Abstract
Hemorrhagic transformation (HT) critically impacts outcomes in acute ischemic stroke (AIS) patients post-endovascular thrombectomy (EVT). Building upon the validated utility of post-EVT dual-energy CT (DECT), this study focused on developing and integrating a DECT-based predictive model with key clinical variables to achieve precise, individualized quantification of HT risk. This retrospective study analyzed 116 thrombectomy treated AIS patients stratified by HT status. Post-EVT DECT within 24 h assessed CT values (in Hounsfield Units, HU) of ischemic lesions on mixed energy images; CT values (in HU) on virtual non-contrast (VNC) images and on Sn80 keV and Sn150 keV monoenergetic images; absolute iodine concentrations (AIC, in mg/mL); and relative iodine concentrations (RIC, in %, where RIC = lesion AIC/sigmoid sinus AIC), using follow-up imaging and clinical criteria as the gold standard for HT.. HT patients exhibited higher NIHSS (median 14.5 vs. 9.0) and lower ASPECTS (9 vs. 13) than non-HT (nHT) counterparts, with elevated glucose (GLU, 8.26 vs. 6.45 mmol/L) and lower systolic blood pressure (SBP, 147.5 vs. 156.5 mmHg) (all P < 0.050). DECT-derived parameters demonstrated diagnostic utility, with both iodine overlay maps (IOM) and VNC positivity (χ2 = 60.331, P < 0.001) and dual negativity (χ2 = 58.870, P < 0.001) showing significant intergroup discrimination. Among 42 patients with IOM hyperdensity, RIC differed significantly between subgroups (t = − 2.566, P = 0.014), with elevated RIC independently associated with HT risk (OR = 1.040, 95% CI 1.003–1.078; P = 0.034). RIC alone exhibited strong predictive capacity for HT (AUC = 0.890, 95% CI 0.822–0.957). A nomogram-based model incorporating NIHSS, ASPECTS, and RIC achieved excellent HT prediction in both training (AUC = 0.947, 95% CI 0.903–0.991) and validation cohorts (AUC = 0.902, 95% CI 0.786–1.000), with stable calibration (training: P = 0.655; validation: P = 0.175) and clinical utility on decision curve analysis. Integration into stroke protocols may guide anticoagulation and secondary prevention decisions.
Similar content being viewed by others
Introduction
Stroke, the predominant global cerebrovascular disorder, is classified into ischemic (80%) and hemorrhagic subtypes. Current management of acute ischemic stroke (AIS) prioritizes time-sensitive revascularization to salvage the ischemic penumbra. The 2018 American Heart Association/American Stroke Association (AHA/ASA) guidelines maintain intravenous alteplase as a Class IA recommendation within 4.5 h of symptom onset1, though delayed presentations often preclude thrombolysis due to hemorrhagic risks. Endovascular thrombectomy (EVT) has become standard for large-vessel occlusion within 6–24 h, achieving superior recanalization rates (modified thrombolysis in cerebral infarction [mTICI] 2b/3: 71–88%) versus (vs.) pharmacotherapy2. However, hemorrhagic transformation (HT) complicates 43% of cases within 72 h post-intervention3, independently predicting poor functional outcomes
Post-EVT HT, was defined as secondary intracranial hemorrhage resulting from cerebral ischemia–reperfusion injury in AIS. Its multifactorial pathogenesis includes device-induced endothelial injury4, blood–brain barrier (BBB) disruption during ischemia–reperfusion, activation of collateral circulation, systemic fibrinolysis, and other systemic contributors such as inflammatory/oxidative stress and collateral dysregulation5,6. Non-contrast CT (NCCT), CT angiography, and CT perfusion constitute the baseline imaging modality for diagnosing AIS prior-EVT. While NCCT remains standard for detecting intracranial hemorrhage via hyperdense lesions, its utility is limited by overlapping radiological features between true erythrocyte extravasation and iodine contrast extravasation (ICE)7,8, particularly in areas of neurovascular permeability post-intervention. This diagnostic uncertainty impacts critical decisions regarding antithrombotic resumption and blood pressure modulation, as ICE and hemorrhage require divergent management strategies.
