-year mortality risk in women. Am. J. Epidemiol. 159, 481–490. https://doi.org/10.1093/aje/kwh057 (2024).
Ministerio de Desarrollo Social y Familia. Situación educacional de la población chilena. Encuesta CASEN. (2006). https://observatorio.ministeriodesarrollosocial.gob.cl/storage/docs/casen/2022/Resultados_Educacion_Casen2022.pdf (2024).
Ministerio de Salud de Chile. Tramos del FONASA. https://nuevo.fonasa.gob.cl/tramos/ (2024).
Servicio de Impuestos Internos. Dólar observado 2022–2023. https://www.sii.cl/valores_y_fechas/dolar/dolar2023.htm (2023).
Dalton, S. O. et al. Socioeconomic position, stage of lung cancer and time between referral and diagnosis in Denmark, 2001–2008. Br. J. Cancer 105, 1042–1048. https://doi.org/10.1038/bjc.2011.342 (2011).
de Lemos, L. L. P. et al. Stage at diagnosis and stage-specific survival of breast cancer in Latin America and the caribbean: A systematic review and meta-analysis. PLoS One 14, e0224012. https://doi.org/10.1371/journal.pone.0224012 (2019).
Werutsky, G. et al. Socioeconomic impact of cancer in Latin America and the Caribbean. Arch. Med. Res. 53, 818–825. https://doi.org/10.1016/j.arcmed.2022.11.013 (2022).
Clegg, L. X. et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National longitudinal mortality study. Cancer Causes Control 20, 417–435. https://doi.org/10.1007/s10552-008-9256-0 (2009).
Ruan, Y. et al. The association between neighborhood-level income and cancer stage at diagnosis and survival in Alberta. Cancer 130, 563–575. https://doi.org/10.1002/cncr.35098 (2024).
Denny, L. et al. Social inequalities in cancer risk factors and health-care access in Reducing Social Inequalities in Cancer (ed Vaccarella, S.) 95–107 (IARC, 2019).
Panzone, J. et al. The impact of household income on prostate cancer diagnosis, treatment, and outcomes. J. Clin. Oncol. 41, 5038–5038. https://doi.org/10.1200/JCO.2023.41.16_suppl.5038 (2023).
Instituto Nacional de Estadísticas (INE). Mujeres en Chile y Mercado laboral. Participación laboral femenina y brechas salariales. https://www.ine.gob.cl/docs/default-source/ocupacion-y-desocupacion/publicaciones-y-anuarios/publicaciones/mujeres-en-chile-y-mercado-del-trabajo---participaci%C3%B3n-laboral-femenina-y-brechas-salarialesa.pdf?sfvrsn=ade344d4_3 (2015).
Ministerio de Salud de Chile. Orientaciones de la estrategia lugares de trabajo promotores de la salud. https://entornospromotoresdelasalud.minsal.gob.cl/wp-content/uploads/2024/03/ORIENTACIONES-LTPS.pdf (2024).
Observatorio de igualdad de género de América Latina y el Caribe. Indicador de proporción del tiempo dedicado al trabajo doméstico y de cuidado no remunerado según sexo. https://oig.cepal.org/es/indicadores/proporcion-tiempo-dedicado-al-trabajo-domestico-cuidado-no-remunerado-segun-sexo (2023).
UN Women. Unpaid care and domestic work: Issues and suggestions for Vietnam. https://asiapacific.unwomen.org/sites/default/files/Field%20Office%20ESEAsia/Docs/Publications/2017/01/Unpaid-Care-and-Domestic-Work-EN.pdf (2016).
de Almeida, R. J. et al. Impact of educational level and travel burden on breast cancer stage at diagnosis in the state of Sao Paulo, Brazil. Sci. Rep. 12, 8357. https://doi.org/10.1038/s41598-022-12487-9 (2022).
Acknowledgements
The authors would like to thank Dr. MarÃa Luisa Vázquez (Principal Investigator) and Dr. Ingrid Vargas (Co-Principal Investigator) of the EquityCancer-LA Project, both affiliated with the Health Policy and Health Services Research Group, Health Policy Research Unit, Consortium for Health Care and Social Services of Catalonia, Barcelona, Spain, as well as the entire project team for their support during the development of this manuscript. Camilo Guerrero-Nancuante was awarded a doctoral scholarship by the National Agency for Research and Development (ANID) - Human Capital Subdivision / National Doctorate / 2022 / 21220560.
Funding
This work was funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 965226 on the call topic SC1-BHC-17-2020, Global Alliance for Chronic Diseases - Prevention and/or early diagnosis of cancer.
Author information
Authors and Affiliations
Contributions
C.GN, P.E, I.AB, and ML.G. conceptualized and designed the study. P.E. and I.AB. acquired the data and organized the database. C.GN and ML.G. analyzed the data and wrote the original draft. All authors reviewed the draft and made substantial contributions to the work. All authors reviewed and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study was approved by the Ethics Committees of the Metropolitan North Health Service (No. 062-12021) and the Metropolitan South Health Service (No. 39-16052022).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guerrero-Nancuante, C., Eguiguren, P., Abarca-Baeza, I. et al. Socioeconomic position and cancer stage at diagnosis in a fragmented Latin American health system. Sci Rep 15, 40813 (2025). https://doi.org/10.1038/s41598-025-24564-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-24564-w