Abstract
To develop a postoperative recurrence/metastasis risk prediction model for cervical cancer based on circulating tumor cells (CTCs) and tumor fibrosis distance (TFD), and to validate its biological mechanisms through animal experiments. This model aims to guide preoperative risk stratification and personalized treatment strategies. A total of 148 patients with stage IB–IIA cervical cancer who underwent radical surgery between 2020 and 2022 were retrospectively enrolled. Preoperative CTC counts, TFD measurements, and clinicopathological data were collected. Independent prognostic factors were identified using Cox regression analysis. The model’s performance was assessed using receiver operating characteristic (ROC) curves, and risk stratification was evaluated with Kaplan–Meier survival analysis. Additionally, an orthotopic tumor model was established in 60 Sprague-Dawley rats divided into six groups (Control, Model, HTFD, LTFD, HCTCs, and LH). Tumor burden, CD4+/CD8 + ratios, IL-6, TNF-α, MDA, and SOD levels were measured to explore the model’s underlying metabolic and immune mechanisms. Patients with poor outcomes had significantly higher CTC counts (30.50 vs. 23.74/5 mL, P < 0.001) and lower TFD values (5.47 vs. 5.98 mm, P < 0.001). CTCs ≥ 28/5 mL (OR = 6.63) and TFD ≤ 5.7 mm (OR = 3.37) were identified as independent predictors of poor prognosis. The combined model yielded an AUC of 0.91, with a sensitivity of 90.6%, specificity of 84.5%, and a negative predictive value (NPV) of 94.7%. Patients were stratified into low-, intermediate-, and high-risk groups, with corresponding clinical management recommendations. In high-risk rat groups, elevated CTC release, reduced CD4+/CD8 + ratios, increased IL-6 and TNF-α levels, and significant oxidative damage were observed, supporting the biological plausibility of the clinical model. Unlike conventional models that mainly rely on clinicopathological features, our model incorporates both CTCs and TFD, providing superior discrimination for recurrence risk and offering a novel integrative approach for postoperative surveillance. This dual validation underscores the translational potential of the model, which may be incorporated into individualized postoperative monitoring strategies and multidisciplinary decision-making.
Similar content being viewed by others
Introduction
Cervical cancer (CC) remains one of the leading causes of cancer-related deaths among women worldwide, with an incidence of approximately 13.1 per 100,000 and a five-year survival rate of up to 68%1,2. Persistent infection with high-risk human papillomavirus (HPV) is the primary etiological factor3. Although surgery, chemotherapy, radiotherapy, and targeted or immunotherapy have been widely integrated into cervical cancer treatment, therapeutic outcomes remain variable due to tumor heterogeneity and drug resistance, particularly in the context of immunotherapy4,5,6. Moreover, while most early-stage patients can resume normal life following treatment, a subset still experiences recurrence or metastasis, leading to poor prognosis7,8.
According to the FIGO staging system, the recurrence rate ranges from 11 to 22% in stage IB–IIA and up to 28–64% in stage IIB–IV. The five-year survival rate for patients with recurrence or metastasis drops sharply to 3.2–17%8. Limited therapeutic options after surgery, radiotherapy, or chemotherapy contribute significantly to mortality in advanced cervical cancer9. In terms of prevention, primary (HPV vaccination), secondary (screening), and tertiary (diagnosis and treatment) measures have significantly reduced the incidence and mortality in developed countries10,11. However, limited access to vaccination and screening in developing countries continues to result in high incidence rates and a younger age of onset12. Such disparities may indirectly influence recurrence risk and model applicability, as patients from areas with lower vaccination coverage may present with more aggressive tumor biology and higher recurrence rates. Although postoperative pathological features such as tumor size, lymph node metastasis, lymphovascular space invasion (LVSI), and FIGO stage are associated with recurrence/metastasis risk13, these parameters are not available preoperatively, hindering early identification and management of high-risk patients. Previous models based on tumor size, lymphovascular invasion, or FIGO stage had limited preoperative applicability, while serum SCC-Ag provided only moderate predictive accuracy. These limitations highlight the need for novel preoperative biomarkers. Given these challenges, researchers have increasingly turned to minimally invasive biomarkers such as circulating tumor cells (CTCs) and novel imaging-derived indicators like tumor fibrosis distance (TFD). Several studies have demonstrated the prognostic relevance of CTCs in cervical cancer, with higher CTC counts correlating with lymph node metastasis, advanced FIGO stage, and poor survival. These findings support the utility of CTCs as a noninvasive prognostic marker. TFD refers to the shortest distance from the tumor invasive front to the surrounding stromal boundary, reflecting the degree of tumor–stromal interaction. Unlike the conventional surgical margin, which represents the resection boundary, TFD captures microstructural invasiveness within the cervical stroma and thus constitutes a distinct histopathological parameter. These may overcome the limitations of conventional pathological features by offering preoperative prognostic information.
