Abstract
Amniotic membrane transplantation (AMT) is an effective treatment for refractory macular hole closure, yet the underlying mechanism supporting that effectiveness remains unclear. Here, we investigated the role of retinal regeneration in this process using an in vivo rabbit retinal-hole (RH) model in which AMT was performed on one of the holes. Histological and immunofluorescence examinations with anti-glial fibrillary acidic protein and anti-glutemine synthetase antibodies were conducted to evaluate cell migration and hole closure. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to analyze the tissues after implantation. Optical coherence tomography scans revealed that AMT promoted RH closure. Histological analyses demonstrated that retinal pigment epithelium (RPE) cells migrated onto the AM, and immunofluorescence analyses showed that retinal glial cells, including Müller cells, contributed to RH closure. SEM and TEM findings revealed cellular coverage and extracellular matrix formation at the AMT site, suggesting successful integration with the surrounding retinal tissue. Furthermore, the loss of mitochondrial cristae within the RPE caused by the creation of RH was suppressed by AMT. In summary, AMT promoted RH closure in a rabbit model by serving as a scaffold for retinal cell migration and by preserving RPE integrity, although regeneration of the retinal-layers was not observed.
Similar content being viewed by others
Introduction
A macular hole (MH) is an anatomical opening in the fovea that causes significant visual impairment and metamorphopsia. It has been reported that the Müller cell cone contributes to the pathogenesis of a MH1. Although vitrectomy has improved the closure rate of MHs2, and additional internal limiting membrane (ILM) peeling has been shown to further increase closure rates3, refractory cases such as large, long-standing MHs or those associated with high myopia continue to challenge vitreoretinal surgeons. Reported advanced surgical techniques for refractory MH include the inverted ILM flap technique4, autologous lens capsule transplantation5, autologous retinal transplantation6, and amniotic membrane (AM) transplantation (AMT)7,8. Among these, AMT has attracted attention for retinal tissue reconstruction because it is an implanted material with no limitation on the available tissue volume.
The AM is the innermost layer of the placental sac, and is a translucent elastic tissue that contains no vascular components. It consists of an epithelial layer, a basement membrane, and a stromal layer facing the chorionic membrane, and it can be used for the treatment of a variety of tissue-related disorders9,10. AM has been used in the field of ophthalmology since the 1940s to treat patients afflicted with severe ocular surface diseases, and studies have shown that it possesses anti-inflammatory11,12, antifibroblastic13, antimicrobial14, and angiogenic properties15, with very low immunogenicity16. Although some reports suggest that AM might have potential for retinal tissue remodeling17 beyond ocular surface diseases, the exact mechanism underlying such remodeling has not been elucidated.
While clinical reports have demonstrated the effectiveness of AMT in closing refractory MHs7,8, and Caporossi et al.18 further reported tissue ingrowth above the transplanted AM using multimodal imaging, the underlying closure mechanisms remain unclear. In particular, how the transplanted AM interacts with host retinal tissue at the cellular and ultrastructural levels has not been clarified. To address this issue, our study aimed to clarify how AM functions as a scaffold to promote retinal hole closure by conducting in vitro assays with human Müller cells and in vivo experiments using a rabbit retinal hole model. Specifically, we uniquely combined scanning electron microscopy (SEM) to evaluate the ultrastructural interface between AM and retinal tissue with transmission electron microscopy (TEM) to investigate cell interactions and mitochondrial changes.
Materials and methods
Cell culture
Dulbecco’s Modified Eagle Medium (DMEM)/F12, fetal bovine serum (FBS), TrypLE™ Express Enzyme (1X), and Penicillin/Streptomycin (Pe/St) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). The human Müller glial cell line (MIO-M1 cells) was obtained from the laboratory of Astrid Limb19. Cells were cultured on T25 flasks or 6-well tissue culture plates. MIO-M1 cells were thawed and cultured in DMEM/F12, 10% FBS, and 1% Pe/St. Cells at a low passage number (≤ 5) were used for all experiments.