Dual-energy CT (DECT) overcomes conventional CT limitations in differentiating HT from ICE through material decomposition algorithms that exploit differential X-ray attenuation at dual energy levels, enabling precise iodine-blood product discrimination9,10. Bonatti et al. demonstrated significant attenuation differences between HT and ICE on DECT-derived virtual non-contrast (VNC) imaging11, corroborated by Wang et al. reporting 92% diagnostic accuracy12. This technological advancement facilitates rapid and low-radiation acquisition while resolving diagnostic ambiguities critical for anticoagulation decisions and secondary prevention. Standardization of DECT protocols remains imperative to optimize its clinical translation in post-EVT management.
While emerging studies validate DECT reliability in qualitative HT assessment13,14,15,16, quantitative parameter standardization remains unresolved. Current research on post-EVT HT predictors exhibits methodological heterogeneity, with inconsistent conclusions regarding neuroimaging and clinical variables. Crucially, predictive models integrating DECT-derived parameters are absent despite their pathophysiological relevance. In this bicentric study, we leveraged post-EVT DECT-derived parameters to construct a predictive model for HT, with the goal of addressing these existing knowledge gaps and ultimately helping to inform anticoagulation management strategies in clinical practice.
Material and methods
Study population
This bicentric retrospective study analyzed 116 large vessel occlusion (LVO) patients with successful mechanical thrombectomy (mTICI ≥ 2b) from May 2021 to February 2025. Inclusion criteria:(1) first-ever AIS; (2) age ≥ 18 years; (3) computed tomography angiography (CTA)/digital subtraction angiography (DSA) -confirmed LVO > 6 h post-onset; (4) within 24-h post-procedural DECT; (5) follow-up CT within 7 days. After exclusions [prior stroke/surgery (n = 21), pre-existing hemorrhage (n = 11), incomplete imaging (n = 59), lost follow-up (n = 7)], 116 patients were qualified for analysis.
This retrospective study was approved by the Institutional Review Board of Dongguan Songshan Lake Tungwah Hospital. Due to the retrospective nature of the study, the Institutional Review Board of Dongguan Songshan Lake Tungwah Hospital waived the need of obtaining informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Facilities
Postoperative neuroimaging was acquired using a third-generation DECT system (Somatom Force, Siemens Healthineers, Erlangen, Germany) configured for intracerebral hemorrhage assessment. Scans were performed within 24-h post-EV T with patients in the supine position, covering an anatomical range from the mandible to the cranial vertex aligned parallel to the orbitomeatal (OM) reference line. The dual-energy protocol employed simultaneous acquisition at 80 kVp/310 mAs (Tube A) and 140 kVp/207 mAs (Tube B), with helical scanning parameters including = 0.5 mm slice thickness/inter-slice spacing, pitch factor = 0.7, gantry rotation time = 1.0 s, and 200 × 200 mm field of view (FOV). Pre-thrombectomy NCCT scans were obtained using a 120 kVp/340 mAs protocol while maintaining identical geometric parameters. All volumetric datasets were automatically transferred to a SyngoVia workstation (Siemens Healthineers) for post-processing, where a trained radiologist performed three-material decomposition using the manufacturer’s proprietary hemorrhage differentiation algorithm. This process generated VNC images, iodine overlay map (IOM), and Sn50/Sn180 keV monoenergetic images. Additionally, it produced mixed energy CT images, which were reconstructed through linear fusion of data acquired from 80 and 140 kVp X-ray beams using equal weighting (50% each). This fusion technique enhances tissue contrast resolution while minimizing beam-hardening artifacts.