Therefore, this study aims to develop a predictive model for postoperative recurrence and metastasis based on preoperative clinical and imaging features, to support early risk stratification and guide individualized treatment planning.
Materials and methods
Patient selection and ethical approval
This multicenter retrospective cohort study included cervical cancer patients (FIGO stage IB–IIA, no distant metastasis) who underwent radical hysterectomy with pelvic lymph node dissection between January 2020 and January 2022 at three hospitals: The First Affiliated Hospital of Shandong Second Medical University, Jinan Fourth People’s Hospital, and Peking University Medical Lunan Hospital. Eligible patients had histologically confirmed squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma, with no history of neoadjuvant chemoradiotherapy. Exclusion criteria included concurrent malignancies, severe infections, hepatic or renal insufficiency, hematologic or immune disorders, recent (within 3 months) use of hormones or immunosuppressants, follow-up duration < 1 month, or in-hospital postoperative death. All procedures involving human participants or their data were conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. All methods were performed in accordance with relevant guidelines and regulations.
Clinical and laboratory data collection
Baseline data were extracted from electronic medical records, including age, BMI, histological subtype, preoperative FIGO stage, maximal tumor diameter on imaging, parametrial invasion, myometrial invasion, pelvic lymph node status, LVSI, and postoperative adjuvant therapy. Within 48 h before surgery, 5 mL of peripheral blood was collected for routine blood tests (neutrophils, lymphocytes, platelets), squamous cell carcinoma (SCC) antigen, and CTCs. CTCs were enriched using immunomagnetic beads coated with anti-EpCAM and anti-cytokeratin antibodies, while CD45 was employed to exclude leukocytes. Only EpCAM⁺/CK⁺/DAPI⁺/CD45⁻ cells were counted as CTCs, thereby minimizing false-positive results. Preoperative TFD was measured on MRI, and postoperative pathological specimens were used to confirm stromal invasion depth. When discrepancies > 1 mm were detected, two blinded radiologists and one pathologist re-evaluated the case to ensure consistency. The risk score was weighted according to β coefficients from multivariate regression, where CTCs showed a stronger effect size (OR = 6.63) compared with TFD (OR = 3.37), justifying the assignment of 2 and 1 points, respectively.
Animal experiment design
Sixty SPF-grade female Sprague-Dawley rats (6–8 weeks old, 180–220 g) were randomly assigned into six groups (n = 10 each): Control, Model, HTFD (complete resection with TFD ≥ 5 mm), LTFD (residual tumor with TFD ≤ 1 mm), HCTCs (high CTC load with complete resection), and LH (high CTC load with residual tumor). After one week of acclimatization, rats received a 0.5 mL intravaginal injection of 1 × 10^7/mL HeLa cells. HeLa cells were selected because they are the most widely used human cervical cancer cell line with stable tumorigenicity, making them ideal for translational relevance. The grouping strategy was designed to reflect the key risk categories defined by the clinical model (high/low TFD, high/low CTCs). When tumor volume reached 100–200 mm³ with clear margins (3–4 weeks post-injection), surgical interventions were performed. Rats were anesthetized with intraperitoneal sodium pentobarbital (40 mg/kg). Postoperative monitoring included respiratory and temperature control, prophylactic penicillin, ibuprofen for analgesia, and daily wound evaluation. Survival was recorded up to 60 days. Rats that died naturally or lost > 20% body weight were euthanized using CO₂. All animal experiments were approved by the Animal Care and Use Committee of Shandong Second Medical University (approval no. XYZ2024-017), and conducted in accordance with institutional guidelines and national regulations for animal welfare. This study is reported in accordance with the ARRIVE guidelines.