Preparation of human AM (hAM)
hAM was obtained and prepared according to our previously reported standard method20,21. With proper informed consent in accordance with the tenets of the Declaration of Helsinki for research involving human subjects and on approval by the Institutional Review Board of Kyoto Prefectural University of Medicine, Kyoto, Japan, hAMs were obtained at the time of elective cesarean section in volunteers who were seronegative for human immunodeficiency virus, human hepatitis B and C, and syphilis. Briefly, under sterile conditions, the hAM was washed with sterile phosphate-buffered saline (PBS) containing antibiotic–antimycotic liquid (i.e., penicillin [10,000 U/mL], streptomycin [10,000 μg/mL], and amphotericin B [25 μg/mL]), and then cut into pieces approximately 4 × 4 cm in size. The hAM was then denuded of amniotic epithelial cells by incubation with 0.02% ethylenediaminetetraacetic acid (Nacalai Tesque, Inc., Kyoto, Japan) at 37 °C for 2 h. Each membrane sample was placed in a sterile vial containing DMEM and glycerol (1:1, vol/vol), with the vials then frozen at -80 °C. The samples were then thawed immediately prior to use by warming the container to room temperature.
Cell morphology and adhesion molecules of Müller glial cells adhering to hAM
hAMs were attached to 24-well plates with the epithelial side-up (epithelial group) and the stromal side-up (stromal group) (n = 4 per group). Then, MIO-M1 cells (1.0 × 105) were seeded in each group and cultured for 24 h. At the time of cell adhesion, the cells were visualized with phalloidin staining, and the aspect ratio was measured using Image J software (National Institutes of Health, Bethesda, MD, USA). Western blotting was performed to examine the amount of vinculin, an adhesion protein, and quantified using ImageLab software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The vinculin/β-actin ratio was used for comparison.
Migration assay of Müller Glial cell on hAM
In this study, a room temperature vulcanization silicon rubber (Shin-Etsu Chemical Co., Ltd., Tokyo, Japan) was used as a cell migration barrier. As in the above-described adhesion experiment, two groups of 12-well plates with hAM attached (i.e., one group with the epithelial side-up [epithelial group] and the other group with the stromal side-up [stromal group]) were arranged, and a 3-mm-diameter silicone rubber (7.1 mm2) was placed in the center (n = 3 in each group) as a cell migration barrier. In each group, 1.0 × 105 MIO-M1 cells were then seeded and the silicone rubber was removed after 24 h once confirming that the cells had reached at least 80% confluence. Phase contrast microscopy images were taken immediately after removal and at 72 h later, and immunofluorescence studies (phalloidin) were performed. Cell migration capacity was determined by analyzing the before and after images using ImageJ software and subtracting the region of free area.
Animals
All animals used in this study were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and with the experimental procedure approved by the Committee for Animal Research at Kyoto Prefectural University of Medicine. Male Japanese white rabbits (2.5–3.0 kg) were purchased from Shimizu Laboratory Supplies Co., Ltd., Kyoto, Japan. The procedures complied with the approved guidelines and the ARRIVE guidelines (https://arriveguidelines.org).
Development of the rabbit MH model and AMT
In one eye of each rabbit, a standard 25-gauge vitrectomy using the CV-30000 (NIDEK, Co., Ltd., Gamagori, Japan) with a wide-angle noncontact viewing system (Resight™; Carl Zeiss Meditec AG, Jena, Germany) was performed with intramuscular injection of xylazine hydrochloride (5 mg/mL) and ketamine hydrochloride (50 mg/mL). The retina was carefully aspirated with a vitreous cutter and adjusted to a 2-mm diameter to form two RHs, one hole was left untreated and a 2-mm-diameter piece of hAM was implanted in the other hole with the stromal side positioned at the bottom of the RH. The wound was closed to complete the procedure, and the operated eye was kept in a resting position facing the sky for 1 h. Optical coherence tomography (OCT) (NIDEK) examinations were then performed at 4-wks postoperative (n = 3).
