Abstract
Our study investigates the effect of vonoprazan on albuminuria in diabetic kidney disease (DKD) patients compared to lansoprazole through a randomized controlled trial. Participants were randomized to receive either lansoprazole 30 mg or vonoprazan 20 mg. Specifically, 100 patients were included for H. pylori eradication, and 40 patients for other indications. The albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) were measured at baseline and three months later. Both vonoprazan and lansoprazole significantly reduced ACR from baseline levels after 3 months (p < 0.05). However, the reduction in albuminuria was significantly greater in the vonoprazan group compared to the lansoprazole group, regardless of H. pylori diagnosis or eradication success (p < 0.05). Treatment type was a significant predictor, with a standardized beta of 0.555. Patients receiving vonoprazan experienced a 19.2-unit increase in the percentage change of ACR compared to those receiving lansoprazole (β = 19.219, 95% CI [13.62-24.817]). Additionally, vonoprazan-treated patients showed significant improvement in eGFR after 3 months compared to baseline levels (p < 0.05), with this improvement being superior to that observed with lansoprazole (p < 0.05). Vonoprazan may have a direct renal effect, warranting further research to elucidate its mechanism in reducing albuminuria.
Similar content being viewed by others
Introduction
Proton pump inhibitors (PPIs) have been linked to acute kidney injury (AKI), chronic kidney disease (CKD), and progression of existing kidney disease. Despite this, (PPIs) are widely used as acid inhibitory agents to treat gastroesophageal-related disorders in patients with diabetic kidney disease (DKD), who often receive multiple medications which can cause gastric irritation or ulcers1.
Diabetic kidney disease (DKD) is one of the most prevalent complications of diabetes, affecting approximately 40% of cases, and is the leading contributor to end-stage renal disease (ESRD)2. Although therapeutic strategies have advanced, the reduction of proteinuria remains a critical yet evolving goal in nephrology, requiring further investigation to optimize outcomes. Zhang et al. (2024) highlight the need for multidrug approaches reinforcing the call for continued research into effective proteinuria-lowering therapies. The promising effects of combination therapy with two or more agents in reducing albuminuria and preserving renal function warrant further exploration3.
Lansoprazole stands out from other (PPIs) due to its unique pharmacological properties, such as potential anti-inflammatory effects, reduced oxidative stress, anticancer potentials, and anti-diabetic benefits4. There are conflicting results regarding PPI use and the subsequent risk of developing or progressing albuminuria or eGFR decline in patients with diabetes5,6,7,8,9. However, various studies have demonstrated that eradicating H. pylori using triple therapy consisting of lansoprazole, amoxicillin, and clarithromycin significantly reduces proteinuria in diabetic, non-diabetic, and primary glomerulonephritis patients10,11,12,13.
In May 2022, the FDA approved the use of the potassium-competitive acid blocker vonoprazan as an alternative antisecretory agent for the treatment of H. pylori infection and other gastrointestinal disorders14. No data exist in the literature regarding its effect on proteinuria.
The aim of this study is to determine whether vonoprazan has a differential effect on albuminuria in patients with DKD compared to lansoprazole.
Materials and methods
Study design and patient’s enrolment
This is a a prospective randomized controlled single blinded parallel trial conducted at the outpatient clinics of Cairo University Hospital on patients with DKD recruited between January 2024 and April 2024. The study was registered in Pan African Clinical Trails Registry at (25/11/2024) with registration ID number: PACTR202411502424899 and CONSORT reporting guidelines was also used to complete the CONSORT Checklist15.
Ethical committee approval
This study was approved by the Research Ethics Committee of the Faculty of Medicine at Cairo University in December 2023 (approval number: N-394–2023). Informed written consent was obtained from all participants.
Randomization method
randomly assigning patients, ensuring a 1:1 allocation ratio for both study groups. Allocation Concealment was maintained by using sealed, opaque envelopes to prevent selection bias. Stratification and Blocking: The protocol does not specify any stratification or blocking procedures for the randomization process.