Physiological and immunological measurements
Body weight and food intake were recorded daily. Tumors were weighed within 3 min after resection. Tissue samples were fixed, stored in ethanol, or flash-frozen in liquid nitrogen. Blood samples were collected at baseline and on postoperative days 7, 14, and 28 to measure serum superoxide dismutase (SOD, xanthine oxidase method), malondialdehyde (MDA, thiobarbituric acid method), and glutathione (GSH, DTNB colorimetry). Serum IL-6, TNF-α, and IL-10 levels were quantified using commercial ELISA kits. Peripheral blood T cell subsets (CD3⁺, CD4⁺, CD8⁺) were analyzed by flow cytometry (BD FACSCanto II; 10,000 events per sample), and data were processed using FlowJo software.
Follow-up, endpoint Definition, and statistical analysis
Patients were followed from the date of surgery until the date of documented recurrence/metastasis, death, or last contact. The primary endpoint was disease-free survival (DFS), defined as the interval from surgery to first documented local recurrence, distant metastasis, or death from any cause. Median follow-up was 12 months. Kaplan–Meier methods were used to estimate DFS and log-rank tests to compare groups.Data were recorded in Excel and analyzed using SPSS 24.0. Continuous variables were expressed as mean ± standard deviation, and categorical variables as percentages. ROC curves and the maximum Youden index were used to determine optimal cut-off values for CTCs and TFD. Variables with P < 0.10 in univariate analysis were entered into a multivariate Cox regression model to identify independent predictors. A two-sided P value < 0.05 was considered statistically significant.
Technical roadmap
This study was conducted following a structured framework encompassing model development, validation(Figure 1).
Results
Baseline characteristics
A total of 148 patients were included, with 32 (21.6%) classified in the poor prognosis group and 116 (78.4%) in the favorable prognosis group. No significant differences were observed in age (57.34 vs. 57.50 years), BMI (24.58 vs. 24.66), or maximum tumor diameter (4.77 vs. 4.67 cm) (all P > 0.05). However, significant differences were found in histological subtype and clinical stage: squamous cell carcinoma was more common in the favorable group (80.2% vs. 65.6%, χ² = 3.912, P = 0.048), and stage I was more frequent in the favorable group (56.0% vs. 31.3%, χ² = 4.087, P = 0.043) (Table 1).
Correlation of CTCs and TFD with clinical features
CTC levels were weakly positively correlated with tumor diameter (r = 0.18, P = 0.032), while TFD showed no significant correlation with tumor size (r = − 0.15, P = 0.067). Advanced FIGO stages were associated with increased CTC counts (r = 0.41, P < 0.001) and decreased TFD values (r = − 0.37, P < 0.001). Lymph node metastasis correlated with elevated CTC levels (r = 0.33, P = 0.001) and reduced TFD (r = − 0.29, P = 0.003). No significant association was found between histological subtype and either marker (P > 0.05).
Predictive performance of individual indicators
CTCs: The ROC curve showed an AUC of 0.85 (95% CI: 0.78–0.92), with an optimal cutoff value of ≥ 28 cells/5 mL, yielding a sensitivity of 84.4%, specificity of 79.3%, Youden index of 0.637, positive predictive value (PPV) of 68.2%, and negative predictive value (NPV) of 90.1%.
TFD: The AUC was 0.72 (95% CI: 0.63–0.81), with an optimal cutoff of ≤ 5.7 mm, a sensitivity of 71.9%, specificity of 68.1%, Youden index of 0.400, PPV of 54.8%, and NPV of 82.4%.