After sacrificing the rabbits with an intravenous overdose of 6.5% pentobarbital sodium solution (80 mg/kg; Nacalai Tesque, Inc., Kyoto, Japan), the operated eye of each rabbit was excised and fixed in Super Fix (Kurabo Industries Ltd., Osaka, Japan). The fixed eye was then embedded in optimal cutting temperature compound (Tissue-Tek® O.C.T. Compound; Sakura Finetek Japan Co., Ltd., Tokyo, Japan), after which the tissue was cut into 7-μm sections for histological examination and immunohistochemistry.
Immunofluorescence examination
Immunofluorescence staining was performed with mouse anti-glutamine synthetase (GS)-6 (1:1000; Sigma-Aldrich, Burlington, MA, USA) and anti-glial fibrillary acidic protein (GFAP) (1:500; Chemicon; Sigma-Aldrich) antibodies overnight at 4 °C. The sections were then incubated with a secondary antibody (Alexa Fluor 488 and goat anti-mouse IgG; 1:1000; Invitrogen™, Carlsbad, CA, USA) for 1 h at room temperature, and then mounted with Vectashield® Antifade Mounting Medium (Vector Laboratories, Inc., Burlingame, CA, USA) containing DAPI (4',6 diamidino-2-phenylindole).
Scanning electron microscopy (SEM) analysis
For SEM analysis, the obtained tissue samples that were first fixed in 0.1 mol phosphate buffered 2% glutaraldehyde were subsequently post-fixed in 2% osmium tetroxide for 2 h in an ice bath. The specimens were then dehydrated in a graded ethanol and dried by frozen t-butyl alcohol in a vacuum. Next, the freeze-dried specimens underwent SEM examination (JEM-7500F; JEOL Ltd., Tokyo, Japan) after being coated with an osmium plasma ion coater device.
Transmission electron microscopy (TEM) analysis
For TEM analysis, tissue samples were fixed in 0.1-mol phosphate-buffered 2% paraformaldehyde and 2% glutaraldehyde in an ice bath. The samples were then post-fixed in 2% osmium tetra-oxide for 2 h in an ice bath. Next, the specimens were dehydrated in a graded ethanol and embedded in the epoxy resin. Ultrathin sections were obtained via the ultramicrotome technique, and then stained with uranyl acetate for 15 min and lead staining solution for 2 min before undergoing TEM analysis at 100 kV (H-7600 Transmission Electron Microscope; Hitachi High-Technologies Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis of the data was performed and basic descriptive statistics were calculated on all of the gathered data, with the values reported as medians with interquartile ranges (IQR). Results were compared between the two groups by Mann–Whitney U test. Box plots were constructed such that the boxes represent the interquartile range, the horizontal lines within the boxes indicate the medians, and the whiskers denote the minimum and maximum values. A P-value of < 0.05 was considered statistically significant.
Results
Müller cell adhesion on AM
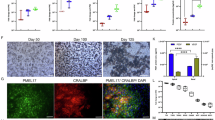
We first examined the cell morphology and adhesion molecules of the in vitro cultured human Müller cell line MIO-M1 cells adherent to AM. At 24 h after cell seeding, cells in the epithelial side-up group exhibited intracellular extension of actin fibers along the long axis, with clearly visible pseudopodia in the adhesive image. In contrast, cells in the stromal side-up group appeared round, and actin fibers were observed mainly in the pericellular area (Fig. 1A). The aspect ratio of cells in the epithelial group showed a median of 6.53 (IQR: 4.63–9.44), which was significantly higher than that in the stromal group 1.21 (IQR: 1.13–1.37) (p < 0.05, Mann–Whitney U test) (Fig. 1B).
Evaluation of cell morphology and adhesion proteins during adhesion of Müller cells on amniotic membrane (AM) (n = 4 per group). (A) Representative images comparing the morphology of MIO-M1 cells cultured on AM with epithelial side-up and stromal side-up using actin staining. (B) Quantification of the aspect ratio of the cells in both groups. (C) Western blot analysis showing vinculin protein expression in cells adhered to AM in both orientations. (D) Quantification of vinculin protein levels normalized to β-actin, analyzed using ImageJ software. Scale bar, 50 μm. Data are presented as box plots, where boxes represent the interquartile range (IQR), horizontal lines indicate the median, and whiskers denote the data range. *P < 0.05, Mann–Whitney U test.
