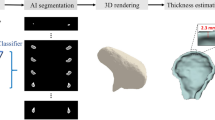
Abstract
Accurate volumetric assessment of intraosseous lesions is crucial in various fields, including bone defect evaluation, surgical outcome prediction, treatment monitoring, and 3D model design. High volumetric accuracy is essential for CBCT in digital dentistry applications. However, there is a notable lack of studies investigating the accuracy of volume determination using CBCT. In this study, we examined the factors affecting CBCT volumetric accuracy, namely voxel size, lesion location, and segmentation techniques, to improve diagnostic protocols and optimize the clinical applications of this imaging modality. 28 artificial bone defects were created in the dry rabbit mandible in two regions (Anterior and Posterior). CBCT imaging was performed with standardized positioning at two voxel sizes (0.1 and 0.2 mm). regarding micro-CT imaging as the gold standard. Images were analyzed in DICOM format using ImageJ after preprocessing, and semi-automatic segmentation was conducted via Otsu thresholding with a manually defined external defect border. In Avizo, a ResNet18-encoded U-Net architecture (Avizo’s Backboned U-Net implementation) was trained for the multiclass segmentation of the bone, background, and lesions. The volume calculations were based on the voxel counts. Volumetric measurements from CBCT showed no statistically significant difference from the micro-CT gold standard (p > 0.05). However, a significant underestimation of volume was observed when using a larger voxel size (0.2 mm) compared with a smaller voxel size (0.1 mm), irrespective of the segmentation software used (p < 0.05). The choice of software (ImageJ vs. Avizo’s deep learning-assisted tool) did not significantly affect the measurements of the porosity. The location of the defect (anterior vs. posterior) also had no significant impact on the accuracy. CBCT is a reliable tool for the volumetric assessment of mandibular bone defects and demonstrates strong agreement with micro-CT. Clinically, our findings suggest that selecting a smaller voxel size (0.1 mm) is paramount for maximizing measurement accuracy in applications requiring high precision, such as surgical planning and 3D model fabrication. The implementation of a deep learning-assisted segmentation model proved to be a viable and efficient alternative to conventional semi-automatic methods, highlighting its potential to streamline the digital workflow in dentistry without compromising accuracy.
Similar content being viewed by others
Introduction
In modern clinical and research settings, accurate volumetric assessment of intraosseous lesions is recognized as a fundamental requirement1. Precise volume measurement plays a crucial role not only in diagnosis and treatment planning, such as determining the need for surgical intervention, resection, bone grafting, or prosthetic reconstruction, but also in monitoring volumetric changes to assess healing progress and disease progression2,3,4. Consequently, the utilization of advanced imaging modalities, particularly cone beam computed tomography (CBCT), has emerged as a key tool in volumetric evaluation.
Accurate linear and volumetric measurements are also essential for three-dimensional (3D) treatment planning, precise design of palatal plates, and fabrication of patient-specific 3D anatomical models5,6,7,8. Recent advancements in three-dimensional (3D) printing and modeling technologies have enabled the creation of highly detailed, patient-specific anatomical models. These models serve as educational tools for both patients and students and aid in anticipating potential surgical challenges and improving procedural outcomes. The accuracy of the initial imaging data is critical for constructing these models, as even minor discrepancies can impact the final printed structure7,8.
Furthermore, the precise assessment of volume in structures such as the upper airway, maxillary sinus, and other key anatomical regions in dentistry and maxillofacial surgery necessitates tools capable of detecting subtle changes and tracking clinical progression9,10,11,12. Therefore, continuous volumetric monitoring is important for guiding clinical decision-making.
A key consideration is the choice between CBCT and conventional computed tomography (CT). While traditional CT offers superior soft tissue visualization and Hounsfield Unit calibration, its higher radiation dose and cost limit its utility for repeated assessments and dedicated 3D modeling in dentistry13,14,15,16. CBCT, with its high spatial resolution, lower radiation dose, and rapid acquisition, has become the preferred modality for these applications. Its capability for precise volumetric assessment is critical for generating accurate 3D anatomical models, although this requires further validation5,6,7,8.
Previous studies have extensively validated the accuracy of CBCT in linear and angular measurements, establishing its reliability for various clinical applications2. However, with the increasing adoption of CBCT for advanced 3D modeling and treatment planning, its accuracy in volumetric assessment remains an area that requires further investigation3. This research gap highlights the need for a comprehensive evaluation of CBCT’s capability in precise volume measurement to enhance clinical outcomes and optimize treatment protocols4.
Several factors influence the volumetric accuracy of the CBCT. Among the most critical are:
-
Voxel Size: The voxel size, defined by its depth, width, and height in a 3D image, directly affects the image resolution and noise levels. While a higher resolution enhances detail, it also increases the radiation dose, necessitating an optimal balance17,18.
-
Object Positioning in Field of View (FOV): The positioning of the scanned object in the FOV significantly affects the image quality, artifact formation, and beam scattering. Since uniform X-ray exposure is not achieved across the entire FOV, this can lead to measurement errors19,20.
-
Software and Segmentation Techniques: Segmentation, whether manual, semi-automated, or fully automated, is a crucial step in accurately defining the lesion volumes. Manual segmentation is time-consuming and clinically impractical, whereas fully automated algorithms have not yet achieved the required precision for clinical use. Therefore, semi-automated methods, which allow user control while improving efficiency, are particularly valuable17. Various software platforms offer advanced segmentation capabilities, and as demonstrated in previous studies, these software choices impact both the user experience and measurement accuracy21.
