Abstract
This study aimed to explore the potential association between serum osteocalcin (OCN) and hepatic steatosis (HS) in patients with chronic hepatitis B (CHB). In this cross-sectional study, a total of 263 CHB patients were enrolled and their baseline profiles were analyzed from January 2020 to January 2022. HS was defined based on the controlled attenuation parameter (CAP) value. Specifically, patients with a CAP value exceeding 248 dB/m were diagnosed with HS. Among the 263 patients, 76 with HS and 187 without HS. The levels of serum OCN were lower in with HS than in without HS participants (P = 0.002). Scatter plots with Pearson correlation coefficients indicated that there was a negative correlation among OCN levels with CAP (r = − 0.134, P = 0.030), ALB (r = − 0.21, P < 0.001), and GLB (r = − 0.139, P = 0.025). Logistic regression analysis showed that the OCN levels (OR, 0.92; P = 0.005) were negatively associated with HS, and age (OR, 1.04; P = 0.012), GLB(OR, 0.93; P = 0.039), ALT (OR, 1.05; P = 0.002), and γ-GT (OR,1.04; P = 0.001) positively associated with HS. In stratified analysis, the association remained significant in the age (P = 0.020), OCN (P = 0.026), GLB (P = 0.037), ALT (P = 0.020), and γ-GT (P < 0.001) in CHB patients. Our finding suggested that serum OCN was independently and negatively associated with HS in CHB patients.
Similar content being viewed by others
Introduction
Beyond its structural and protective functions, bone has emerged as a dynamic endocrine organ capable of orchestrating systemic metabolic regulation through osteokine secretion1. Accumulating evidence identifies osteocalcin (OCN) – a bone-specific protein synthesized by osteoblasts – as a pivotal endocrine regulator that integrates skeletal remodeling with whole-body energy homeostasis2,3,4. In addition, OCN has been discovered to stimulate beta cells in the pancreas to secrete insulin, as well as to direct fat cells to emit adiponectin, thereby augmenting insulin sensitivity5,6. These findings position skeletal-derived OCN as mediator in the bone-pancreas-adipose axis, offering novel therapeutic targets for metabolic disorders.
Globally, over 400 million people are affected by chronic hepatitis B virus (CHB) infection, posing a serious public health challenge7. If untreated, patients with CHB have a 15%−40% life-time risk of developing severe complications, such as liver cirrhosis, hepatic decompensation and hepatocellular carcinoma (HCC)8. Therefore, current therapeutic strategies prioritize sustained viral suppression and its complications to improve the long-term prognosis. Notably, hepatic steatosis (HS) coexists in 14–70% of CHB cases, with emerging evidence suggesting its synergistic role in accelerating advanced liver disease9,10,11. Moreover, a recent study further identify that the presence of metabolic syndrome is closely associated with HS, and is an independent risk factor for cirrhosis and HCC among patients with CHB12. These findings underscore the critical need for systematic evaluation and targeted management of HS in CHB populations to optimize clinical interventions.
Metabolic-associated fatty liver disease (MAFLD), defined by HS involving ≥ 5% hepatocytes, represents a clinical spectrum ranging from bland steatosis to nonalcoholic steatohepatitis13. While the mechanistic underpinnings connecting chronic liver disease and osteoporosis remain incompletely understood, emerging evidence suggests metabolic dysfunction-associated MAFLD elevates osteoporosis risk, with histological severity inversely correlating with bone mineral density14,15,16. The pathophysiological interplay between MAFLD and reduced OCN levels has been postulated to involve chronic low-grade inflammatory cascades and dysregulation of bone turnover biomarkers17. Furthermore, nationwide cohort studies indicate that those with persistent HBV infection are more likely to suffer from osteoporosis in the future18. A retrospective cohort study of CHB patients in which long-term safety of oral nucleoside analogs was assessed revealed that the use of nucleotide analogs increased the risk of hip fracture19. A mechanism is that nucleotide analogs cause a reduction in osteoblast gene expression which causes defective osteoblast function leading to decreased bone formation20. On the contrary, in a study conducted by Tien et al.21 there was no increase in the risk of osteoporosis with TDF treatment. OCN, a bone formation marker regulating energy metabolism, shows conflicting associations with HS in existing studies22,23. However, this association remains unexplored in CHB patients. Therefore, the purpose of our study was to investigate the relationship between serum OCN and HS in CHB patients.