Image analysis
All imaging series were temporally anonymized and independently evaluated by two blinded junior radiologists for HT using predefined criteria: (1) postoperative hyperdense lesion on mixed energy CT images compared to preoperative scans, indicating hemorrhage or ICE; (2) Concurrent IOM and VNC hyperdense congruency indicated hemorrhage with ICE, and isolated IOM hyperdensity denoted sole ICE; (3) lesion persistence on follow-up CT/MRI confirming HT. Furthermore, the infarcted areas were assessed based on the modified ASPECTS score17, and HT was classified according to the ECASS II-Heidelberg criteria. Diagnostic discrepancies between the two assessments underwent adjudication by a senior neuroradiologist to establish consensus findings.
Measurement
Comprehensive quantitative measurements were performed, the measured parameters included: CT values (in Hounsfield Units, HU) of ischemic lesions on mixed energy images from the standard blended series; CT values (in HU) on VNC images and on Sn50 keV and Sn180 keV monoenergetic images; absolute iodine concentrations (AIC, in mg/mL); and relative iodine concentrations (RIC, in %). All attenuation values are reported in HU, while iodine concentrations are quantified in mg/mL or as a percentage. RIC was calculated as:
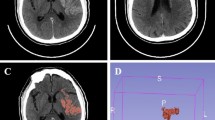
Standardized circular region of interest (ROI) (30 mm2) were centered on the sigmoid sinus (avoiding motion artifacts) and cerebral lesions (excluding partial-volume effect layers). Additionally, it should be emphasized that for volumetric measurements of hyperdense areas on mixed energy CT images, measurements should be performed on the slice with the largest diameter. All patients underwent triplicate measurements per lesion, with mean values recorded. Intraobserver and interobserver reproducibility were rigorously assessed through duplicate measurements conducted with a 7-day washout period to minimize recall bias. Final consensus values were derived from averaged independent evaluations, demonstrating excellent agreement [intraclass correlation coefficient (ICC) = 0.92, 95% confidence interval(CI) 0.85–0.96] using a two-way mixed-effects model for absolute agreement. The images of a typical case were shown in Fig. 1.
DECT and MRI finding exhibition. A 46-year-old female presented with acute left hemiparesis persisting over 3 h. (A) Post-EVT mixed energy CT revealed a mixed-attenuation area in the right basal ganglia that was indeterminate between hematoma and ICE. The mixed energy CT value was measured at the center of the lesion. (B) DECT analysis using IOM revealed contrast extravasation, (C) while VNC confirmed acute hemorrhage and ICE. the CT value on the VNC image was also measured. (D) Quantitative IOM showed AIC (0.80 mg/mL) and a RIC ratio of 203%. The CT values on Sn50 keV and Sn180 keV monoenergetic images were automatically calculated using the SyngoVia workstation. (E–F) Follow-up MRI at 5 days exhibited HT characterized by hemosiderin deposition on SWI.
Statistical methods
The cohort was stratified into HT and non-hemorrhagic transformationn (nHT) groups based on predefined diagnostic criteria. Comprehensive statistical analyses of clinical baseline characteristics, DECT parameters, and risk factors were performed using SPSS v.26.0 (IBM, USA) and R3.6.1. Continuous variables with normal distribution were presented as Mean ± standard deviation and analyzed by independent Student’s t-test. Skewed continuous variables were reported as Median (1st Quartile, 3rd Quartile) and compared using Mann–Whitney U nonparametric test. Categorical variables were expressed as frequencies with percentages (n, %), and between-group differences were assessed through Pearson’s chi-square test or Fisher’s exact tests. Patients were randomly allocated into training and validation sets at a 7:3 ratio. A logistic regression model with forward conditional selection was developed to identify determinants of HT. The predictive performance of the model was evaluated using receiver operating characteristic (ROC) curve analysis, and a nomogram was subsequently constructed. The calibration curve was utilized to verify model stability, while clinical utility was quantified through decision curve analysis (DCA). A P < 0.050 indicated significance.