Combined predictive model
The combined model incorporating CTCs and TFD achieved an AUC of 0.91 (95% CI: 0.86–0.96), with a sensitivity of 90.6%, specificity of 84.5%, Youden index of 0.751, PPV of 76.3%, and NPV of 94.7%, significantly outperforming individual predictors (Table 2; Fig. 2).
Risk model development and validation
A logistic regression model was constructed to predict recurrence/metastasis in cervical cancer (Table 3). CTCs ≥ 28 cells/5 mL (OR = 6.63, P < 0.001) and TFD ≤ 5.7 mm (OR = 3.37, P = 0.002) were identified as independent risk factors. Based on the β coefficients, a risk score ranging from 0 to 3 was developed: 2 points were assigned for elevated CTCs and 1 point for reduced TFD.
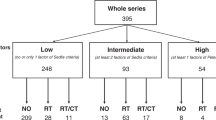
Patients were stratified into three risk groups
Low-risk group (< 10% recurrence/metastasis): routine follow-up every 6 months was recommended, considering the indolent disease course and cost-effectiveness;
Intermediate-risk group (10%–30%): intensified surveillance every 3 months, including imaging evaluations, was advised to detect early signs of progression;
High-risk group (> 30%): prophylactic chemoradiotherapy and monthly multidisciplinary team (MDT) assessments were initiated. This approach integrates emerging evidence on tumor heterogeneity to reduce metastatic burden through proactive intervention (Table 4).
The Hosmer–Lemeshow goodness-of-fit test confirmed good calibration of the model (χ² = 3.21, P = 0.665).
Validation in animal models
In the tumor model group, rat body weight significantly decreased (from 201.9 to 146.2 g). In the high CTC + low TFD (combined intervention) group, postoperative weight recovered only to 162.9 g, indicating pronounced metabolic impairment. In contrast, rats in the high TFD group maintained stable body weight (202.2 g), suggesting that sufficient surgical margins may mitigate tumor-induced metabolic disturbance (Fig. 3).
The tumor mass was highest in the model group (8.38 g), while the high TFD group had significantly reduced tumor mass (1.30 g), supporting the efficacy of complete tumor resection. Rats in the low TFD, high CTC, and combined intervention groups had persistently high tumor burdens (6.75 g), indicating that either insufficient margins or elevated CTCs could sustain tumor growth, with their combination producing the most severe burden—consistent with the high-risk classification in the predictive model (Fig. 3).
In the combined intervention group, CD4⁺ T cell levels decreased by 37.8%, CD8⁺ T cells by 19.9%, and the CD4⁺/CD8⁺ ratio dropped from 2.39 (high TFD group) to 2.04, indicating marked immunosuppression and T cell exhaustion. Inflammatory cytokine analysis showed that IL-6 levels increased by 130%, TNF-α by approximately 400%, and IL-1β by nearly 400% in the combined group, reflecting a strong systemic inflammatory response consistent with tumor-driven inflammation.
Oxidative stress markers further supported this: SOD activity was significantly reduced, MDA levels were elevated, and reactive oxygen species (ROS) accumulated, indicating substantial antioxidant system damage. In contrast, rats in the high TFD group showed normalized inflammatory cytokine levels, SOD recovery to 78 U/mg, and significantly reduced MDA levels (Fig. 4), confirming the anti-inflammatory and antioxidant regulatory role of sufficient TFD in preventing CTC-induced oxidative injury.
Discussion
This study established a preoperative risk prediction model that integrates CTCs and TFD, demonstrating robust performance in identifying cervical cancer patients at high risk of recurrence and metastasis after radical surgery. Multivariate analysis revealed that CTCs ≥ 28/5 mL and TFD ≤ 5.7 mm were independently associated with poor outcomes, increasing the risk by 6.6- and 3.4-fold, respectively. The combined model achieved an area under the curve (AUC) of 0.91 and a NPV of 94.7%, indicating its strong potential for clinical application.