Artificial intelligence, particularly deep learning models, has shown promising results in interpreting medical images, reducing reading times, and improving diagnostic accuracy22. Tasks such as segmentation of maxillofacial structures have been achieved with high accuracy using convolutional neural networks (CNNs)23. However, there is no research exploring the use of neural networks in intraosseous lesion segmentation. The evolution of Convolutional Neural Networks (CNNs) has enabled effective pattern recognition through backpropagation. Despite the challenges in training deep networks, innovations such as Deep Belief Networks and Semi-supervised learning have advanced practical applications24. Among these architectures, ResNet-18 stands out because of its residual learning framework, which performs well even with small datasets, and its success in Image Classification tasks. Initially trained on ImageNet, ResNet-18 can be adapted for medical applications through Transfer Learning. Its residual learning framework is specifically designed to mitigate the vanishing gradient problem, enabling effective training even on smaller datasets, which is a significant advantage in medical imaging, where annotated data are often limited25.
Emerging technologies, such as micro-computed tomography (micro-CT), have been introduced as the gold standard for evaluating volumetric measurement accuracy. Micro-CT provides a highly detailed reference dataset, enabling the validation and refinement of CBCT segmentation algorithms to enhance lesion volume estimation26,27,28,29.
The primary objective of this study is to determine the accuracy of CBCT in measuring bone lesion volumes. To achieve this, we will compare the volumetric measurements of anterior and posterior mandibular lesions using two different voxel sizes and two different software in rabbit mandible model. This approach aims to elucidate the influence of voxel size, software, and lesion location on measurement accuracy. A key novel aspect of this work is the implementation and evaluation of a deep learning-assisted segmentation approach using a ResNet18-encoded U-Net architecture for the volumetric assessment of intraosseous mandibular defects in CBCT images, an application that, to our knowledge, has not been previously explored. These findings will contribute to refining imaging methodologies, developing more precise diagnostic protocols, and enhancing the clinical and research applications of CBCT in bone lesion assessment.
Methods & materials
Ethics approval
This study utilized dry anatomical specimens (rabbit mandibles) in accordance with the principles of the Declaration of Helsinki and international guidelines for non-living tissue research; No live animals were used in this study. These specimens had previously been employed in unrelated research projects and were later purchased by our research team. Permission to use these specimens, along with approval for all stages of the study, was obtained from the Ethics Committee of Babol University of Medical Sciences (Approval Code: IR.MUBABOL.AEC.1403.015).
Sample Preparation
The sample size for this study was determined a priori using a power analysis based on effect sizes reported in previous comparable studies investigating CBCT volumetric accuracy30,31. These studies, with a statistical power of 80% to 95% and effect sizes (Cohen’s d) ranging from 0.35 to 0.62, utilized sample sizes between 25 and 30 lesions. To ensure adequate power to detect significant differences between our experimental protocols (voxel sizes, software), a target sample size of 28 lesions was selected.
Seven dry mandibular specimens from New Zealand rabbits were selected using a simple sampling method. Subsequently, bilateral bone defects were created on each mandible in the posterior and anterior regions using a tungsten carbide bur (Zhengzhou Smile Dental Equipment Co., Ltd., Henan, China). Ultimately, a total of 28 artificial bone defects were produced32.
CBCT scan
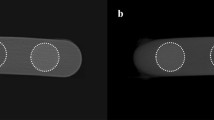
To acquire the images, the specimen was first mounted at the center of a stabilization plate, which was then fixed to the device. The device’s laser lights for the midsagittal, coronal, and axial planes were adjusted to align parallel with the specimen’s planes and to pass through its center. To standardize the position of all specimens during exposure, the laser beams were marked on the plate and used as a guide for subsequent exposures. CBCT imaging of the rabbit mandible was performed using the X-MIND TRIUM device (Acteon, Olgiate Olona, Italy) with the following settings: FOV 11 × 8 cm, focal spot 0.5 mm, 80 kVp, 6 mA, and a scan time of 7 s. For each specimen, two scans were acquired with identical parameters but using two different voxel sizes: 0.1 and 0.2 mm (Figs. 1 and 2) .
Micro-CT scan
Ten specimens were randomly selected from the sample pool. The created bone cavity was isolated as a bone block from the mandible using a disk with a 5-mm distance from the borders, and then sent to the preclinical laboratory at Tehran University for micro-CT imaging. The micro-CT scan was performed using the LOTUS InVivo device (Behin Negareh Co., Ltd., Tehran, Iran) available in the preclinical laboratory at Tehran University of Medical Sciences, under the following exposure settings: 50 kV, 0.15 mA, and a total scan time of 60 min. The voxel size was 20 microns in the transverse direction and 40 microns in the axial direction. The acquired three-dimensional data were reconstructed using LOTUS inVivo-REC (Behin Negareh Co., Ltd., Tehran, Iran) with the standard Feldkamp, Davis, Kress (FDK) algorithm.