Results
Clinical and DECT characteristics of AIS patients
HT patients demonstrated significantly elevated National Institutes of Health Stroke scale (NIHSS) scores on admission (Z = 4.775, P < 0.001) and higher blood glucose levels (GLU; Z = 3.023, P = 0.003) compared to nHT counterparts. Conversely, reduced Alberta stroke program early CT scores (ASPECTS; Z = − 5.614, P < 0.001) and lower systolic blood pressure (SBP; Z = − 2.254, P = 0.024) were observed in the HT patients. Notably, no statistically significant differences (P > 0.050) emerged in other baseline characteristics between the cohorts (Table 1) .
There were 22 HT + ICE patients (18.96%) with IOM-positive and VNC-positive results, 9 HT + ICE patients (7.76%) with IOM-positive and VNC-negative results, and 5 HT-alone patients (4.31%) with IOM-negative and VNC-negative results. Notably, no nHT/nICE patients showed IOM-positive and VNC-positive results, representing a statistically significant difference compared to HT patients. Additionally, 69 nHT /nICE patients (59.48%) exhibited IOM-negative and VNC-negative results, which also differed significantly from HT patients (Table 2).
Discriminative ability of DECT-derived parameters between HT and contrast extravasation
To further investigate contrast-related parameters, a subgroup analysis of 42 patients with IOM hyperdensity (n = 42, 31 HT vs. 11 nHT) revealed significantly elevated RIC (t = − 2.566, P = 0.014) on ipsilateral lesion sides. While quantitative comparisons revealed nominally increased values in HT group for mixed energy attenuation, monoenergetic images (Sn80/Sn150), VNC, and AIC, these differences failed to reach statistical significance (all P > 0.050). Notably, contralateral hemispheric analysis demonstrated no statistically significant differences across all DECT-derived parameters (P > 0.050) (Table 3 and Fig. 2).
Univariable logistic regression identified RIC as a significant impact factor of HT, with elevated RIC values demonstrating increased odds of HT occurrence [odds ratio (OR) = 1.023, P = 0.024]. None of the other DECT-derived parameters including mixed energy attenuation, monoenergetic images (Sn80/Sn150), VNC, and AIC showed significant discriminative capacity in models (all P > 0.050). Further multivariable logistics regression analysis suggested that RIC was an independent impact factor of HT (OR = 1.040, P = 0.034) (Table 4).
ROC curve analysis based on DECT-derived parameters to predict HT
The ROC curve analysis demonstrated that the RIC parameter exhibited strong discriminatory ability in differentiating HT patients from nHT patients, achieving an area under curve (AUC) of 0.890 (95%CI 0.822–0.957) with the highest specificity among the evaluated parameters. Comparatively, the AIC showed acceptable diagnostic performance with an AUC of 0.860 (95%CI 0.784–0.937). In contrast, while VNC displayed the highest sensitivity, its diagnostic utility was limited by poor AUC value of 0.569 (95%CI 0.449–0.689) (Table 5 and Fig. 3).
Independent factors for predicting HT patients from nHT patients
According to univariable logistics regression analysis, lower ASPECTS on admission was related to a higher probability of HT (OR = 1.035, P < 0.001). higher values of RIC(P < 0.001), NIHSS on admission (P < 0.001), and GLU (P = 0.022) were correlated with a higher probability of HT (Table 6). Further multivariable logistics regression analysis suggested that higher values of RIC, and NIHSS on admission (P < 0.001) were independently associated with a higher probability of HT (OR = 1.030 and 1.252, respectively, P < 0.050). Conversely, lower ASPECTS on admission was independently related to an elevated risk of HT (OR = 0.672, P = 0.026) (Table 7).
The prediction model using DECT-derived parameters to predict HT
A prediction model incorporating NIHSS on admission, ASPECTS on admission, and RIC was developed using a nomogram (Fig. 4). The calibration curve, and DCA of the nomogram model are presented in Figs. 5 and 6, respectively. According to ROC analyses, the AUC values for the training and validation cohorts were 0.947 (95%CI 0.903–0.991) and 0.902 (95%CI 0.786–1.000), respectively. The calibration curves of both cohorts indicated acceptable model stability (P = 0.655 and 0.175, respectively). The DCA results demonstrated clinically useful predictive performance in both cohorts.