Blinding procedures
For this single-blinded study, participants, data collectors, and those assessing the outcomes were blinded to the treatment assignments, but the health care providers are not blinded. Blinding was maintained throughout the study by ensuring consistent handling procedures for both treatment groups. Any information that could reveal the group allocation was concealed from participants, data collectors, and outcome assessors. Unblinding Procedures: will only conducted under specific conditions where it was necessary, such as in the case of serious adverse events requiring knowledge of the treatment allocation.
Study protocol
Inclusion criteria
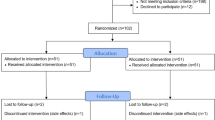
Patients with DKD who had an albumin/creatinine ratio exceeds 500 mg/day for at least six months before inclusion and a glomerular filtration rate (GFR) greater than 30 ml/min were enrolled. The albuminuria threshold (> 500 mg/day) was sustained throughout the six-month period and reconfirmed at the time of enrolment. In the initial phase, 100 patients with (DKD) who Met the ACR and eGFR criteria and had a confirmed Helicobacter pylori infection were included. In the subsequent phase, an additional 40 DKD patients with the same ACR and eGFR criteria were enrolled based on their prescription of proton pump inhibitors (PPIs) for indications unrelated to H. pylori infection. All patients were receiving stable treatment with either the maximum tolerated dose of ACE inhibitors or ARBs. 151 DKD patients were assessed for eligibility criteria, of them 140 patients were randomized. The flowchart is presented in Fig. 1
Exclusion criteria
Patients with malignancies, liver disease, cardiac failure, acute coronary syndrome, pregnancy, lactation, active infection within the last two months, a history of acid-suppressive drug use within the last three months, a history of upper gastrointestinal system disease or surgery, previous eradication therapy for H. pylori, use of SGLT2 inhibitors and other drugs that can affect proteinuria, a GFR less than 30 ml/min, uncontrolled hypertension requiring new or changing doses of antihypertensive drugs within the last three months, and those unable to provide informed consent.
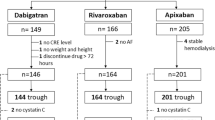
The inclusion of patients in this study was conducted in two stages. Initially, 100 patients diagnosed with DKD and positive H. pylori were included. These patients were randomly assigned (1:1) using sealed envelopes, with 50 patients in each group. One group received amoxicillin 1000 mg, clarithromycin 500 mg, and lansoprazole 30 mg twice daily, while the other group received vonoprazan 20 mg, amoxicillin 1000 mg, and clarithromycin 500 mg twice daily. All patients were treated for 14 days as eradication therapy.
The patients in each group were further divided into two subgroups based on their response to the H. pylori eradication treatment into (Stool H. pylori antigen negative or Stool H. pylori antigen positive).
For these 100 patients, the following were recorded: age, sex, body mass index (BMI), systolic and diastolic blood pressure, serum creatinine, estimated glomerular filtration rate (eGFR), serum albumin, total protein, urinary albumin/creatinine ratio (mg/g), HbA1c, fasting blood glucose, and 2-hour postprandial blood glucose. These measurements were taken at baseline and again after three months after treatment.
Next, we included an additional 40 patients with DKD but had another indication for PPI. These patients were randomly assigned to receive either lansoprazole 30 mg or vonoprazan 20 mg for one month, with 20 patients in each group. The same randomization method described earlier was used. For these 40 patients, the albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR) were measured at baseline and after three months.
The expected duration of participation for each subject was 4 months, including a screening (baseline) period, a treatment period and a follow-up period.
This approach allowed us to evaluate the effect of these drugs on albuminuria, independent of the presence of H. pylori or eradication therapy.
In Egypt, vonoprazan is available as a standalone drug, unlike in the USA where it is only available in combination with other H. pylori eradication therapies.