Image interpretation
The images obtained from the CBCT and Micro-CT scans were initially examined for quality assurance using OnDemand3D Dental (Cybermed Inc., Seoul, Korea) and LOTUS inVivo-REC (Behin Negareh Co., Ltd., Tehran, Iran), respectively. Images saved in DICOM (Digital Imaging and Communications in Medicine) format. To calculate the defect volumes, the images were imported as stacks into ImageJ (National Institutes of Health, Bethesda, USA) and Avizo Amira (Thermo Fisher Scientific, Waltham, Massachusetts, USA). All the process was done independently by both an oral and maxillofacial radiology resident and a specialist and the average of the two measurements considered as the final defect volume.
ImageJ software
In the pre-processing phase, the Auto-Contrast tool was applied to all slices. Subsequently, the application of the Contrast Limited Adaptive Histogram Equalization (CLAHE), Edge Enhancement, and Non Local Means Denoising algorithms improved both the contrast and overall quality of the images (Fig. 3). Segmentation was performed using thresholding via the Otsu method, which resulted in binary images. Each defect was then segmented semi-automatically by drawing a straight line from the posterior to the anterior to serve as the external border of the defect and by selecting the Reslice option, thereby generating a secondary stack of slightly sagittal sections. Following inversion, the voxels corresponding to the defect appeared in white, and any extraneous areas were manually removed. The defect volumes were calculated from the inverted binary images by counting the voxels with a gray value of 255 and multiplying by the voxel size (either 0.1–0.2 mm)(Fig. 4).
Demonstration of the image pre-processing workflow in ImageJ on CBCT images acquired with a 0.2 mm voxel size. (a, b) Original images of posterior and anterior defects. (c, d) Images after application of the Contrast Limited Adaptive Histogram Equalization (CLAHE) and Edge Enhancement algorithms. (e, f) Final images after application of the Non-Local Means Denoising filter to reduce noise while preserving edges.
Semi-automatic segmentation steps for volume calculation in ImageJ. (a) Binary mask of the mandible generated by Otsu thresholding. (b) Manual drawing of a straight line to define the outer contour of the defect. (c) An inverted reslice stack of the defect. (d) A cropped 3D model visualizing the anterior defect. (e) A reoriented 3D view of the segmented defect. (f) An overlay of the final segmented defect (green area) on the original binary mask.
Avizo software
In the Avizo software, pre-processing was initially performed in a fully automated sequence using the following algorithms: Automatic Brightness and Contrast Adjustment, Edge Enhancement, and a Deep Learning U-Net model (DL Training Noise to Void 2D) to reduce image noise in preparation for segmentation33(Fig. 5). A ResNet18-encoded U-Net architecture (Avizo’s Backboned U-Net implementation) was trained for multiclass segmentation of bone, background, and lesions on 64 image slices that weren’t included in the study, to perform multi-class segmentation and No data augmentation techniques were applied during training34. (Fig. 6). Following this, the trained segmentation model was applied to CBCT images, and user-guided corrections were implemented to refine the segmentation (Figs. 7 and 8). The accompanying figure illustrates the workflow of the semi-automatic segmentation process conducted using the Avizo software (Fig. 9).
User-guided correction of the deep learning segmentation output in Avizo. (a) Initial defect segmentation generated by the trained model. (b) Addition of false-negative areas (red) using a brush tool. (c) Subtraction of false-positive areas (indicated by arrow) using an eraser tool. (d) The final, refined segmentation of the defect after manual corrections.
Statistical analysis
In this study, the effect of four different CBCT protocols, combining two voxel sizes (0.1 and 0.2 millimeters) with two software packages (ImageJ and Avizo) in two locations (Anterior and Posterior), was compared against the standard micro-CT method on 28 samples. The statistical analyses performed included paired t-tests, repeated measures ANOVA, the Bonferroni test, calculation of the intra-class correlation coefficient (ICC), and evaluation of the mean absolute error (MAE) by SPSS26 software. To assess the discrepancy between the different methods and the gold standard, the Mean Absolute Error (MAE) index was employed.

Results
This study demonstrated that both voxel size and software influence CBCT-based bone defect volume measurements. Volumetric measurements for all CBCT protocols and the micro-CT gold standard are presented in Table 1. Using a 0.1 mm voxel size, both software packages produced values close to the micro-CT standard. However, using a 0.2 mm voxel size, measurements were consistently lower, suggesting reduced accuracy with larger voxel sizes.
Normality tests (Kolmogorov–Smirnov and Shapiro–Wilk) confirmed that all measurements—across both voxel sizes (0.1 mm and 0.2 mm) and software tools (ImageJ, Avizo), as well as micro-CT—followed a normal distribution (p = 0.200 for Kolmogorov–Smirnov and p = 0.749 for Shapiro–Wilk). This confirmed the appropriateness of using parametric tests (e.g., t-test, one-way ANOVA, and repeated measures ANOVA).
The intra-class correlation coefficient (ICC = 0.98, p = 0.001) indicated excellent agreement among the different measurement methods, reinforcing the reliability of the results. These findings highlight that voxel size is a more significant factor than software type in determining measurement accuracy (Table 2).
As Table 3 demonstrated comparing each measurement protocol to micro-CT showed no statistically significant differences among the four CBCT-based protocols. The 0.1 mm voxel protocol using Avizo had the smallest mean difference from micro-CT (7.41 ± 4.61 µL), while the 0.2 mm voxel protocol using ImageJ had the largest (7.95 ± 6.07 µL). However, these differences were not statistically significant.