Discussion
Intraprocedural vascular injury, which occurs in approximately 60% of thrombectomy procedures18, acts synergistically with multiple underlying mechanisms to potentiate the risk of HT. This is corroborated by the 31.03% incidence of HT (36/116) observed in our cohort. Furthermore, ICE, observed in up to 43% of EVT cases3, serves as both a biomarker and accelerator of HT: meta-analyses confirm its strong association with HT development and poor neurological recovery19,20, while our data revealed HT progression in 73% (31/42) of patients with post-EVT ICE. among which 11 of these 31 ICE patients subsequently developed symptomatic intracerebral hemorrhage (sICH), consistent with the findings reported by Clémence et al21. These findings underscore the clinical imperative to differentiate ICE from true hemorrhage, as their overlapping imaging features yet divergent management pathways directly influence therapeutic decisions and long-term prognosis22,23.
Given the high prevalence of postprocedural ICE and the limited diagnostic accuracy of Conventional CT24, Dekeyzer et al. recommend repeated cerebral CT scans for precise HT diagnosis25. Nevertheless, this strategy escalates radiation exposure and patient burden. DECT addresses this limitation by reliably differentiating HT from ICE, thereby mitigating unnecessary repeat imaging and associated radiation risks.
In our cohort, all 22 patients with high-density lesions on both IOM and VNC images were in the HT group (100%, 22/22), demonstrating significant differentiation from the nHT group (χ2 = 60.331, P < 0.05), Conversely, among patients with ICE alone, 9 out of 20 subsequently developed HT, which consistent with the results reported by Ebashi et al14. However, quantitative analysis revealed that VNC CT values lacked discriminative power for HT (Z = − 0.560, P = 0.577), contrasting with prior literature. This discrepancy likely originates from methodological disparities: Ebashi et al14 included patients without high-density areas on NCCT in their analysis and did not specify whether patients with delayed HT beyond 24 h were included, whereas our cohort contained limited delayed HT cases (4.3%, 5/116) that disproportionately influenced small-sample analyses. These findings suggests that relying solely on CT imaging features or CT values of lesions to predict HT may not be a reliable strategy.
DECT not only differentiates cerebral hemorrhage from ICE but also quantifies lesion iodine concentration26. Bonatti et al. reported that AIC > 1.35 mg/mL in infarcted areas post-EVT predicted HT with 100% sensitivity and 67.60% specificity11, while Ma et al. proposed a higher threshold of AIC > 2.70 mg/mL (75.70% sensitivity, 65.50% specificity)27. In contrast, our cohort demonstrated lower AIC values in HT subgroups, likely due to delayed DECT timing (within 24 h post-EVT), allowing partial contrast absorption via cerebral circulation4. Our data similarly showed no significant AIC variation (Z = − 0.823, P = 0.410), consistent with Byrne et al28. These findings suggest limited reliability of AIC alone for HT prediction when DECT is performed within 24 h post-EVT. However, RIC reduces variability in AIC values influenced by individual factors29. Byrne et al. used the superior sagittal sinus as a reference, reporting RIC > 100% predicted HT with 94.7% sensitivity and 43.4% specificity28. As is well recognized, factors influencing the spontaneous density of the venous sinus primarily include age, platelet (PLT) count, hemoglobin and red blood cell (RBC) count, particularly under conditions of anemia. In our study cohort, no statistically significant differences were observed in these clinical characteristics between HT and nHT patients. Therefore, our study employed the sigmoid sinus, which offers larger diameter and higher iodine content, improving measurement accuracy. Results showed RIC had 66.20% sensitivity and 97.20% specificity for HT prediction. While Byrne emphasized sensitivity for early postoperative assessment, we contend that prioritizing specificity becomes of greater clinical significance within 24 h post-EVT—a timeframe during which ICE begins to resolve. Enhanced specificity not only improves differentiation between hemorrhage and ICE but also facilitates evidence-based therapeutic decision-making. This strategy effectively balances diagnostic precision with pragmatic clinical demands throughout the critical peri-interventional period.