Safety considerations
Any adverse event (AE) observed by the study personnel or reported by the participant will be recorded promptly. Participant’s unique identifier, Date and time of the event, Description of the event, Severity (mild, moderate, severe), Duration (start and end dates), Relationship to study medication (related, possibly related, unrelated), Actions taken (medication adjustment, additional treatment, study withdrawal), Outcome (resolved, ongoing, unresolved) will be documented.
The trial is being conducted in compliance with the Declaration of Helsinki. All participants gave written informed consent prior to any trial-related activities.
Outcome measured
Primary outcome
The change in ACR from baseline and after 3 months of treatment.
Secondary outcome
The change in eGFR from baseline and after 3 months of treatment.
The estimated glomerular filtration rate (eGFR) was calculated using the 2021 CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula: [eGFR = 142×min(Scr/κ,1)α×max(Scr/κ,1) −1.200 × 0.9938 Age× Sex Factor]16.
Variables: Scr = serum creatinine (mg/dL), κ (kappa) = 0.7 for females, 0.9 for males, α (alpha) = − 0.241 for females, − 0.302 for males, Sex Factor = 1.012 for females, 1.000 for male, Age = age in years, min(Scr/κ, 1) = the smaller of Scr/κ or 1, max(Scr/κ, 1) = the larger of Scr/κ or 1.
H. pylori was diagnosed by stool antigen test at baseline and two weeks after treatment completion. Normoalbuminuria is defined as Urinary albumin-to-creatinine ratio (UACR) less than 30 mg/gCr, Microalbuminuria: Urinary albumin-to-creatinine ratio (UACR) between 30 and 300 mg/gCr and Macroalbuminuria: Urinary albumin-to-creatinine ratio (UACR) greater than 300 mg/gCr17.
Sample size estimation
This study aimed to evaluate the effects of the newly FDA-approved drug vonoprazan on proteinuria and to compare its effect with lansoprazole. As this intervention is new and has never been used before, it will be a pilot study. The suggested total sample size is 100 (50 per group). According to Connelly (2008), extant literature suggests that a pilot study sample should be 10% of the sample projected for the larger parent study. However, Hertzog (2008) cautions that this is not a simple or straightforward issue to resolve because these types of studies are influenced by many factors. Treece and Treece (1982) also suggested 10% of the project sample size18.
Statistical analysis
Analysis of data was done by IBM computer using SPSS (statistical program for social science version 23) as follows: Description of quantitative variables as Mean, SD, Median and IQR according to shapiro test of normality. Description of qualitative variables as number and percentage. Chi-square test was used to compare qualitative variables between groups. Fisher exact test was used when one expected cell or more are less than 5.Independent t test was used instead of to compare quantitative variables between two groups in parametric data Mann Whitney test was used instead of unpaired t-test in non-parametric data (SD > 30%mean). Wilcoxon signed rank test used to compare quantitative data before and after treatment Backward Linear regression used to find predictors for readmission. P value > 0.05 was considered insignificant, P < 0.05 was considered significant.
Results
A total of 140 patients were included in this study.
The 100 patients with DKD & positive H pylori
The mean age of the participants was 53.24 ± 10.87 years (female: 46%; male: 54%). Demographic and laboratory findings were comparable at baseline, as shown in Table 1. No reported adverse effects in both groups. After 3 months of follow-up, patients who received either vonoprazan or lansoprazole as part of H. pylori eradication therapy exhibited significant reductions in albuminuria, fasting blood glucose (FBG), 2-hour postprandial glucose (2 h PPG), and HbA1c levels (p ≤ 0.05). However, vonoprazan demonstrated superior improvement in serum creatinine and corresponding eGFR compared to lansoprazole, with statistical significance (p < 0.001). 72% of patients treated with Vonoprazan tested negative for H. pylori, compared to 56% of those treated with Lansoprazole. While Vonoprazan appears to have a higher percentage of negative results compared to Lansoprazole, the difference is not statistically significant, p = 0.096.