Materials and methods
Study population and criteria
We retrospectively collected data from 485 patients clinically diagnosed with CHB between January 2020 and January 2022. Chronic HBV infection is characterized by a sustained positive hepatitis B surface antigen (HBsAg) test result for a period exceeding 6 months24.
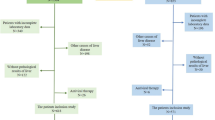
Inclusion criteria included: (1) Patients were adults (≥ 18 years) regardless of gender, ethnicity; (2) Patients with clinically proved CHB. Exclusion criteria included: (1) Non-hepatitis B (n = 168); (2) Incomplete research data (n = 21); (3) Excessive alcohol consumption of greater than or equal to 210 g/week (n = 10); (4) Acute hepatitis, biliary obstruction, liver congestion, liver tumors, and ascites (n = 19); (5) Patients with chronic renal insufficiency (n = 5); (6) Patients with hepatobiliary surgery (n = 3); (7) Patients with medication for diabetes, hypertension or dyslipidaemia (n = 6) (Fig. 1).
Finally, this cross-sectional study involved 263 patients. All individuals underwent FibroScan for NAFD and blood tests to assess lipid metabolism as well as hepatic and renal function. Each of the participants filled out a standard questionnaire on their gender, age, drinking history, health conditions, and drug history.
HS: hepatic steatosis.
Laboratory investigations
Each person’s venous blood samples were taken following an overnight fast lasting between 8 and 12 h. At the Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine’s laboratory, all blood samples underwent testing. Serum levels of the OCN were measured with ELISA kits from Immunodiagnostic Systems (Boldon, UK). Blood test parameters, including albumin (ALB), globulin (GLB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (γ-GT), total bile acid (TBA), blood urea nitrogen (BUN), and creatininee (CRE), were measured using the Sysmex 2100 whole blood cell analyzer (Sysmex, Kobe, Japan). We used EIA (AxSYM AFP, Abbott Japan, Tokyo, Japan) to analyze alpha fetoprotein (AFP) levels. The hepatitis B markers were tested using polymerase chain reaction.
Definition of HS based on abdominal controlled Attenuation parameter (CAP)
The validated non-invasive tests of LSM and CAP have been employed to accurately diagnose hepatic fibrosis and steatosis25. For the quantification of liver steatosis, measurements were conducted with FibroScan® on all participants (model 502, Echosens, Paris, France) by a trained technician. The presence of more than 5% macrovesicular HS was an essential requirement for MAFLD26. The CAP scores were classified into the following categories: mild (CAP 248–267 dB/m), moderate (CAP 268–279 dB/m) and severe (CAP > 279 dB/m)27. The quantitative diagnostic of HS in this investigation was established as CAP ≥ 248 dB/m in accordance with the aforementioned studies.
Statistical analysis
SPSS (version 26.0, IBM, Armonk, NY, United States) and GraphPad Prism (version 9.5.0, 525, La Jolla, CA, USA) software were employed for all statistical analyses. The categorical variables were represented by their corresponding number and percentage (n, %), while the continuous variables were represented by means ± standard deviation or as the median (quartile) in accordance with the observed distribution. Chi-square tests were employed to contrast categorical variables, while independent sample tests or nonparametric tests based on normality of distribution were employed to compare continuous variables. Scatter plots with Pearson correlation coefficients were used to examine whether clinical variables were potentially associated with OCN levels in CHB patients. Univariate and multivariate logistic regression were then used to investigate clinical variables potentially linked to HS. Subgroup analysis was subsequently performed based on categories of age, OCN, ALB, GLB, AST, ALT, and γ-GT after adjustment for gender, age, AFP, BUN, CRE, ALB, GLB, AST, ALT, γ-GT and LSM. P values less than 0.05 were considered statistically significant.