Current evidence suggests considerable variability in HT diagnosis when DECT is performed immediately versus within 24 h post-EVT in AIS patients, though no standardized guidelines exist regarding optimal DECT timing30,31. In our study, post-EVT DECT was performed at approximately 20 h—coinciding with the peak incidence period for HT—yet our results indicate that scan timing may not significantly influence HT prediction. However, further prospective studies are warranted to validate this observation. Notably, unlike previous reports11,28,32, our analysis revealed no correlation between hyperdense lesion volume on mixed energy CT and HT development, a finding notably consistent with the observations by Huang et al30. This discrepancy is potentially attributable to methodological variations across studies, including differences in post-EVT DECT acquisition time.
Beyond DECT parameters, clinical assessment tools remain indispensable for HT risk stratification. Elevated NIHSS scores and reduced ASPECTS have been consistently validated across thrombectomy cohorts, reflecting the interplay between baseline stroke severity, infarct burden, and hemorrhagic susceptibility33,34 Our analysis showed significant intergroup differences in NIHSS and ASPECTS on admission (Z = 4.775; − 5.614, P < 0.050), with logistic regression confirming both as independent HT risk factors—higher NIHSS (OR = 1.252, P < 0.050) and lower ASPECTS (OR = 0.672, P < 0.050) correlated with increased HT likelihood.
Our predictive model incorporated NIHSS, ASPECTS, and RIC in infarct regions, integrating pre- and post-thrombectomy imaging features of AIS patients. The model demonstrated strong goodness-of-fit for HT prediction in both training and validation cohorts, indicating practical clinical utility. ROC and DCA revealed robust predictive performance across cohorts, supporting its high efficacy for HT risk stratification. These findings highlight the model’s potential for clinical implementation, warranting further validation in real-world settings.
Limitations
Firstly, the baseline data included in this study are limited. Potential risk factors such as a history of atrial fibrillation, the number of intraoperative puncture attempts, and other relevant variables could not be incorporated due to incomplete patient records. Secondly, a certain degree of sampling error or selection bias is unavoidable in this retrospective analysis. Thirdly, the sample size is relatively small, which may limit the generalizability of the findings. Fourthly, absence of external validation. Future studies will aim to address these limitations by expanding the sample size, incorporating more comprehensive baseline data, and minimizing potential biases to enhance the robustness of the results.
Conclusion
Post-EVT ICE in AIS patients has been identified as a critical determinant of HT and neurological recovery. DECT enables 24 h post-EVT differentiation between ICE and HT, reducing repeated imaging and radiation exposure. Given significant interpatient variability, sole reliance on CT attenuation, AIC or RIC for HT prediction remains suboptimal. Our analysis demonstrated that predictive models incorporating NIHSS, ASPECTS, and RIC parameters achieved better HT predictive accuracy. This approach prioritizes actionable diagnostic accuracy for therapeutic decision-making.
Data availability
The data used to support the findings of this study are available from the corresponding author on reasonable request.
Abbreviations
- AIS:
-
Acute ischemic stroke
- EVT:
-
Endovascular thrombectomy
- HT:
-
Hemorrhagic transformation
- BBB:
-
Blood–brain barrier
- NCCT:
-
Non-contrast CT
- ICE:
-
Iodine contrast extravasation
- DECT:
-
Dual-energy CT
- VNC:
-
Virtual non-contrast
- IOM:
-
Iodine overlay map
- AIC:
-
Absolute iodine concentration
- RIC:
-
Relative iodine concentration
- CI:
-
Confidence interval
- ROC:
-
Receiver operating characteristic
- DCA:
-
Decision curve analysis
- NIHSS:
-
National Institutes of Health Stroke scale
- GLU:
-
Glucose
- ASPECTS:
-
Alberta stroke program early CT scores
- SBP:
-
Systolic blood pressure
- OR:
-
Odds ratio
- AUC:
-
Area under curve
References
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 50(12), e344–e418 (2019).