Overall, the percentage decrease in albuminuria, regardless of treatment success, was significantly higher in the vonoprazan group compared to the lansoprazole group (30.85 vs. 8.88, p < 0.001). Among patients successfully treated for H. pylori (negative), the decrease in albuminuria was also significantly higher in the vonoprazan group (30.39 vs. 7.26, p < 0.001). Even among patients who remained H. pylori positive, vonoprazan still outperformed lansoprazole significantly (p = 0.001). (Table 2).
Within each group and the entire H pylori study population, there was no statistically significant difference in the decrease in albuminuria % change decrease after eradication treatment between those who remained H. pylori positive and those who became negative, (Table 3).
We conducted Backward multiple linear regression model c to find predictors for ACR percentage of change. The age, BMI and treatment Type were found to be the significant predictors among variables entered in the model. Treatment type was found to be the highly significant predictor where standardized beta was 0.555. patients receiving Vonoprazan were more liable to increase by 19.2 units in Percentage of change of ACR in comparison to Lansoprazole (β = 19.219 95th CI (13.62–24.817.62.817)) While increase in Age and BMI was associated with decrease in the percent of change of ACR (β −0.327 95th CI (−0.592-0.062) and − 1.896 95th CI(−3.063-0.729) respectively), Table (4).
The 40 patients with DKD & other PPI indications
After 3 months of follow-up, no reported adverse effects in both groups. There was no statistically significant difference in the reduction of albuminuria between Vonoprazan and Lansoprazole (p = 0.076). Both drugs significantly reduced the Albumin-to-Creatinine Ratio (ACR) from baseline levels compared to 3 months post-treatment (p < 0.05). However, the percentage reduction in ACR was significantly greater in the Vonoprazan group (p < 0.001, Table 5). Additionally, patients treated with Vonoprazan showed a significant improvement in their estimated Glomerular Filtration Rate (eGFR) after 3 months compared to baseline levels (p = 0.017), and this improvement was superior to that observed with Lansoprazole, with statistical significance (p = 0.001), Table 5.
Discussion
There is no prior evidence or specific studies linking vonoprazan to improvements or deteriorations in proteinuria associated with diabetic kidney disease. In our study, patients with diabetic kidney disease and positive H. pylori showed a significant reduction in the Albumin-to-Creatinine Ratio (ACR) from baseline levels after 3 months of treatment with either vonoprazan or lansoprazole. This effect is logical and supported by numerous studies indicating that the eradication of H. pylori significantly reduces proteinuria in patients with diabetic kidney disease9,10.
The type of drug used for eradication in our study resulted in different outcomes. The reduction in albuminuria was significantly higher in the vonoprazan group compared to the lansoprazole group. This can be attributed to vonoprazan’s enhanced efficacy on H+,K + ATPase, which is approximately 350 times more potent than that of lansoprazole19.
This increased potency not only appears in the magnitude of proteinuria reduction but also in the higher percentage of treated patients achieving eradication compared to lansoprazole in our study.
New evidence from our research indicates that vonoprazan decreased albuminuria regardless of H. pylori eradication status. This effect was also observed in patients who received vonoprazan for indications other than H. pylori infection, suggesting a unique direct renal effect of this drug class on albuminuria in patients with diabetic kidney disease.
While H-K ATPase is not directly associated with albumin excretion, we propose that vonoprazan may have an effect similar to SGLT2 inhibitors, considering that renal ion transporters are integral to the complex pathophysiological mechanisms of diabetic kidney disease (DKD) and the recent clinical benefits of sodium–glucose cotransporter 2 (SGLT2) inhibitors in DKD highlight the crucial role of renal ion homeostasis in disease progression20,21.