Results
Demographic characteristics
In total, 263 subjects (mean age, 45 ± 11 years) were identified in the analysis. The total incidence of HS was 28.90% (76/263). The levels of serum OCN were lower in 76 HS participants than in 187 non-HS participants (16.63 ± 5.74 ng/mL vs. 19.61 ± 9.02 ng/mL, P = 0.002). Differences were found in terms of gender, age, ALT, γ-GT, BUN, CRE, and OCN. However, no statistical differences were observed in demographic variables, such as AFP, ALB, GLB, AST, LSM, HBV-DNA (+), HBeAg (+), and NAs between these two groups (Table 1).
Scatter diagram: relationship between OCN levels and age, CAP, ALB, GLB, AST, ALT
We examined whether these parameters were associated with OCN levels in CHB patients. Scatter plots with Pearson correlation coefficients indicated that there was a negative correlation among OCN levels with CAP (r = − 0.134, P = 0.030), ALB (r = − 0.21, P < 0.001), and GLB (r = − 0.139, P = 0.025). However, there was no statistically significant correlation between OCN levels and age, AST, and ALT (all P > 0.05) (Fig. 2).
(A) Scatter diagram between OCN levels and age; (B) Scatter diagram between OCN levels and controlled attenuation parameter (CAP); (C) Scatter diagram between OCN levels and albumin (ALB); (D) Scatter diagram between OCN levels and globulin (GLB); (E) Scatter diagram between OCN levels and aspartate aminotransferase (AST); (F) Scatter diagram between OCN levels and alanine aminotransferase (ALT).
Table 2 Univariable and multivariable logistic regression analyses of correlative factors of HS.
Setting the presence of visceral HS as the dependent variable, the multivariable logistic regression analyses showed that serum OCN levels (OR, 0.92; 95% CI, 0.87–0.98; P = 0.005) was negatively associated with HS, whereas age (OR, 1.04; 95% CI, 1.01–1.08; P = 0.012), GLB(OR, 0.93; 95% CI, 0.87–1.00.87.00; P = 0.039), ALT (OR, 1.05; 95% CI, 1.02–1.08; P = 0.002), and γ-GT (OR, 1.04; 95% CI, 1.01–1.06; P = 0.001) were positively correlated (Table 2).
Subgroup analysis
The analysis of subgroups was carried out using logistic regression and plotted in a forest plot (Fig. 3). We performed subsequently subgroup analyses based on categories of age, OCN, ALB, GLB, AST, ALT, and γ-GT. Subgroup analysis was conducted according to age (median 45 years), OCN (mean 18 ng/mL), ALB (mean 45 g/L), GLB (mean 30 U/L), AST (mean 27 U/L), ALT (mean 29 g/L), and γ-GT (mean 27 g/L). All values were rounded to be integers. In stratified analysis, the association remained significant in the age (P = 0.020), OCN (P = 0.026), GLB (P = 0.037), ALT (P = 0.020), and γ-GT (P < 0.001) groups in CHB patients. However, in the stratified analysis of ALB, AST still had no association with HS (all P > 0.05).
The association was adjusted for age, gender, age, AFP, BUN, CRE, ALB, GLB, AST, ALT, γ-GT, LSM and OCN. OR, odds ratio; CI, confidence intervals. HS = hepatic steatosis; OCN = osteocalcin. ALB = albumin; GLB = globulin; ALT = alanine aminotransferase; AST = aspartate aminotransferase; γ-GT = γ-glutamyl transpeptidase.