Inoue, M. et al. expanding the treatable imaging profile in patients with large ischemic stroke: Subanalysis from a randomized clinical trial. Stroke 55(7), 1730–1738 (2024).
Elfil, M. et al. Endovascular thrombectomy for large vessel occlusion in acute ischemic stroke patients with concomitant intracranial hemorrhage. J. Clin. Neurosci. 134, 111093 (2025).
Hong, J. M. et al. Hemorrhagic transformation after ischemic stroke: Mechanisms and management. Front. Neurol. 12, 703258 (2021).
Chen, X. Y. et al. Blood-brain barrier permeability by CT perfusion predicts parenchymal hematoma after recanalization with thrombectomy. J. Neuroimag. 34(2), 241–248 (2024).
Hamann, G. F. et al. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J. Cereb. Blood Folw Metab. 22(5), 526–533 (2002).
Jadhav, A. P. et al. Care of the post-thrombectomy patient. Stroke 49(11), 2801–2807 (2018).
Vitt, J. R., Trillanes, M. & Hemphill, J. C. III. Management of blood pressure during and after recanalization therapy for acute ischemic stroke. Front. Neurol. 10, 138 (2019).
Grkovski, R. et al. A novel dual-energy CT method for detection and differentiation of intracerebral hemorrhage from contrast extravasation in stroke patients after endovascular thrombectomy: Feasibility and first results. Clin. Neuroradiol. 33(1), 171–177 (2023).
Ebaid, N. Y. et al. Diagnostic accuracy of dual-energy computed tomography in the diagnosis of neurological complications after endovascular treatment of acute ischaemic stroke: a systematic review and meta-analysis. Br J. Radiol. 97(1153), 73–92 (2024).
Bonatti, M. et al. Iodine extravasation quantifification on dual-energy CT of the brain performed after mechanical thrombectomy for acute ischaemic stroke can predict haemorrhagic complications. AJNR Am. J. Neuroradiol. 39(3), 441–447 (2018).
Wang, Z. S. et al. Early diagnosis and prediction of intracranial hemorrhage using dual-energy computed tomography after mechanical thrombectomy in patients with acute ischemic stroke. Clin. Neurol. Neurosurg. 203, 106551 (2021).
Liu, K. Q. et al. The role of dual energy CT in evaluating hemorrhagic complications at different stages after thrombectomy. Front. Neurol. 11, 583411 (2020).
Ebashi, R. et al. Significance of simulated conventional images on dual energy CT after endovascular treatment for ischemic stroke. J. Neurointerv. Surg. 11(9), 898–902 (2019).
DiNitto, J. et al. Flat-panel dual-energy head computed tomography in the angiography suite after thrombectomy for acute stroke: A clinical feasibility study. Interv. Neuroradiol. 2, 15910199231157462 (2023).
Chrzan, R. et al. Dual energy computed tomography in differentiation of iodine contrast agent staining from secondary brain haemorrhage in patients with ischaemic stroke treated with thrombectomy. Neurol. Neurochir. Pol. 56(1), 68–74 (2022).
Rodriguez, M. P. et al. AC-ASPECTS, ACh-ASPECTS, and H-ASPECTS: New imaging scales to assess territorial and total cerebral hemispheric ischemic injury. Front. Neurol. 15, 1397120 (2024).
Luo, J. Q. et al. Clearance rate of contrast extravasation after endovascular therapy is associated with functional outcome and mediated by cerebral edema. J. Cereb. Blood Flow Metab. 45(1), 66–76 (2025).
Yang, F. et al. Prognostic value of contrast staining on dual-energy CT after endovascular therapy in acute ischemic stroke: a meta-analysis. BMC Neurol. 23(1), 326 (2023).
Heo, J. et al. Prediction of cerebral hemorrhagic transformation after thrombectomy using a deep learning of dual-energy CT. Eur. Radiol. 34(6), 3840–3848 (2024).
