Abstract
Melatonin receptor (MTR) plays a crucial role in mediating melatonin signaling. Therefore, it is necessary to study functions and characteristics of MTR. In this study, full length cDNA of MTRI in Procambarus clarkii was cloned. The phylogenetic tree revealed that MTRI amino acid sequence of P. clarkii had the closest relationship with Homarus americanus. The temporal and spatial distribution expression results indicated that MTRI was highly expressed in gonadal tissues and eyestalk. RNA interference (RNAi) was used to identify the content of related hormones and the relative expression of genes in gonads of P. clarkii. The results showed that melatonin had an important effect on gonad of P. clarkii. Due to the specificity of the reproductive system in female and male crayfish, the siRNA interference efficiency was different, GIH, GTH, 5-HT and MT showed the opposite trend. Levels decreased in females but increased in males. However, the sex hormones E2 and T decreased, indicating that the silencing of MTRI will hinder gonadal development. This study provides a theoretical basis for further study of the role of melatonin in crustacean gonads.
Similar content being viewed by others
Introduction
Melatonin (MT) is a hormone widely found in various organisms1. In mammals, melatonin is secreted by the pineal gland and retina. Melatonin exerts biological effects by activating receptors to regulate the animal’s biological clock, movement, body temperature and reproduction2,3. Melatonin biosynthesis has four main steps: firstly, tryptophan hydroxylation reaction to produce 5-hydroxytryptophan (5-HTP). Secondly 5-HTP decarboxylation to produce 5-hydroxytryptamine (5-HT), then converted into N-acetyl-5-hydroxytryptamine (NAS). Finally, it is methylation to produce melatonin. Crustaceans do not have pineal gland but found melatonin in their bodies, involved in regulating their immune, antioxidants and reproduction4,5,6.
Melatonin receptors (MTR) exhibit high binding specificity for melatonin7. Melatonin binds to membrane-associated MTR upon entering the extracellular fluid, forming a hormone-receptor complex8. This specific binding is determined by molecular structures of melatonin and melatonin receptor. The indole ring, methoxy and other groups in melatonin interact with the binding site of the melatonin receptor to form a stable complex9. Currently identified MTR are all G protein-coupled receptors with 7 transmembrane domains10. Melatonin receptor mainly activates the downstream signal transduction pathway by coupling with G protein. These pathways further regulate gene expression, protein synthesis, and metabolic processes, controlling an organism’s physiological functions. Previous studies about melatonin mainly focused on mammals, and showed potential physiological functions and clinical application value in many aspects, but its mechanism in crustaceans remains to be studied.
Melatonin serves as a critical regulator of growth and reproduction in diverse animal species. Extensive research has established the involvement of multiple hormones in crustacean development, including gonadotropin-releasing hormone (GnRH), Gonadotropin (GTH), Gonad-Inhibiting Hormone (GIH), progesterone (P) and testosterone (T)11. Studies have shown that synthetic GnRH can shorten the ovarian maturation and oviposition cycle of Penaeus monodon and Macrobrachium rosenbergii12,13. It has been reported in Macrobrachium kistnesis, Parapenaeopsis hardwickiin and Potamon dehaani that GTH is secreted by the cerebral ganglia of crustaceans and induces ovarian growth14,15,16. Treerattrakool et al. knocked out the GIH gene from the nerve tissues of P.monodon. GIH knockout led to a significant increase in the level of Vg transcript in the shrimp ovary17. Emerging evidence highlights melatonin’s multifaceted role in ovarian physiology, including steroid production, follicular development, oocyte maturation and ovulation18. Ciani et al. found that temporal regulation of melatonin receptor expression has been observed in Salmo salar, where pituitary mRNA levels exhibit diurnal oscillations preceding gonadal development19. Khan and Thomas observed that the appropriate concentration of melatonin stimulates the pituitary gland of Micropogonias undulatus to release GTH to promote gonadal development20. Ying et al. found that melatonin in food is involved in the regulation of mTOR signaling pathway in Cherax damageor, regulating follicular growth, development and coordinating amino acid metabolism21. Fu et al. through transcriptome analysis of Eriocheir sinensis revealed that melatonin receptors are as important as other sex hormones for gonadal development and are involved in reproductive development22.
Studying the sex differences of animals is essential for understanding reproductive strategies and gonadal development mechanisms. The differences in physiology and behavior between male and female individuals reflect the adaptive differentiation of both sexes under reproductive and sexual selection pressures. The function of melatonin in vertebrate Hyla cinerea varies by sex23. The expression level of Larimichthys crocea MTR was significantly higher in males than in female24. The reproductive endocrine system of crustaceans is significantly different from that of vertebrates, and the regulatory mechanism of sex dimorphism has not been understood.