Discussion
OCN, synthesized and secreted by osteoblasts, critically regulates bone mineralization and demonstrates robust osteogenic potential in clinical and histological studies3,28. Emerging evidence highlights OCN’s dual role in bone turnover and systemic metabolism: insulin signaling stimulates OCN secretion, which reciprocally enhances pancreatic β-cell function and tissue insulin sensitivity5. These metabolic properties have propelled OCN into the spotlight as a key regulator of glucose-lipid homeostasis29. HS is known to exacerbate hepatic insulin resistance. Intriguingly, even subtle alterations in liver lipid content can exert substantial impacts on whole - body glucose metabolism30. The body’s sensitivity to insulin is closely associated with the concentration of OCN in peripheral blood. Insulin resistance is considered a key pathophysiological factor in HS. The sensitivity of the body to insulin is strongly correlated with the amount of OCN in peripheral blood, and insulin resistance is thought to be a pathophysiological cause of HS31. The metabolic benefits of OCN secreted by osteoblasts have been well-documented. Additionally, in terms of the association between hepatitis B and osteoporosis, a population - based cohort study has indicated that chronic HBV infection is associated with an increased risk of future osteoporosis18. Since the discovery that OCN plays a role in regulating glycolipid and energy metabolism, numerous investigations have been carried out to explore the link between OCN and HS. This background motivated us to examine the relationship between OCN and HS in patients with CHB.
A case-control study revealed a marked decrease in serum OCN levels among those with biopsy-confirmed MAFLD compared to healthy controls22. Similarly, our results also showed that in CHB patients, the serum OCN levels in the HS group were significantly lower than those in the non - HS group, which is in line with the results of other researchers32,33. Another prior study demonstrated that in both pre - and post - menopausal women without osteopenia or osteoporosis, serum OCN levels were independently and negatively associated with the presence of HS23. Also, we could observe that serum OCN levels were independently correlated with HS in CHB patients. Moreover, after adjusting for other covariates, the study identified an inverse relationship between blood OCN levels and the presence of HS. In the subgroup analysis stratified by OCN levels, a significant association between serum OCN and HS persisted. These results suggest that OCN may play a role in the development of HS in CHB patients.
The complexity of the connection between serum OCN levels and HS is yet to be determined, as the causes of lower OCN levels in those with the condition remain a mystery. An experimental study showed that the onset of insulin resistance in rats fed a high - fat diet reduces osteoblast proliferation and differentiation and increases osteoblast apoptosis34. Based on this, it is hypothesized that HS may affect osteoblast activity, leading to a decrease in OCN. Additionally, HS and CHB - related chronic inflammation can have detrimental effects on bone health. Numerous liver conditions, such as cirrhosis, hepatitis, and alcoholic liver disease, have been associated with interleukin-1, interleukin-6, and tumor necrosis factor, for example. These inflammatory cytokines can potentially boost osteoclast activity, hinder their apoptosis, and promote osteoclast genesis35,36. Consequently, the inhibition of OCN production leads to a decrease in serum OCN levels in these individuals37. Adiponectin, an endocrine hormone that lowers bone mineral density by preventing osteoprotegerin production in osteoblasts, may also contribute to an increased risk of HS38. Another possible explanation for the relationship between blood OCN levels and HS is that OCN may increase insulin activity and adiponectin production, reduce fat mass, and improve insulin sensitivity5, may thereby protecting against the development of HS. In recent years, there has been a growing research interest in exploring whether antiviral treatment poses a risk of osteoporosis among chronic hepatitis B (CHB) patients. A cohort study was conducted to evaluate the long-term safety of oral nucleos(t)ide analogs in a large sample of 53,500 CHB patients, which included 46,454 untreated patients and 7,046 patients who received treatment. During a 3-year follow-up period, the study revealed that exposure to nucleotide analogs was associated with a significantly higher risk of hip fracture compared to nucleoside analogs19. In another study, bone mineral density and fracture risk score were compared to healthy controls in CHB patients treated with TDF. In patients with CHB treated with TDF, the hip BMD score decreased while the fracture risk score increased39. It has been hypothesized that tenofovir disoproxil fumarate may induce osteoporosis through two main mechanisms. Firstly, it can inhibit DNA synthesis in osteoclasts within the bone. Secondly, it may lead to proximal renal tubule dysfunction, known as Fanconi syndrome, in the kidneys20. In contrast, a study21 assessing BMD measurements only once after ≥ 18 months of TDF therapy reported no significant bone mineral density changes. Furthermore, a retrospective cohort study40 by the same research group observed stable serum phosphorus levels—an indirect marker of bone metabolism—in TDF-treated patients during short-to-medium follow-up periods (≤ 3 years). These findings suggest minimal perturbations in osteocalcin (OCN) levels during this timeframe, aligning with data indicating no significant OCN alterations with short-to-medium-term TDF use. Our results confirm that serum OCN remains an independent negative predictor of HS after accounting for NAs. Given these findings, it is also recommended that CHB patients undergoing treatment with nucleotide analogs should be closely monitored. If risk factors for osteoporosis are identified, appropriate preventive and therapeutic measures for osteoporosis should be promptly implemented41.