Next, the levels of vinculin protein, a membrane cytoskeletal protein of focal adhesion plaques that links integrin adhesion molecules to the actin cytoskeleton, were compared. The original, uncut membrane is shown in Supplementary Fig. 1, and the cropped blots used for densitometric analysis are presented in Fig. 1C and D. The vinculin/β-actin ratio showed a median of 1.13 (IQR: 0.98–1.22) in the epithelial group and 0.22 (IQR: 0.18–0.27) in the stromal group (Fig. 1C, D). These results demonstrated that protein expression of vinculin was significantly higher in the epithelial group than in the stromal group (P < 0.05, Mann–Whitney U test).
Migration of Müller cells on AM
Since Müller cells reportedly contribute to the closure of the MH22, we investigated their migration on AM via an in vitro examination. As schematized in Fig. 2A, a 3-mm silicone disk was placed centrally on hAM (epithelial- or stromal-side up). MIO-M1 cells were seeded around the disk and, after 24 h, the disk was removed to initiate migration (0 h). Migration was quantified from phase-contrast images (Fig. 2B) as the reduction of the initial cell-free area using ImageJ (n = 3 per group). The median migration area of the epithelial side-up group and the stromal side-up group were 1.06 mm2 (IQR: 0.79–1.36 mm2) and 0.039 mm2 (IQR: 0.018–0.065 mm2), respectively (P < 0.05, Mann–Whitney U test) (Fig. 2C). These findings indicated that the migration capacity of Müller cells was significantly greater in the epithelial side-up group.
Evaluation of the migration of Müller glial cells on AM. (A) Schematic illustration of the method used for the cell migration assay (n = 3 per group). (B) Representative images of the area of MIO-M1 cells that migrated on the epithelial and stromal side of the AM as measured by ImageJ software (yellow line). (C) Quantification and comparison of the migration area of MIO-M1 cells between the epithelial side-up group and the stromal side-up group. Data are shown as box plots (median, IQR, and range). *P < 0.05, Mann–Whitney U test.
AM transplantation (AMT) in a retinal hole (RH) in rabbits (n = 3). (A) Fundus of the rabbit eye with two 2-mm-diameter RHs created, one treated with AMT (yellow arrowheads) and the other left untreated (white arrowheads). (B) Optical coherence tomography (OCT) findings at 4-weeks postoperative in 3 treated eyes: untreated cases (top row), AMT cases (bottom row).
AMT in the rabbit RH model
Finally, we examined the potential of AM as a scaffold for RHs in rabbits. RHs of nearly the same diameter were created in all locations (Fig. 3A). Postoperative inflammation was minimal in all cases. OCT findings at 4-weeks postoperative revealed that the untreated holes remained open in 3 of 3 eyes, whereas the AM-transplanted holes were closed, with high-intensity tissue at the bottom of the hole and overlying tissue of the same intensity as the surrounding retina in 3 of 3 eyes (Fig. 3B).
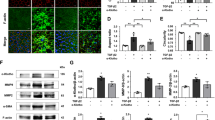
Histological examination of hematoxylin and eosin-stained section showed that the thick AM stroma was attached to the bottom of the hole, and the transplanted AM area is outlined by yellow dotted lines. The AM was covered with retinal tissue without layer structure (Fig. 4A). Immunofluorescence staining showed that GS-6 and GFAP were expressed in the cells on the AM, indicating that retinal glial cells, including Müller cells, had migrated to and coated the AM (Fig. 4B, C). The edge of the transplanted AM in Fig. 4B is demarcated with white dotted lines, and enlarged views of this region are shown in Fig. 4D–F, illustrating GS-6–positive cells migrating from the adjacent normal retina onto the AM.