Potassium channels within the renal tubules are vital in the mechanisms that lead to proteinuria occurrence or progression in DKD. Alterations in K + channel activity can impact hemodynamic resistance, kidney blood flow, and glomerular filtration pressure, thereby affecting protein excretion in the urine. These channels help mitigate angiotensin-related podocyte injury and intrarenal pressure, and inhibit TGF-β1 signalling, thus reducing proteinuria and kidney injury18. Recent evidence suggests that impaired potassium balance in nephrotic syndrome is related to proteinuria22,23,24.
Ensuring optimal kidney function through proper acid-base and electrolyte balance can potentially alleviate stress on the glomeruli, indirectly impacting albuminuria levels.
Vonoprazan may have similar effects to lansoprazole (LPZ), such as potential anti-inflammatory properties, reduced oxidative stress, and anti-diabetic benefits, which could lead to a reduction in albuminuria4,19. This might explain the significant improvement in fasting blood glucose, 2-hour postprandial glucose, and HbA1c levels observed in our patients receiving vonoprazan and lansoprazole.
Despite current clinical treatments for diabetic kidney disease (DKD) that aim to alleviate kidney damage by addressing direct and indirect metabolic and hemodynamic factors, such as using SGLT2 inhibitors to improve proteinuria and eGFR in patients with CKD25, our study cannot attribute albuminuria reduction to these drugs. This is because we excluded patients using SGLT2 inhibitors and other drugs that can affect proteinuria from our enrollment criteria.
The mechanisms of action for vonoprazan and other PPIs are entirely different, evident by the improvements in serum creatinine and corresponding eGFR in vonoprazan groups, compared to lansoprazole, despite the known risk of PPI nephrotoxicity19.
However, the renal effects of vonoprazan cannot be fully explained by these suggested mechanisms. Further research is necessary to elucidate the precise role of H-K ATPase in diabetic kidney disease and its potential impact on albuminuria. Understanding these mechanisms could pave the way for new therapeutic interventions. Future researches explore the molecular, mechanistic, and pharmacological basis of vonoprazan’s effect on proteinuria in diabetic kidney disease are recommended. While the current findings are clinically intriguing, a deeper understanding of the drug’s renal actions at the cellular and biochemical levels would provide valuable insight and strengthen the translational relevance of this work.
Strengths and limitations
This study is the first to explore the effect of the newly approved drug vonoprazan on albuminuria in diabetic kidney disease. However, the relatively small sample size and unmeasured confounders remain limitations especially in H Pylori negative patients.
Conclusion
Our work suggests that vonoprazan has pleiotropic beneficial renal effects beyond its gastrointestinal benefits. Further research is needed to elucidate the exact mechanism by which vonoprazan decreases albuminuria in DKD.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Parmar, M. P. et al. Impact of proton pump inhibitors on kidney function and chronic kidney disease progression: a systematic review. Cureus. 15(12), e49883 (2023).
Chen, Y., Lee, K., Ni, Z. & He, J. C. Diabetic kidney disease: Challenges, Advances, and opportunities. Kidney Dis. (Basel). 6, 215–225 (2020).
Zhang, Y. et al. A new perspective on proteinuria and drug therapy for diabetic kidney disease. Front. Pharmacol. 15, 1349022 (2024).
Lin, M. H. et al. Association between clinical use of Lansoprazole and the risk of type 2 diabetes mellitus: a pharmacoepidemiological cohort study. Diabetol. Metab. Syndr. 15(1), 96 (2023).
Hayashino, Y. et al. Association of proton pump inhibitor use with the risk of the development or progression of albuminuria among Japanese patients with diabetes: A prospective cohort study [Diabetes distress and care registry at Tenri (DDCRT 16)]. Diabetes Res. Clin. Pract. 138, 1–7 (2018).
Davis, T. M., Drinkwater, J. & Davis, W. A. Proton pump inhibitors, nephropathy, and cardiovascular disease in type 2 diabetes: the Fremantle diabetes study. J. Clin. Endocrinol. Metabolism 102(8), 2985–2993 (2017).