The repeated measures ANOVA revealed a statistically significant main effect for the measurement method (p = 0.028), suggesting that the selected imaging protocol significantly influences volumetric outcomes (Table 4). Importantly, the interaction effect between the measurement method and defect location (anterior vs. posterior) was not statistically significant (p = 0.239). This indicates that the accuracy of CBCT-based measurements did not differ between anterior and posterior regions in the FOV, supporting the application of a consistent protocol across locations. This repeated measures ANOVA evaluated:
-
The significant differences were attributable to the measurement method, not the defect location.
-
CBCT protocols varied in accuracy depending on voxel size.
-
Since defect location did not influence measurement accuracy, a single standardized protocol may be applied regardless of region.
-
Post-hoc pairwise comparisons (e.g., Bonferroni tests) are recommended to identify the most accurate protocol.
Bonferroni post-hoc comparisons (Table 5) showed statistically significant differences between:
0.1 mm ImageJ and 0.2 mm ImageJ (p = 0.013), 0.1 mm ImageJ and 0.2 mm Avizo (p = 0.016), 0.1 mm Avizo and 0.2 mm ImageJ (p = 0.032), 0.1 mm Avizo and 0.2 mm Avizo (p = 0.028).
These findings indicate that voxel size, more than software, is a determining factor in measurement accuracy. The largest mean differences were between the 0.1 mm ImageJ and 0.2 mm Avizo protocols (7.118 µL) and between the 0.1 mm and 0.2 mm ImageJ protocols (6.9 µL). In contrast, comparisons between different software at the same voxel size (e.g., 0.1 mm ImageJ vs. 0.1 mm Avizo) and comparisons with micro-CT did not show significant differences (p > 0.05).
The repeated measures ANOVA confirmed that defect location (anterior vs. posterior) had no statistically significant effect on volumetric accuracy (p = 0.239). To further explore potential trends, the Mean Absolute Error (MAE) for each protocol was calculated separately for each location and is presented in Table 6. In the posterior region, the 0.1 mm voxel size protocols resulted in the lowest MAE values (ImageJ: 6.07 ± 1.67 µL, p = 0.340; Avizo: 6.13 ± 3.02 µL, p = 0.412). Conversely, in the anterior region, the lowest MAE was unexpectedly associated with the 0.2 mm voxel size protocols (ImageJ: 5.07 ± 5.05 µL, p = 0.140 ; Avizo: 5.20 ± 4.75 µL, p = 0.162). It is critical to note that these differences in MAE between regions for any given protocol were not statistically significant (all p > 0.05), as shown in Table 6.
Discussion
This study aimed to assess the accuracy of cone-beam computed tomography (CBCT) in the volumetric evaluation of intraosseous lesions, using micro-computed tomography (micro-CT) as the reference standard. Additionally, the study examined the impact of voxel size, software, and lesion location on measurement accuracy. This investigation holds clinical significance as precise volumetric analysis is essential for treatment planning, surgical monitoring, and three-dimensional modeling in digital dentistry. The findings revealed no significant differences between CBCT and micro-CT measurements, although CBCT slightly underestimated lesion volumes. Voxel size significantly affected accuracy, whereas software choice and lesion location had minimal impact. Overall, CBCT can provide reliable volumetric assessments of intraosseous lesions when appropriate imaging parameters are employed. Micro-CT remains the gold standard for volumetric measurements due to its high spatial resolution, facilitating precise evaluation of bone defects35. Tayman et al. (2019) compared CBCT and micro-CT in both linear and volumetric measurements, identifying significant differences in volumetric assessments, particularly at voxel sizes of 0.75 mm and 0.2 mm. CBCT tended to underestimate lesion volume compared to micro-CT, a finding corroborated by our study, although the difference was not statistically significant. Their results underscore micro-CT’s superior accuracy, especially for complex structures such as furcations and dehiscences. Despite CBCT’s limitations in detecting small defects, it performs adequately in linear measurements. In our study, although minor differences between CBCT and micro-CT results were observed, they were not statistically significant35. Ahlowalia et al. reported an excellent correlation between CBCT and micro-CT in measuring artificial periapical lesion volumes, supporting our findings36. Similarly, Koc et al. found that CBCT measurements remained accurate when slice thickness was below 0.5 mm31.
Silveira et al. (2015) evaluated simulated internal root resorption and found that smaller voxel sizes (0.076 mm, 0.1 mm, and 0.2 mm) improved volumetric accuracy. They reported a significant difference between 0.1 mm and 0.2 mm voxel sizes, supporting our findings. In our study, the mean volume with a 0.1 mm voxel size was greater than with 0.2 mm and more closely aligned with micro-CT values37. Although this difference compared to micro-CT was not statistically significant, it supports the clinical adequacy of a 0.2 mm voxel size. Noise amplification at smaller voxels, however, may offset the theoretical resolution advantage. This explains why in anterior lesions, 0.2 mm voxels sometimes performed comparably. Significant difference between 0.1 and 0.2 mm voxel sizes aligns with findings by Koc et al. and Petro et al., who noted that partial volume effects are more prominent in smaller structures when thicker slices are used. At a voxel size of 0.2 mm, each CBCT voxel covers a larger area, causing signal averaging at lesion borders—especially those with irregular or gradual transitions—leading to volume underestimation. Additionally, lower resolution results in stair-step artifacts along lesion margins, obscuring fine details. In contrast, 0.1 mm voxels better preserve boundary curvature, improving volumetric accuracy31. In contrast, Yilmaz et al. (2019) reported no significant differences between 0.1, 0.15, and 0.2 mm voxel sizes, but this still supports the broader conclusion that CBCT can yield reliable volumetric measurements when optimized38.