Histological examination and immunofluorescence staining after AMT in a RH in rabbits. (A) Hematoxylin and eosin staining of the section. The transplanted AM is outlined by yellow dotted lines. (B, C) Immunofluorescence staining using anti-glutamine synthetase 6 (GS-6) and anti-glial fibrillary acidic protein antibodies. (C) Higher-magnification image showing GFAP expression. (D–F) Enlarged views of the area outlined in (B), showing GS-6 (D), DAPI (E), and merged (F). Scale bar, 100 µm.
SEM was used to examine three areas where AMT was performed after a RH was created (Fig. 5A). The results showed that the area outside the hole had an ILM and an extracellular matrix (ECM) overlying the ILM that appeared to be a thin mesh of vitreous cortex. Moreover, we found that there was a gap between the AMT region and the host retina, that the cells and ECM were elongated to bridge the gap, and that the hAM was densely covered with cells and ECM in the central region of the AMT (Fig. 5B).
Scanning electron microscopy (SEM) analysis of the AMT in a rabbit RH. (A) SEM analysis was performed on tissue samples obtained by punching out a 3-mm-diameter section, including the area where AMT was performed (white arrowheads). (B) SEM images show three specific regions: (a) normal tissue outside the AMT region, (b) the peripheral edge of the AMT region, and (c) the central area of the AMT region. The gap referred to in the text is clearly indicated by a black arrow. Each region was observed at two magnifications, with the upper row at 3,000× magnification and the lower row at 10,000× magnification. Scale bar (upper row), 3 µm; Scale bar (lower row), 1 µm.
Finally, TEM was performed to investigate retinal pigment epithelium (RPE) cells in three regions: (1) the normal retinal area, (2) the untreated RH area, and (3) the AMT area (Fig. 6). In the normal retinal area, the RPE cells were aligned in a monolayer with intact tight junctions, and the photoreceptor outer segments contacted the RPE cells. In the untreated RH area, photoreceptor cells were absent, the RPE cells were greatly enlarged, and intercellular junctions were disrupted. In the AMT area, no photoreceptor cells were observed, and the RPE was arranged in a monolayer, with some overlaying. Over the RPE layer, tissue without cellular components, which appeared to be hAM, was observed (Fig. 6A). At 20,000 × magnification, mitochondrial cristae density in RPE cells was compared among the three regions. Cristae density was slightly decreased in the AMT area compared to the normal area, whereas the untreated area showed a marked reduction, indicating a significant disruption of the internal structure (Fig. 6B).
Transmission electron microscopy (TEM) analysis of the retinal pigment epithelium (RPE) cells in the three groups. (A) Representative TEM images of three regions: the normal retinal area (left), the untreated RH area (middle), and the AMT area (right). Images were taken at three different magnifications: 2,500× (top row), 5,000× (middle row), and 20,000× (bottom row). (B) Representative TEM images at 20,000× magnification showing the mitochondrial structure within the RPE cells in each group.
Discussion
In this study, our findings first revealed that both the adhesion and migration capacity of Müller cells were higher on the basement membrane side of the hAM than on the stromal side. Based on that finding, we discovered that in rabbit RHs that when hAM was inserted with the stromal side positioned at the bottom of the RH, retinal glial cells, including Müller cells, migrated onto the basement membrane of the hAM and the hole subsequently closed. Furthermore, our findings revealed impairment of RPE intercellular junctions and disruption of the mitochondrial structure within the RPE cells in the untreated RH area, whereas these disturbances were suppressed in the AMT area.
The structure of hAM consists of epithelial cells, the basement membrane, and the stromal layer in contact with the chorionic membrane. In this study, hAM was denuded of epithelial cells, leaving the basement membrane exposed, which allowed us to investigate how the side of the AM affects the adhesion and migration of Müller cells. We found that both cell adhesion and migration were enhanced on the basement membrane side. The basement membrane of AM is composed of an ECM such as collagen I, III, IV, V, and VII, laminin, and fibronectin23,24,25, which may serve as a scaffold for cells. Although the mechanism of MH closure is not fully clear, Shiode et al.21 reported that in the inverted ILM flap technique, the inverted ILM and surrounding tissue release cytokines that promote retinal glial cell migration and hole closure. Although not a hole closure mechanism similar to the inverted ILM flap technique, RH creation may have led to the release of cytokines from surrounding cells, thereby inducing retinal glial cell migration onto the AM basement membrane, which then acted as a scaffold and promoted closure.