This study has some several limitations that must be acknowledged and resolved. Firstly, the cross-sectional nature of the study did not seek to explore the mechanisms behind the observed associations. Secondly, some potential bias may have been masked, such as the effect of various antiviral treatment regimens and the duration of antiviral medication on osteoporosis, might have gone unnoticed. Thirdly, we used CAP rather than liver biopsy, which is the conventional gold-standard method for determining the degree of hepatic steatosis and diagnosis HS. Additionally, due to missing data, various variables—including BMI, serum lipidemia, serum TNF, and HBV-related indicators—were left out of the analysis.
Conclusion
In summary, we confirmed that serum OCN levels were independently and inversely linked with HS in Chinese patients suffering from CHB, and considerably lower in HS patients than in those without. Future enormous-scale prospective clinical studies will be necessary to find out how serum OCN in HS patients’ functions.
Data availability
The data used to support the findings of the study can be made available upon request to the corresponding author.
Abbreviations
- HS :
-
Hepatic steatosis
- MAFLD:
-
Metabolic-associated fatty liver disease
- AFP:
-
Alpha fetoprotein
- ALB:
-
Albumin
- GLB:
-
Globulin
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- γ-GT:
-
γ-Glutamyl transpeptidase
- LSM:
-
Liver stiffness measurement
- CAP:
-
Controlled attenuation parameter
- BUN:
-
Blood urea nitrogen
- CRE:
-
Creatinine
- OCN:
-
Osteocalcin
- NASH:
-
Nonalcoholic steatohepatitis
References
DiGirolamo, D. J., Clemens, T. L. & Kousteni, S. The skeleton as an endocrine organ. Nat. Rev. Rheumatol. 8 (11), 674–683 (2012).
Musso, G., Paschetta, E., Gambino, R., Cassader, M. & Molinaro, F. Interactions among bone, liver, and adipose tissue predisposing to diabesity and fatty liver. Trends Mol. Med. 19 (9), 522–535 (2013).
Zoch, M. L., Clemens, T. L. & Riddle, R. C. New insights into the biology of osteocalcin. Bone 82, 42–49 (2016).
Kim, Y. S., Paik, I. Y., Rhie, Y. J. & Suh, S. H. Integrative physiology: defined novel metabolic roles of osteocalcin. J. Korean Med. Sci. 25 (7), 985–991 (2010).
Lee, N. K. et al. Endocrine regulation of energy metabolism by the skeleton. Cell 130 (3), 456–469 (2007).
Liu, J. M., Rosen, C. J., Ducy, P., Kousteni, S. & Karsenty, G. Regulation of glucose handling by the skeleton: insights from mouse and human studies. Diabetes 65 (11), 3225–3232 (2016).
Zhang, H. et al. First-In-Human study on Pharmacokinetics, Safety, and tolerability of single and multiple escalating doses of Hepenofovir, a novel hepatic targeting prodrug of Tenofovir in healthy Chinese subjects. Front. Pharmacol. 13, 873588 (2022).
Liaw, Y. F. & Chu, C. M. Hepatitis B virus infection. Lancet 373 (9663), 582–592 (2009).