Sonmez et al. (2018) found that software choice significantly affected results, while voxel size did not, both findings contrasting with our results, where voxel size played a more decisive role and software showed minimal impact. However, consistent with our findings, cavity location had no significant effect on measurement accuracy. this difference may stem from variations in segmentation methods, which were not clearly described in their study21.in another study Adisen et al. (2015) evaluated CBCT’s accuracy in measuring lesion volumes and found no significant inter-observer differences when using ITK-SNAP software1. In Ozdede et al. (2024) study voxel size had no impact on Observer 1, but significantly affected Observer 2’s results both statistically and clinically. The study also noted a decrease in inter-observer correlation as voxel size increased, emphasizing the importance of considering voxel resolution and observer error in volumetric accuracy30. El-Beblawy et al. (2024) reported significant underestimation by all software studied, with statistically significant discrepancies, In contrast to our findings39. Furthermore, as noted by Sonmez et al., the absence of detailed segmentation and volume calculation protocols in their study may have contributed to the discrepancies, given the influence of segmentation methods on volumetric measurements.
Park et al. (2017) demonstrated that volumetric errors in CBCT remain within statistically and clinically acceptable limits, confirming the method’s reliability under standardized conditions. Their study showed that tube voltage significantly affects volumetric accuracy, whereas tube current does not. Additionally, the object’s distance from the center of the field of view (FOV) was a key factor influencing volumetric error. Specifically, the farther the object was from the image center, the greater the volumetric error observed40. Interestingly, larger voxels (0.2 mm) sometimes reduced noise-related artifacts in anterior lesions, which may explain their comparable performance to 0.1 mm voxels in this region. Park et al. (2017) noted that smaller voxel sizes increase noise, leading to segmentation errors, whereas larger voxel sizes may reduce noise and improve accuracy. Although lesion location’s impact on volumetric accuracy hasn’t been directly studied, it is likely that noise and artifacts vary across the FOV, resulting different image quality in different positions in FOV. Future research should explore this further40,41. Although previous studies have not directly examined the effect of lesion location on volumetric accuracy, it is well established that noise and artifact distribution are not uniform across the entire FOV. In the posterior mandible of rabbits, image quality may be superior due to reduced interference from anterior teeth and their associated artifacts. However, future studies should specifically investigate these factors impacts.
Semi-automatic and artificial intelligence (AI)-based segmentation techniques can enhance result precision, as delineating lesion boundaries remains challenging in certain cases42. In the present study, efforts were made to minimize human error and improve measurement accuracy by implementing AI-driven image quality enhancement tools, noise reduction algorithms, and advanced semi-automatic segmentation protocols. Although these methods improved workflow efficiency, they did not yield statistically superior results compared to traditional segmentation.
Dot et al. (2024) proposed and evaluated a novel tool for multiclass DentoMaxillofacial (DMF) CT and CBCT image segmentation called DentalSegmentator. They used the pre-trained nnU-Net network. The results demonstrated the robustness and generalizability of the model for the segmentation of routine CT and CBCT scans acquired in several cases of use such as orthognathic surgery planning, guided implant surgery, impacted teeth visualization, and digital orthodontics23. We adapted the ResNet18 architecture for simultaneous semantic segmentation and classification of mandibular defects in CBCT images, demonstrating promising performance. ResNet18’s residual learning framework mitigated gradient vanishing in our small dataset, while the U-Net decoder preserved spatial resolution for precise boundary delineation, critical for low-contrast intraosseous defects. These findings highlights the potential of lightweight, task-specific deep learning architectures for applications in different aspects of digital dentistry. while ResNet18-U-Net improved efficiency, its accuracy was statistically comparable to used method in ImageJ, suggesting the need for larger training datasets or domain-specific pretraining.
Xu et al. (2025) conducted a study to evaluate automatic segmentation of bone graft in maxillary sinus via distance constrained Network guided by prior anatomical knowledge. They used a deep neural network to conquest major challenges due to the complex local appearance, including blurred boundaries, lesion interference, implant and artifact interference, and bone graft exceeding the maxillary sinus43. In our study we used ResNet18 which due to its residual learning framework, This model overcame challenges, where regions have similar intensities and unclear boundaries. The model streamlined the segmentation process and reduced time requirements, although manual corrections were still required.