In this present study, the creation of RHs resulted in disruption of intercellular junctions and mitochondrial damage in RPE cells, while AMT suppressed these changes. Cristae are folds of the inner mitochondrial membrane that increase surface area for aerobic respiration26,27. These results imply that persistent macular holes may disrupt mitochondrial energy metabolism in RPE cells, whereas AMT promotes its preservation. Healthy functioning RPE cells are essential for photoreceptor maintenance28,29, and maintaining RPE function through MH closure is an important aspect of future photoreceptor regeneration. Previous studies by Rizzo et al.8 and Caporossi et al.7,18 reported that an AM plug closed MHs and improved visual function. They also suggested that the AM plug stimulates retinal proliferation and intraretinal tissue restoration, potentially contributing to recovery of retinal function. In our study, Müller cells migrated onto the AM and covered the hole, raising the possibility that their latent regenerative potential may contribute to tissue repair. Recent studies have indicated that Müller glia possess a capacity for reprogramming and neuronal regeneration30,31, supporting a potential role of AMT in facilitating Müller cell–mediated retinal regeneration. Other clinical studies have similarly demonstrated the efficacy of AMT for refractory MHs, with evidence of integration of the transplanted tissue into host retina. Although their findings did not clarify the anti-inflammatory properties of AM plugs, our results are consistent with their report in demonstrating functional preservation.
One limitation of this present study was that the postoperative observation period was only 4 weeks. While the mechanism of RH closure by AMT and the short-term effect on RPE was confirmed, the longer-term reconstruction of retinal tissue, including photoreceptor cells, requires further investigation. Another limitation concerns the study design in the animal model. In this study, two RHs were created in close proximity within the same eye, which raises the possibility that diffusible factors released from the transplanted AM might have influenced the untreated retinal hole. While this approach has the advantage of reducing the number of animals used, the present results should be interpreted with caution in light of these potential effects, and future studies should also consider comparisons using separate eyes. Moreover, since rabbit eyes do not have a macula, the exact mechanism by which the macula closes requires the use of animal eyes that have a macula32 or macular-like structures33,34.
Conclusion
In conclusion, the findings in this study indicate that hAM is a promising transplantation material for the closing of MHs. However, further research with longer-term observation period is required to better elucidate retinal tissue regeneration, including photoreceptor cells.
Data availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Gass, J. D. M. Muller cell cone, an overlooked part of the anatomy of the fovea centralis. Arch. Ophthalmol. 117, 821–823 (1999).
Kelly, N. E. & Wendel, R. T. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch. Ophthalmol. 109, 654–659 (1991).
Tognetto, D. et al. Internal limiting membrane removal during macular hole surgery: Results of a multicenter retrospective study. Ophthalmology 113, 1401–1410 (2006).
Michalewska, Z., Michalewski, J., Adelman, R. A. & Nawrocki, J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 117, 2018–2025 (2010).
Chen, S. N. & Yang, C. M. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina 36, 163–170 (2016).
Grewal, D. S. & Mahmoud, T. H. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 134, 229–230 (2016).
Caporossi, T., Tartaro, R., De Angelis, L., Pacini, B. & Rizzo, S. A human amniotic membrane plug to repair retinal detachment associated with large macular tear. Acta. Ophthalmol. 97, 821–823 (2019).
Rizzo, S. et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina 39, S95-103 (2019).
Davis, J. S. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med. J. 14, 542–549 (1910).
Stern, M. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituing skin grafts. J. Am. Med. Assoc. 60, 973 (1913).