Yang, H., Juang, S. Y. & Liao, K. F. Proton pump inhibitors use and risk of chronic kidney disease in diabetic patients. Diabetes Res. Clin. Pract. 147, 67–75 (2019).
Chenchula, S. et al. Association and mechanisms of proton pump inhibitors use with Type-2 diabetes mellitus incidence in adults: A systemic review and Meta-Analysis. Curr. Diabetes. Rev. 20(10), 1–2 (2024).
Raj, J. P., Pinto, R. W., Tomy, S. K. & Kulkarni, S. M. Diabetic nephropathy and proton pump inhibitors–pilot case-control study. Indian J. Nephrol. 32(2), 127–131 (2022).
Timucin, A. et al. Effects of Helicobacter pylori eradication on proteinuria: a prospective study. Wien Klin. Wochenschr 124(7–8), 241–244 (2012).
Bahar, C. et al. The effects of Helicobacter pylori eradication on proteinuria in patients with primary glomerulonephritis. Int. J. Nephrol. 2014, 180690 (2014).
Fatih, D. et al. The effect of Helicobacter pylori eradication on proteinuria in patients with primary glomerulonephritis. Arch. Med. Sci. 11(4), 764–769 (2015).
Wei, P. et al. Association between Helicobacter pylori infection and kidney damage in patients with peptic ulcer. Ren. Fail. 41(1), 1028–1034 (2019).
Onge, E. & Phillips, S. T. Vonoprazan: a new potassium-competitive acid blocker. J. Pharm. Technol. 39(3), 139–146 (2023).
Schulz, K. F. & Altman, D. G., Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials.
18-National Kidney Foundation.CKD-EPI Creatinine Eq. (2021). Available from: https://www.kidney.org/ckd-epi-creatinine-equation-2021
Molitch, M. E., DeFronzo, R. A., Franz, M. J. & Keane, W. F. Nephropathy in diabetes. Diabetes Care 27, 79 (2004).
Hertzog, M. A. Considerations in determining sample size for pilot studies. Res. Nurs. Health. 31, 180–191 (2008).
Ishida, M. et al. Vonoprazan-associated nephrotoxicity: extensive real-world evidence from spontaneous adverse drug reaction reports. Kidney Int. 102(3), 666–668 (2022).
Liu, J., Li, X., Xu, N., Han, H. & Li, X. Role of ion channels in the mechanism of proteinuria. Experimental Therapeutic Med. 25(1), 27 (2022).
Hu, H., Liang, W. & Ding, G. Ion homeostasis in diabetic kidney disease. Trends in Endocrinology & Metabolism. 35(2), 142–150 (2024).
Persson, P. B. Potassium homeostasis during nephrotic syndrome: signalling via filtered protein?. J. Physiol. 589(Pt 14), 3417 (2011).
Fila, M. et al. Inhibition of K + secretion in the distal nephron in nephrotic syndrome: possible role of albuminuria. J. Physiol. 589(14), 3611–3621 (2011).
Guo, J., Zhang, C., Zhao, H., Yan, Y. & Liu, Z. The key mediator of diabetic kidney disease: potassium channel dysfunction. Genes Dis. 11(4), 101119 (2024).
Ahmed, R. M., Rakha, N. K., Yousry, A. & Soliman, A. R. Potential impact of sodium glucose co-transporter (SGLT2) inhibitors on cholesterol fractions in stage 3 chronic kidney disease. Egypt. J. Intern. Med. 36(1), 83 (2024).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All Authors contributed to study design, screening of all citations from full-text papers retrieved, data collection, and final revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, R.M., Soliman, A.R. & Mohammed, A. Vonoprazan attenuates proteinuria in diabetic kidney disease through potential direct renal mechanism. Sci Rep 15, 41446 (2025). https://doi.org/10.1038/s41598-025-25376-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25376-8