This study focuses on quantifying bone defect volumes in CBCT and evaluating the impact of voxel size, anatomical positioning, and advanced software on measurement precision. The findings hold direct relevance to digital dentistry and 3D modeling applications. Below, two key domains to which this research contributes are examined:
1. Enhancement of Bone Volume Measurement Techniques for Surgical Analyses and Outcome Monitoring.
Accurate volumetric assessment of bone lesions and their longitudinal changes remains a significant challenge in surgical treatment planning. Previous studies have examined variables such as image analysis software and scan quality. Travessas et al. (2022) demonstrated that intra-cavity content and software selection have a greater impact on CBCT volumetric accuracy than voxel size. Our findings corroborate that error margins attributable to varying voxel sizes are relatively limited, confirming CBCT’s reliability in quantifying bone defects across both tested voxel dimensions3. However, adjacent anatomical structures may introduce measurement inaccuracies, warranting further investigation. Volumetric monitoring of bone changes during treatment is pivotal in digital dentistry. Zheng et al. (2025) established that CBCT-based 3D analysis using 3D Slicer software enables precise volumetric evaluation of jaw cysts, providing a robust foundation for clinical decision-making13. Our findings align with such applications, validating CBCT’s accuracy in assessing bone defect volumes using ImageJ and Avizo Amira software. Nevertheless, as demonstrated by Travessas et al., variations in software algorithms and tissue characteristics may alter precision. These outcomes highlight CBCT’s potential as a critical modality for longitudinal treatment monitoring and digital surgical planning3.
2. Optimization of CBCT Imaging for Generating 3D Models in Treatment Evaluation and Planning.
A pivotal application of CBCT in digital dentistry lies in constructing 3D models for surgical planning and 3D printing. Kamio et al. (2023) identified threshold determination for distinguishing bone from soft tissues in CBCT as a critical challenge, emphasizing that scan quality and CBCT accuracy directly influence the precision of resultant Standard Triangle Language (STL) models44. This matter represents importance of accurate raw data and segmentation process. Our findings, alongside analogous studies, corroborate the reliability of CBCT-derived volumetric measurements, reinforcing its utility as a trusted imaging modality for generating data essential to 3D modeling and digital surgical applications. However, this necessitates further research to standardize CBCT datasets, as unresolved challenges persist. For instance, uncertainties regarding the validity of CBCT-derived gray values, particularly their calibration against Hounsfield Units (HU), remain under investigation45 .
Rostetter et al. (2015) highlighted persistent quantitative limitations of CBCT compared to multidetector CT (MDCT), particularly in bone density measurements. CBCT demonstrates lower accuracy in absolute metrics such as HU and exhibits irregular anatomical representations due to scatter radiation effects. Clinical reconstructions from CBCT and MDCT showed reproducible internal correlations but were confined to linear measurements and again volumetric measurements was ignored. The authors proposed that standardized HU calibration and manufacturer-level corrections for CBCT systems could mitigate discrepancies and enhance data homogeneity20. These factors may explain volumetric measurement divergences between CBCT and MDCT, as subtle imaging parameter variations can influence lesion boundary delineation.
A key challenge in CBCT-based digital surgical planning involves optimizing protocols that balance radiation dose reduction with sufficient image quality for 3D modeling. Koivisto et al. (2024) demonstrated that select low-dose CBCT protocols yield data with adequate precision for this purpose46. Our study similarly confirms CBCT’s volumetric measurement reliability and evaluates voxel size as a critical determinant of imaging accuracy. These insights provide a foundation for refining CBCT protocols to generate precise 3D models in digital dentistry.
Beyond voxel size, variables such as software algorithms, segmentation techniques, lesion morphology, and regional anatomical features significantly impact measurement precision. Our findings underscore the importance of selecting appropriate voxel dimensions and software configurations, affirming CBCT as a reliable tool for quantifying bone defect volumes.
To mitigate errors such as partial volume effects and artifactual segmentation, further development of advanced, intelligent segmentation algorithms is imperative. Future studies should also address the influence of soft tissue characteristics, artifact presence, and CBCT’s inherent limitations in bone density measurement compared to MDCT. Standardizing imaging protocols and validating gray value consistency across CBCT systems remain critical to improving volumetric accuracy in bone lesion assessment.
Although the rabbit mandible provides a suitable experimental model due to its structural and density similarities with the human jaw, certain limitations should be acknowledged32. The present study was conducted in vitro, without the influence of surrounding soft tissues, patient movement, or other clinical variables that may affect image quality and segmentation accuracy. Additionally, anatomical differences between rabbit and human mandibles, such as bone size and trabecular architecture, may limit direct extrapolation of the results. Nevertheless, the generalizability of our findings remains supported by the consistency of CBCT volumetric measurements across protocols, suggesting that with appropriate imaging parameters, similar accuracy may be expected in human clinical applications. Future studies using human specimens or in vivo models are warranted to further validate these results.
Conclusion
This study demonstrates that CBCT provides reliable volumetric assessment of mandibular bone defects, showing good agreement with the micro-CT gold standard. The principal novelty of this work is the first application of a deep learning segmentation model (ResNet18-encoded U-Net) to poorly marginated mandibular defects in CBCT, offering a promising and efficient approach for this task. Final manual adjustments were necessitated by boundary ambiguity. The results indicate that voxel size is a critical parameter, with a statistically significant difference in measurement accuracy observed between the 0.1 mm and 0.2 mm protocols. In contrast, the choice of segmentation software (ImageJ versus Avizo) and the anatomical location of the defect (anterior versus posterior) had no significant influence on the volumetric outcomes. Thus, the choice between 0.1 mm and 0.2 mm voxel sizes may depend on clinical needs, such as image resolution, processing time, and diagnostic purpose.
Data availability
All data and materials are available upon request from the journal. Request for data can be directed to the submitting author, Mohsen Shalalvand (Mohsenshalalvand@gmail.com) or the corresponding author, Sina Haghanifar (dr_haghanifar@yahoo.com).