Kim, J. S., Kim, J. C., Na, B. K., Jeong, J. M. & Song, C. Y. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp. Eye Res. 70, 329–337 (2000).
Solomon, A. et al. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br. J. Ophthalmol. 85, 444–449 (2001).
Tseng, S. C. G., Li, D. Q. & Ma, X. Suppression of transforming growth factor-beta isoforms, TGF-β receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J. Cell Physiol. 179, 325–335 (1999).
Talmi, Y. P., Sigler, L., Inge, E., Finkelstein, Y. & Zohar, Y. Antibacterial properties of human amniotic membranes. Placenta 12, 285–288 (1991).
Hao, Y., Ma, D. H. K., Hwang, D. G., Kim, W. S. & Zhang, F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 19, 348–352 (2000).
Kle, C. A., Welsh, K. I., Adinolfi, M., Leibowitz, S. & McColl, I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 318, 1003–1005 (1981).
Yang, H., Li, Z., Jin, W. & Yang, A. Application progress of human amniotic membrane in vitreoretinopathy: A literature review. Front. Med. 10, 1206577 (2023).
Caporossi, T. et al. Human amniotic membrane plug to promote failed macular hole closure. Sci. Rep. 10, 1–8 (2020).
Lawrence, J. M. et al. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells 25, 2033–2043 (2007).
Koizumi, N., Inatomi, T., Suzuki, T., Sotozono, C. & Kinoshita, S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 108, 1569–1574 (2021).
Nakamura, T. et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest. Ophthalmol. Vis. Sci. 44, 106–116 (2003).
Shiode, Y. et al. The role of inverted internal limiting membrane flap in macular hole closure. Invest. Ophthalmol. Vis. Sci. 58, 4847–4855 (2017).
Burgeson, R. E., El Adli, F. A., Kaitila, I. I. & Hollister, D. W. Fetal membrane collagens: Identification of two new collagen alpha chains. Proc. Natl. Acad. Sci. U. S. A. 73, 2579–2583 (1976).
Glanville, R. W., Rauter, A. & Fietzek, P. P. Isolation and characterization of a native placental basement-membrane collagen and its component α chains. Eur. J. Biochem. 95, 383–389 (1979).
Stenman, S. & Vaheri, A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J. Exp. Med. 147, 1054–1064 (1978).
Scheffler, I. E. Mitochondria make a come back. Adv. Drug Deliv. Rev. 49, 3–26 (2001).
Revel, J. P., Fawcett, D. W. & Philpott, C. W. Observations on mitochondrial structure angular configurations of the cristae. J. Cell Biol. 16, 187–195 (1963).
Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881 (2005).
Sparrow, J. R., Hicks, D. & Hamel, C. P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 10(802–823), 20 (2010).
Jorstad, N. L. et al. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 548, 103–107 (2017).
Lee, E. J. et al. Restoration of retinal regenerative potential of Müller glia by disrupting intercellular Prox1 transfer. Nat. Commun. 16, 2928 (2025).
Dominik Fischer, M. et al. Detailed functional and structural characterzation of a macular lesion in a rhesus macaque. Doc. Ophthalmol. 125, 179–194 (2012).
Beltran, W. A. et al. Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PLoS ONE 9, 11–17 (2014).
Johansson, U. E., Eftekhari, S. & Warfvinge, K. A battery of cell- and structure-specific markers for the adult porcine retina. J. Histochem. Cytochem. 58, 377–389 (2010).
Acknowledgements
The authors wish to thank John Bush for editing the manuscript.
Funding
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant No. 22K16957.
Author information
Authors and Affiliations
Contributions
H.T. and T.N.: conception and design; H.T., T.M., A.U., T.H, Y.S. and K.K.: collection and assembly of data; H.T., A.U. and T.H: data analysis and interpretation; H.T: writing manuscript text and preparing figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tanaka, H., Miyatani, T., Nakamura, T. et al. Amniotic membrane transplantation promotes retinal hole closure in a rabbit model. Sci Rep 15, 41253 (2025). https://doi.org/10.1038/s41598-025-25162-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25162-6