Numerous studies have validated the prognostic value of CTCs in breast, lung, prostate, and gastrointestinal malignancies14,15,16. In colorectal cancer, Aggarwal et al. found that patients with lower baseline CTCs had significantly longer survival than those with elevated CTCs17, highlighting the utility of CTCs as a broadly applicable prognostic marker. The high NPV (90.1%) suggests that patients with low CTC counts may be spared from unnecessary adjuvant therapy or intensive surveillance. However, the relatively modest PPV (68.2%) underscores the biological heterogeneity of CTCs: some may exist in a quiescent state or lack the functional capacity to form metastases18. In a preoperative setting, we identified both elevated circulating tumor cells (CTCs ≥ 28/5 mL) and a shorter tumor fibrosis distance (TFD ≤ 5.7 mm) as independent predictors of postoperative recurrence/metastasis in early-stage cervical cancer. The high NPV (94.7%) indicates that patients identified as low risk are unlikely to relapse, supporting the safe reduction of overtreatment. Conversely, the modest PPV (76.3%) suggests biological heterogeneity, underscoring the need for additional molecular markers to improve prediction in high-risk patients.
To overcome this limitation, future work may focus on phenotypic and genotypic characterization of CTC subtypes. For instance, epithelial–mesenchymal transition (EMT)-like CTCs have shown higher metastatic potential than purely epithelial ones18. Notably, the dynamic monitoring of CTC changes during and after treatment could also serve as an early indicator of therapeutic response or resistance19. In the “seed and soil” framework, CTCs serve as metastatic seeds, while TFD reflects the microenvironmental soil. Shorter TFD indicates stromal remodeling and local immune suppression that facilitate CTC colonization, consistent with Paget’s hypothesis and recent mechanistic studies. TFD is conceptually distinct from the traditional postoperative “surgical margin”: it is an imaging-derived, preoperative measure of the shortest distance from the tumor edge to the cervical stromal boundary, capturing biology at the invasive front rather than ex vivo margin status20. Consistent with early observations that shorter tumor–stroma separation associates with more aggressive behavior, we found TFD ≤ 5.7 mm to be independently prognostic. Importantly, combining TFD with CTCs improved discrimination from AUC = 0.85 (CTCs alone) and 0.72 (TFD alone) to 0.91, aligning with the “seed-and-soil” paradigm and suggesting that a dual-axis assessment of dissemination potential and microenvironmental permissiveness adds clinical value. From a clinical standpoint, this model offers a practical means of refining postoperative management strategies and minimizing both overtreatment and undertreatment.
To further support the clinical relevance of this model, we conducted comprehensive in vivo validation using a rat cervical cancer model. In the high-risk intervention group (high CTCs and low TFD), animals experienced significant weight loss and maintained high tumor burdens, suggesting profound metabolic disruption. These findings support the role of metabolic reprogramming—specifically the Warburg effect—as a key driver of cancer cachexia21. Notably, inflammatory markers such as IL-6, TNF-α, and IL-1β were significantly elevated, indicating a systemic pro-inflammatory state that may fuel tumor progression. IL-6 has been shown to promote proliferation, angiogenesis, and immune evasion via the JAK/STAT3 pathway. Additionally, IL-6 contributes to muscle protein degradation, a hallmark of cancer cachexia. TNF-α activates the ubiquitin-proteasome system, accelerating proteolysis, while IL-1β disrupts immune cell function and promotes chronic inflammation21. The elevated IL-6 and TNF-α levels in rats mirror inflammatory signatures reported in cervical cancer patients with poor prognosis, indicating consistency between the experimental model and human pathology. The marked reduction in the CD4⁺/CD8⁺ ratio observed in the high-risk group reflects T-cell exhaustion and immunosuppressive remodeling of the tumor microenvironment. These findings suggest that CTCs may not only serve as prognostic indicators but also actively participate in modulating systemic immunity, possibly through PD-L1–related pathways. The high-risk condition modeled by simultaneous CTC elevation and TFD reduction reproduced a systemic phenotype characterized by immune suppression (reduced CD4⁺/CD8⁺ ratio) and a pro-inflammatory milieu (increased IL-6, TNF-α, IL-1β), together with oxidative stress imbalance (ROS and MDA elevation with SOD/GSH depletion). These signatures provide a plausible mechanistic bridge to the clinical observations—linking microenvironmental remodeling and disseminated tumor burden to impaired anti-tumor immunity and metabolic stress that may facilitate recurrence. We view these pathways as hypothesis-generating rather than definitive causal proof, but they offer concrete targets for future correlative and interventional studies.