Clémence, H. et al. Determinants and clinical relevance of iodine contrast extravasation after endovascular thrombectomy: A Dual-energy CT study. AJNR Am. J. Neuroradiol. 45(1), 30–36 (2024).
Qiu, T. et al. Dual-energy computed tomography (DECT) predicts the efficacy of contrast medium extravasation and secondary cerebral hemorrhage after stent thrombectomy in acute ischemic cerebral infarction. Biotechnol. Genet. Eng. Rev. 40(1), 202–216 (2024).
Chen, Z. Y. et al. Contrast extravasation is predictive of poor clinical outcomes in patients undergoing endovascular therapy for acute ischemic stroke in the anterior circulation. J. Stroke Cerebrovasc. Dis. 29(1), 104494 (2020).
Pinckaers, F. M. E. et al. Prognostic implications of intracranial haemorrhage on dual-energy CT immediately following endovascular treatment for acute ischemic stroke. J. Neuroradiol. 51(4), 101168 (2024).
Dekeyzer, S. et al. Distinction between contrast staining and hemorrhage after endovascular stroke treatment:one CT is not enough. Neurointerv. Surg. 9(4), 394–398 (2017).
Nayab, A. et al. Value of dual energy CT in post resuscitation coma. Differentiating contrast retention and ischemic brain parenchyma. Radiol. Case Rep. 17(10), 3722–3726 (2022).
Ma, C. et al. Quantitative intracerebral iodine extravasation in risk stratification for intracranial hemorrhage in patients with acute ischemic stroke. AJNR Am. J. Neuroradiol. 43(11), 1589–1596 (2022).
Byrne, D. et al. Prediction of hemorrhage after successful recanalization in patients with acute ischemic stroke: Improved risk stratification using dual-energy CT parenchymal iodine concentration ratio relative to the superior sagittal sinus. AJNR Am. J. Neuroradiol. 41(1), 64–70 (2020).
Bae, K. T. Intravenous contrast medium administration and scan timing at CT: Considerations and approaches. Radiology 256(1), 32–61 (2010).
Huang, S. Y. et al. Predictive value of clinical and dual-energy computed tomography parameters for hemorrhagic transformation and long-term outcomes following endovascular thrombectomy. Diagnostics (Basel). 14(22), 2598 (2024).
Wang, T. Y. et al. Hemorrhagic transformation assessment based on dual energy CT of immediately and twenty-four hours after endovascular thrombectomy for acute ischemic stroke. Diagnostics (Basel). 13(15), 2493 (2023).
Ahn, S. et al. The clinical utility of dual-energy CT in post-thrombectomy care: Part 2, the predictive value of contrast density and volume for delayed hemorrhagic transformation. J. Stroke Cerebrovasc. 32, 107216 (2023).
Zhong, K. L. et al. Predictive model for the risk of hemorrhagic transformation after rt-PA intravenous thrombolysis in patients with acute ischemic stroke: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 239, 108225 (2024).
Wen, L. H. et al. Risk factors of haemorrhagic transformation for acute ischaemic stroke in Chinese patients receiving intravenous thrombolysis a meta-analysis. Medicine (Baltimore) 99(7), e18995 (2020).
Author information
Authors and Affiliations
Contributions
G.Z., G.Y. and Y.D. conceived and designed the study. G.Z. and F.X. drafted the initial manuscript. W.S., S.Y., D.C. and X.L. contributed to data acquisition. N.C., Z.F. and Z.L. contributed to the analysis of data. R.Z., T.X., D.M. and C.L. verified the data and contributed to the data interpretation. G.Z. and Y.T. revised the manuscript and provided methodological guidance. All authors reviewed and approved the ultimate version of the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, G., Xia, F., Yang, G. et al. A bicentric study on the application of dual energy CT for predicting hemorrhagic transformation post endovascular thrombectomy. Sci Rep 15, 41659 (2025). https://doi.org/10.1038/s41598-025-25574-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25574-4