Fan, J. G., Kim, S. U. & Wong, V. W. New trends on obesity and NAFLD in Asia. J. Hepatol. 67 (4), 862–873 (2017).
Seto, W. K. et al. Association between hepatic Steatosis, measured by controlled Attenuation Parameter, and fibrosis burden in chronic hepatitis B. Clin. Gastroenterol. Hepatol. 16 (4), 575–583 (2018). e2.
Cheng, J. Y. et al. Metabolic syndrome increases cardiovascular events but not hepatic events and death in patients with chronic hepatitis B. Hepatology 64 (5), 1507–1517 (2016).
Chon, Y. E. et al. The relationship between type 2 diabetes mellitus and Non-Alcoholic fatty liver disease measured by controlled Attenuation parameter. Yonsei Med. J. 57 (4), 885–892 (2016).
Sberna, A. L. et al. European association for the study of the liver (EASL), European association for the study of diabetes (EASD) and European association for the study of obesity (EASO) clinical practice recommendations for the management of non-alcoholic fatty liver disease: evaluation of their application in people with type 2 diabetes. Diabet. Med. 35 (3), 368–375 (2018).
Mantovani, A. et al. Systematic review with meta-analysis: non-alcoholic fatty liver disease is associated with a history of osteoporotic fractures but not with low bone mineral density. Aliment. Pharmacol. Ther. 49 (4), 375–388 (2019).
Black, D. M. & Rosen, C. J. Clinical Practice. Postmenopausal osteoporosis. N Engl. J. Med. 374 (3), 254–262 (2016).
Pacifico, L. et al. Adipokines and C-reactive protein in relation to bone mineralization in pediatric nonalcoholic fatty liver disease. World J. Gastroenterol. 19 (25), 4007–4014 (2013).
Syn, W. K. et al. Osteopontin is induced by Hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology 53 (1), 106–115 (2011).
Chen, C. H., Lin, C. L. & Kao, C. H. Association between chronic hepatitis B virus infection and risk of osteoporosis: A nationwide Population-Based study. Med. (Baltim). 94 (50), e2276 (2015).
Wong, G. L. et al. Long-term safety of oral nucleos(t)ide analogs for patients with chronic hepatitis B: A cohort study of 53,500 subjects. Hepatology 62 (3), 684–693 (2015).
Grigsby, I. F., Pham, L., Mansky, L. M., Gopalakrishnan, R. & Mansky, K. C. Tenofovir-associated bone density loss. Ther. Clin. Risk Manag. 6, 41–47 (2010).
Tien, C. et al. Long-term treatment with Tenofovir in Asian-American chronic hepatitis B patients is associated with abnormal renal phosphate handling. Dig. Dis. Sci. 60 (2), 566–572 (2015).
Yusuf, Y., Ramazan, K., Fatih, E. & Nese, I. Serum osteocalcin levels in patients with nonalcoholic fatty liver disease: association with ballooning degeneration. Scand. J. Clin. Lab. Investig. 71(8), 631 (2011).
Sinn, D. H. et al. Association between serum osteocalcin levels and non-alcoholic fatty liver disease in women. Digestion 91 (2), 150–157 (2015).
Hou, J. et al. Chinese society of Hepatology, C.M.A. Chinese society of infectious Diseases, guideline of prevention and treatment for chronic hepatitis B (2015 Update). J. Clin. Transl Hepatol. 5 (4), 297–318 (2017).
Schmid, P. et al. H.I.V.C.S. Swiss, progression of liver fibrosis in HIV/HCV Co-Infection: A comparison between Non-Invasive assessment methods and liver biopsy. PLoS One. 10 (9), e0138838 (2015).
Ferraioli, G., Soares, L. B. & Monteiro Ultrasound-based techniques for the diagnosis of liver steatosis. World J. Gastroenterol. 25 (40), 6053–6062 (2019).
Fabrellas, N. et al. Prevalence of hepatic steatosis as assessed by controlled Attenuation parameter (CAP) in subjects with metabolic risk factors in primary care. A population-based study. PLoS One. 13 (9), e0200656 (2018).