Abbreviations
- AI:
-
Artificial Intelligence
- CBCT:
-
Cone Beam Computed Tomography
- MDCT:
-
Multi Detector Computed Tomography
- CT:
-
Conventional Tomography
- Micro-CT:
-
Micro Computed Tomography
- HU:
-
Hounsfield Unit
- STL:
-
Standard Triangle Language
- FOV:
-
Field Of View
- CLAHE:
-
Contrast Limited Adaptive Histogram Equalization
- ICC:
-
Interclass Correlation Coefficient
- MAE:
-
Mean Absolute Error
- CNN:
-
Convolutional Neural Networks
- DMF:
-
DentinoMaxilloFacial
- DL:
-
Deep Learning
References
Adisen, M. Z. et al. Evaluation of volumetric measurements on CBCT images using Stafne bone cavities as an example. Med. Oral Patol. Oral Cir. Bucal. 20 (5), e580–e586 (2015).
Bouxsein, M. L. et al. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 25 (7), 1468–1486 (2010).
Travessas, J. A. C. et al. Validation and comparison of volume measurements using 1 multidetector computed tomography and 5 cone-beam computed tomography protocols: an in vitro study. Imaging Sci. Dent. 52 (4), 399–408 (2022).
Mallya, S. & Lam, E. White and Pharoah’s Oral Radiology: Principles and Interpretation (Elsevier Health Sciences, 2018).
Zou, Y. P. et al. Precipitation-based silk fibroin fast gelling, highly adhesive, and magnetic nanocomposite hydrogel for repair of irregular bone defects. Adv. Funct. Mater. 33 (29), 2302442 (2023).
Fonseca, C., Cavadas, F. & Fonseca, P. Upper airway assessment in cone-beam computed tomography for screening of obstructive sleep apnea syndrome: development of an evaluation protocol in dentistry. JMIR Res. Protocols. 12 (1), e41049 (2023).
Ünüvar, Y. A. & Köse, E. Evaluation of maxillary sinus volume of class III individuals with different jaw positions by Cone-Beam computed tomography. Turkish J. Orthod. 36 (3), 180 (2023).
Favato, M. N. et al. Impact of human maxillary sinus volume on grafts dimensional changes used in maxillary sinus augmentation: a multislice tomographic study. Clin. Oral. Implants. Res. 26 (12), 1450–1455 (2015).
Koons, G. L., Diba, M. & Mikos, A. G. Materials design for bone-tissue engineering. Nat. Reviews Mater. 5 (8), 584–603 (2020).
Weismann, C. et al. Complete digital workflow for manufacturing presurgical orthodontic palatal plates in newborns and infants with cleft lip and/or palate. J. Funct. Biomaterials. 15 (10), 301 (2024).
Aretxabaleta, M. et al. Accuracy evaluation of additively and subtractively fabricated palatal plate orthodontic appliances for newborns and infants–an in vitro study. Materials 14 (15), 4103 (2021).
Steegman, R. et al. Cone beam computed tomography volumetric airway changes after orthognathic surgery: a systematic review. Int. J. Oral Maxillofac. Surg. 52 (1), 60–71 (2023).
Zheng, X. et al. To evaluate the clinical efficacy of decompression for large cystic lesions in mandible by digital technology. Eur. J. Med. Res. 30 (1), 104 (2025).
Aldaadaa, A., Owji, N. & Knowles, J. Three-dimensional printing in maxillofacial surgery: hype versus reality. J. Tissue Eng. 9, 2041731418770909 (2018).
Patrick, S. et al. Comparison of Gray values of cone-beam computed tomography with Hounsfield units of multislice computed tomography: an in vitro study. Indian J. Dent. Res. 28 (1), 66–70 (2017).
Gaêta-Araujo, H. et al. Cone beam computed tomography in dentomaxillofacial radiology: a two-decade overview. Dentomaxillofacial Radiol. 49 (8), 20200145 (2020).
Panjnoush, M., Kheirandish, Y. & Zeini, N. Effect of Spatial position in the field of view on dimensional changes in cone beam computed tomography. J. Dentistry (Tehran Iran). 14 (5), 282 (2017).
Valizadeh, S. et al. Effect of object position in cone beam computed tomography field of view for detection of root fractures in teeth with intra-canal posts. Iran. J. Radiol. 12 (4), e25272 (2015).
Ibrahim, N. et al. Comparison of anterior and posterior trabecular bone microstructure of human mandible using cone-beam CT and micro CT. BMC Oral Health. 21 (1), 249 (2021).
Rostetter, C. et al. Comparison of in vivo cone-beam and multidetector computed tomographic scans by three-dimensional merging software. Br. J. Oral Maxillofac. Surg. 53 (10), 1021–1026 (2015).
Sönmez, G., Koç, C. & Kamburoğlu, K. Accuracy of linear and volumetric measurements of artificial ERR cavities by using CBCT images obtained at 4 different voxel sizes and measured by using 4 different software: an ex vivo research. Dentomaxillofac Radiol. 47 (8), 20170325 (2018).
Shetty, S. et al. Accuracy of deep learning models in the detection of accessory ostium in coronal cone beam computed tomographic images. Sci. Rep. 15 (1), 8324 (2025).