Oxidative stress also emerged as a key contributor to poor prognosis. Rats in the high-risk group exhibited elevated ROS and MDA levels, along with decreased SOD and GSH activity. These disruptions in redox balance are indicative of oxidative stress–induced damage, which is known to enhance tumor invasiveness, angiogenesis, and immune escape21. Conversely, rats in the high-TFD group displayed near-normal inflammatory and oxidative stress profiles, reinforcing the importance of adequate surgical margins in mitigating systemic tumor-related toxicity. The implications of this model are multifold. First, it offers a low-cost, readily applicable approach to preoperative risk stratification using blood-based and imaging markers. Second, it may complement existing guidelines by identifying high-risk patients who would benefit from intensified surveillance or adjuvant therapy, and conversely, sparing low-risk patients from unnecessary interventions. Third, it opens avenues for precision medicine by aligning surgical decisions with individual tumor biology.
Nonetheless, several limitations merit consideration. A major limitation is the absence of molecular parameters, including HPV subtype, p16, and PD-L1 status, which are increasingly recognized as important prognostic factors. Additionally, external validation in independent cohorts is still needed. Excluding patients with < 1 month follow-up may have introduced selection bias by omitting those with very early recurrence or rapid progression. This limitation should be addressed in future prospective studies. Future studies should aim to incorporate CTC single-cell multi-omics—combining genomic, transcriptomic, and proteomic profiling—to explore signatures associated with EMT, stemness, and immune tolerance. Emerging artificial intelligence (AI) frameworks for cervical imaging analysis, such as deep learning–based margin detection, may be adapted to automate and standardize TFD measurement, thereby enhancing reproducibility.
In summary, the high NPV of our model (94.7%) supports de-escalated management for low-risk patients, while intermediate and high-risk groups warrant closer imaging surveillance and potential adjuvant interventions. The modest PPV highlights the need to incorporate CTC phenotyping and dynamic monitoring to refine positive prediction and optimize treatment selection. Future research should focus on external validation, integration of multi-omics and immune markers, and AI-assisted automation of TFD measurement to improve generalizability and scalability.
Conclusion
Patients with elevated CTC levels and low TFD face significantly increased risks of postoperative recurrence and metastasis in cervical cancer and may benefit from proactive preventive strategies. By accurately stratifying risk before surgery, the model may minimize unnecessary adjuvant treatment, reduce treatment-related morbidity, and ultimately improve patient quality of life.
Future research will incorporate additional biomarkers and multi-omics data to further refine the model at the molecular level. Validation in external, independent cohorts and multicenter trials is planned to assess its generalizability across cancer types. Moreover, efforts will be made to develop rapid CTC and TFD detection platforms to facilitate clinical translation and application.
Data availability
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding author.
References
Joshi, S. et al. A randomised controlled non-inferiority trial to compare the efficacy of ‘HPV screen, triage and treat’ with ‘HPV screen and treat’ approach for cervical cancer prevention among women living with HIV [J]. Nat. Commun. 16 (1), 1888 (2025).
Atahan, I. L. et al. Long-term outcome and prognostic factors in patients with cervical carcinoma: a retrospective study [J]. Int. J. Gynecol. Cancer. 17 (4), 833–842 (2007).
Bruni, L. et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis [J]. Lancet Global Health. 10 (8), e1115–e27 (2022).
Levine, M. D. et al. Glassy cell carcinoma of the cervix: findings from a combined National cancer database analysis and single institution review of treatment patterns and outcomes [J]. Gynecol. Oncol. 173, 15–21 (2023).
Chopra, S., Ranjan, N. & Mittal, P. Postoperative adjuvant radiation for cervix cancer: reflections on the evidence and a peep into the future [J]. Int. J. Gynecol. Cancer. 32 (3), 225–230 (2022).