Ducy, P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 54 (6), 1291–1297 (2011).
Zhou, M. et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur. J. Endocrinol. 161 (5), 723–729 (2009).
Perry, R. J., Samuel, V. T., Petersen, K. F. & Shulman, G. I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510 (7503), 84–91 (2014).
Hwang, Y. C., Jeong, I. K., Ahn, K. J. & Chung, H. Y. Circulating osteocalcin level is associated with improved glucose tolerance, insulin secretion and sensitivity independent of the plasma adiponectin level. Osteoporos. Int. 23 (4), 1337–1342 (2012).
Liu, J. J. et al. Relationship between serum osteocalcin levels and non-alcoholic fatty liver disease in adult males, South China. Int. J. Mol. Sci. 14 (10), 19782–19791 (2013).
Dou, J. et al. Relationship between serum osteocalcin levels and non-alcoholic fatty liver disease in Chinese men. Clin. Exp. Pharmacol. Physiol. 40 (4), 282–288 (2013).
Pramojanee, S. N., Phimphilai, M., Kumphune, S., Chattipakorn, N. & Chattipakorn, S. C. Decreased jaw bone density and osteoblastic insulin signaling in a model of obesity. J. Dent. Res. 92 (6), 560–565 (2013).
Li, H. et al. Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4 + and CD8 + T cells. Blood 116 (2), 210–217 (2010).
Soysa, N. S. & Alles, N. NF-kappaB functions in osteoclasts. Biochem. Biophys. Res. Commun. 378 (1), 1–5 (2009).
Nakchbandi, I. A. & van der Merwe, S. W. Current Understanding of osteoporosis associated with liver disease. Nat. Rev. Gastroenterol. Hepatol. 6 (11), 660–670 (2009).
Lenchik, L. et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33 (4), 646–651 (2003).
Gill, U. S. et al. Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: can the fracture risk assessment tool identify those at greatest risk? J. Infect. Dis. 211 (3), 374–382 (2015).
Guner, R., Kocak Tufan, Z., Yilmaz, G. R. & Mehmet, A. T. Tenofovir disoproxil fumarate May not cause renal and bone toxicity in chronic hepatitis B patients: a retrospective cross-sectional study. Turk. J. Med. Sci. 49 (1), 451–452 (2019).
Kahraman, R. et al. Effects of Long-Term Tenofovir and Entecavir treatment on bone mineral density in patients with chronic hepatitis B. Turk. J. Gastroenterol. 33 (1), 35–43 (2022).
Acknowledgements
We would like to thank the participants in this study for their cooperation. We thank the academic and non-academic staff of Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine for their help. We also would like to thank Editage (www.editage.cn) for English language editing.
Author information
Authors and Affiliations
Contributions
**Yanting He** participated in the acquisition, analysis of the data, preparing the outline of the manuscript, and drafting the initial manuscript. **Lijun Tong** was taking responsibility for the literature review, drafting the initial manuscript. **Yuge Zhou** participated in the acquisition, analysis of the data, and presentation of the results. **Fang Xie** participated in preparing the outline of the manuscript and drafting the initial manuscript. **Ning Tian** supervised the project process, and revised the article critically for important intellectual content. **Weining Xie** designed the study, constructed the research ideas and interpreted the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics and consent
Due to the retrospective nature of the study, (full name of IRB) waived the need of obtaining informed consent. Our research was approved by the Ethics Committee of Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine (Approval number: 2018 − 1254), and it strictly adhered to the principles outlined in the Declaration of Helsinki. Due to the retrospective, observational, and anonymous characteristics of the study, the requirement for obtaining written informed consent from participants was formally waived by the Ethics Committee of Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, Y., Tong, L., Zhou, Y. et al. Serum osteocalcin is associated with the presence of hepatic steatosis in Chinese patients with chronic hepatitis B. Sci Rep 15, 41641 (2025). https://doi.org/10.1038/s41598-025-25625-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25625-w