Dot, G. et al. DentalSegmentator: robust open source deep learning-based CT and CBCT image segmentation. J. Dent. 147, 105130 (2024).
Wang, S. et al. Fully automated deep learning system for osteoporosis screening using chest computed tomography images. Quant. Imaging Med. Surg. 14 (4), 2816–2827 (2024).
Macfadyen, C., Duraiswamy, A. & Harris-Birtill, D. Classification of hyper-scale multimodal imaging datasets. PLOS Digit. Health. 2 (12), e0000191 (2023).
Brüllmann, D. & Schulze, R. Spatial resolution in CBCT machines for dental/maxillofacial applications—what do we know today? Dentomaxillofacial Radiol. 44 (1), 20140204 (2015).
Moudi, E. et al. Evaluation of the cone-beam computed tomography accuracy in measuring soft tissue thickness in different areas of the jaws. J. Indian Soc. Periodontology. 23 (4), 334–338 (2019).
Selvaraj, A. et al. Correlation between Gray values of cone-beam computed tomograms and Hounsfield units of computed tomograms: A systematic review and meta-analysis. Imaging Sci. Dentistry. 52 (2), 133 (2022).
Petersen, L. B. et al. Image and surgery-related costs comparing cone beam CT and panoramic imaging before removal of impacted mandibular third molars. Dentomaxillofacial Radiol. 43 (6), 20140001 (2014).
Ozdede, M. et al. Repeatability and effect of different voxel sizes on linear and volumetric tooth and pulp measurements using cone-beam computed tomography. BMC Oral Health. 24 (1), 1472 (2024).
Koç, A. & Kaya, S. Is it possible to estimate volume of bone defects formed on dry sheep mandibles more practically by secondarily reconstructing section thickness of cone beam computed tomography images? Dentomaxillofac Radiol. 50 (3), 20200400 (2021).
Li, Y. et al. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Translat. 3 (3), 95–104 (2015).
Krull, A., Buchholz, T. O. & Jug, F. Noise2void-learning denoising from single noisy images. in Proceedings of the IEEE/CVF Conference on computer vision and pattern recognition. (2019).
Chen, M. et al. Convolutional neural networks for automated annotation of cellular cryo-electron tomograms. Nat. Methods. 14 (10), 983–985 (2017).
Tayman, M. A. et al. Comparison of linear and volumetric measurements obtained from periodontal defects by using cone beam-CT and micro-CT: an in vitro study. Clin. Oral Invest. 23, 2235–2244 (2019).
Ahlowalia, M. et al. Accuracy of CBCT for volumetric measurement of simulated periapical lesions. Int. Endod. J. 46 (6), 538–546 (2013).
Da Silveira, P. et al. CBCT-based volume of simulated root resorption–influence of FOV and voxel size. Int. Endod. J. 48 (10), 959–965 (2015).
Yilmaz, F. et al. Accuracy of CBCT images in the volumetric assessment of residual root Canal filling material: effect of voxel size. Niger J. Clin. Pract. 22 (8), 1091–1098 (2019).
El-Beblawy, Y. M., Bakry, A. M. & Mohamed, M. E. A. Accuracy of formula-based volume and image segmentation-based volume in calculation of preoperative cystic jaw lesions’ volume. Oral Radiol. 40 (2), 259–268 (2024).
Park, C. W. et al. Volumetric accuracy of cone-beam computed tomography. Imaging Sci. Dent. 47 (3), 165–174 (2017).
Terrabuio, B. R. et al. Cone-beam computed tomography artifacts in the presence of dental implants and associated factors: an integrative review. Imaging Sci. Dentistry. 51 (2), 93 (2021).
Shi, J. Y. et al. Accuracy assessment of a novel semiautomatic method evaluating bone grafts around the dental implant: an in vitro and ex vivo study. Sci. Rep. 10 (1), 14902 (2020).
Xu, J. et al. Automatic segmentation of bone graft in maxillary sinus via distance constrained network guided by prior anatomical knowledge. IEEE J. Biomed. Health Inf. 29 (3), 1995–2005 (2025).
Kamio, T. & Kawai, T. CBCT images to an STL model: exploring the critical factors to binarization thresholds in STL data creation. Diagnostics 13 (5), 921 (2023).
Yadegari, A. et al. Assessment of CBCT Gray value in different regions-of-interest and fields-of-view compared to Hounsfield unit. Dentomaxillofacial Radiol. 52 (8), 20230187 (2023).
Koivisto, J. et al. Assessment of cone-beam CT technical image quality indicators and radiation dose for optimal STL model used in visual surgical planning. Dentomaxillofacial Radiol. 53 (6), 423–433 (2024).
Author information
Authors and Affiliations
Contributions
SH & MS devised acquisition of data, statistical analysis, administrative, technical, and material support. SH &MS&EM devised study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision. AB devised analysis and interpretation of data, administrative, technical, and material support. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All stages of the study were conducted after obtaining approval from the Ethics Committee of Babol University of Medical Sciences (approval code: IR.MUBABOL.AEC.1403.015).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shalalvand, M., Haghanifar, S., Moudi, E. et al. Deep learning-assisted CBCT segmentation provides reliable volumetric assessment of mandibular defects compared with micro-CT for 3D printing and surgical planning. Sci Rep 15, 37561 (2025). https://doi.org/10.1038/s41598-025-24748-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-24748-4