Wu N, Lin F, Ji J, et al. Mitochondrial oxidative stress related LncRNA predict cervical cancer prognosis and immunotherapy response: Molecular structure and protein interaction of ribosomal protein L34 [J]. International Journal of Biological Macromolecules, 2025, 299(140145).
Ludmir, J. & Harish, M. Sehdev. Anatomy and physiology of the uterine cervix. Clin. Obstet. Gynecol. 43 (3), 433–439 (2000).
Lee, K. B., Lee, J. M. & Kim, Y. S. Oncologic outcomes of adjuvant chemotherapy in patients with risk factors after radical surgery in FIGO stage IB–IIA cervical cancer [J]. Gynecol. Oncol. 145, 8–10 (2017).
Shen, Q., Qiu, L., Zhou, Y. et al. Pan-cancer analysis of DCBLD1 and its association with the diagnosis, immunotherapy, and prognosis of cervical cancer [J]. Int. Immunopharmacol., 148, 114167 (2025).
LEE, M. W., SRIPRASERT, I., PHUNG, P. G. et al. Trends and comparisons of palliative care utilization for patients with metastatic gynecologic malignancy [J]. Int. J. Gynecol. Cancer 35, 101631 (2025).
Brinkmann, D. et al. Why do women still develop cancer of the cervix despite the existence of a National screening programme? [J]. Eur. J. Obstet. Gynecol. Reproductive Biology. 119 (1), 123–124 (2005).
Suneja, G. et al. American brachytherapy society: brachytherapy treatment recommendations for locally advanced cervix cancer for low-income and middle-income countries [J]. Brachytherapy 16 (1), 85–94 (2017).
Myers, K. M. et al. The mechanical role of the cervix in pregnancy. J. Biomech. 48 (9), 1511–1523 (2015).
Rzhevskiy, A. S., Sagitova, G. R., Karashaeva, T. A. et al. A comprehensive review and meta-analysis of CTC isolation methods in breast cancer [J]. Crit. Rev. Oncol./Hematol., 206, 104579 (2025).
Lutfi, A., Afghan, M. K. & Kasi, P. M. CTCs and Liquid Biopsies in Patients with Colorectal Cancer [M] (International Review of Cell and Molecular Biology. Academic, 2025).
Pan, L. et al. Distribution of Circulating tumor cell phenotype in early cervical cancer [J]. Cancer Manage. Res. 11 (null), 5531–5536 (2019).
Aggarwal, C. et al. Relationship among Circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer [J]. Ann. Oncol. 24 (2), 420–428 (2013).
Wang, Y. et al. Longitudinal detection of subcategorized CD44v6 + CTCs and circulating tumor endothelial cells (CTECs) enables novel clinical stratification and improves prognostic prediction of small cell lung cancer: A prospective, multi-center study [J]. Cancer Letters, 571(216337). (2023).
Meisels, A. & Fortin, R. Condylomatous lesions of the cervix and vagina. I. Cytologic patterns. Acta Cytol. 20 (6), 505–509 (1976).
Clark, D. J. & Mao, L. Understanding the surgical margin: a molecular assessment[J]. Oral Maxillofacial Surg. Clin. 29 (3), 245–258 (2017).
Moreno-Sánchez, R. et al. Estimation of energy pathway fluxes in cancer cells - Beyond the Warburg effect [J]. Arch. Biochem. Biophys. 739, 109559 (2023).
Author information
Authors and Affiliations
Contributions
ZZG and LB contributed equally to study conception, data acquisition, interpretation, and manuscript writing. LWL was involved in data visualization and manuscript preparation. WZL and YQQ provided senior supervision, contributed to study design refinement and critical manuscript revision, and jointly serve as corresponding authors. All authors reviewed, critically revised, and approved the final submitted version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Biomedical Ethics Review Ethical approval was obtained (Approval No. 2024-YX-250-02). All participants provided written informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Z., Li, B., Li, W. et al. The relationship between CTCs, TFD and postoperative prognosis of cervical cancer patients and the construction of prediction models. Sci Rep 15, 41307 (2025). https://doi.org/10.1038/s41598-025-25068-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25068-3