P. clarkii is an important freshwater economic crustacean in the world. China is the largest aquaculture and consumer country. P. clarkii is highly sensitive to environmental factors (such as temperature and hormone pollutants), and is suitable for studying reproductive regulation mechanisms. At the same time, its low breeding cost, convenient experimental operation, and the improvement of genomic data make it of great value in the study of reproductive biology, endocrinology and ecotoxicology of crustaceans. P. clarkii has become an ideal object for gonadal research because of its typical gonadal structure, short reproductive cycle and strong fecundity. Melatonin as a reproductive hormone, has an important effect on gonadal development by regulating the secretion level of related sex hormones in the body. Because there are few studies on the effect of melatonin on the gonadal development of P. clarkii. In this study, the melatonin receptor of P. clarkii was cloned for the first time, and its effect on gonad was preliminarily discussed. It provides a theoretical basis for the further study of P. clarkii.
Result
Sequence characteristics of MTRI
The full-length cDNA of MTRI in P. clarkii (GenBank accession number, PV159334.1) was 1082 bp. The 5’-UTR is 102 bp, an open reading frame (ORF) of 747 bp, encoding 248 amino acids, and a 3’-UTR of 233 bp (Fig. 1). The deduced amino acid sequence contains 18 negatively charged residues and 33 positively charged residues with an isoelectric point of 9.63. The predicted protein molecular weight is 37.962 KDa, the molecular formula is C1695H2694N478O468S22. The predicted MTRI protein is a hydrophobic protein (Fig. S1). The prediction diagram of the protein transmembrane region is provided in the supplementary file (Fig. S2). Secondary structure and tertiary structure prediction in supplementary file (Figs. S3 and S4). The conserved domain prediction found that the MTRI protein contained rhodopsin-like of the GPCR family.
MTRI amino acid homology comparison and phylogenetic tree construction
Through BLAST sequence alignment on NCBI, it was found that the amino acid sequence of MTRI in P. clarkii was highly similar to that of many crustaceans. The homology of Homarus americanus was 60.28% (XP_042216195.1), Penaeus japonicus was 56.94% (XP_042891455.1), Penaeus vannamei was 55.47% (ROT85071.1), Penaeus chinensis was 51.61% (XP_ 047,471,190.), Penaeus indicus was 51.56% (XP_063586197.1) (Fig. 2).
The phylogenetic tree constructed by MEGA11 revealed two primary evolutionary clades (Fig. 3). The amino acid sequence of MTRI in P. clarkii is clustered with many invertebrates such as crustaceans, and the genetic relationship is the closest. Other mollusks, fish, mammals and other vertebrates are clustered together, and the genetic relationship with P. clarkii is low. This is basically consistent with the traditional classification concept. These results indicated that the amino acid sequence of MTRI was most similar to Homarus americanus.
Temporal and spatial distribution expression
QRT-PCR results showed that the relative expression of MTRI was highest in testis, followed by ovary and eyestalk, and trace expression in heart and muscle (Fig. 4a). The relative expression of MTRI was the highest in stage III and the lowest in stage V of ovarian development. And the expression level in the early stages was significantly higher than that in the late stages (P < 0.05), indicating that MTRI plays an important role in the early stages of oocyte development (Fig. 4b).
(a) Relative expression of MTRI in different tissues of Procambarus clarkii. (b) Relative expression of MTRI in ovary at different stages of Procambarus clarkii. The data were presented as mean ± SD (n = 3). Different labels indicated that there were significant differences between different groups (P < 0.05).
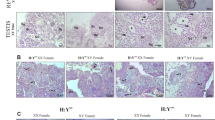
The results of in situ hybridization showed that MTRI detected a large number of mRNA positive signals in the testis and ovary. Early in gonadal MTRI is mainly expressed in the nucleus of oocytes (Fig. 5a–c), and expressed in the nucleus and cytoplasm of spermatocytes (Fig. 6a–c). It is expressed in the nucleus and the cell membrane at late stages of gonadal development, with significantly higher expression in the cell membrane than in the nucleus (Figs. 5d–f and 6d–f).
Changes of related hormones in gonads after MTRI interference
The concentration of GIH in the gonads of female crayfish was significantly higher (P < 0.05) than that of the negative control group before 96 h of MTRI silencing in P. clarkii, and the concentration was significantly lower after 120 h (P < 0.05) (Fig. 7a). The concentration of GTH, 5-HT, MT and E2 (Fig. 7b–e) remained significantly lower than controls until 96 h (P < 0.05), before returning to baseline levels by 120 h (P > 0.05). The concentration of GIH and T in the gonads of male crayfish was significantly lower (P < 0.05) than that of the negative control group before 96 h, and the concentration had no significant difference after 120 h (P < 0.05) (Fig. 8a,e). Compared with the negative control group, concentrations of GTH, 5-HT and MT were all significantly higher until 96 h (P < 0.05) (Fig. 8b–d).
Concentration of gonadal related hormones at different time points after injection of siRNA in female Procambarus clarkii. (a) Gonad-inhibiting Hormone, (b) Gonadotropic hormone, (c) Serotonin, (d) Melatonin, e: Estradiol. The data were presented as mean ± SD (n = 3). * indicates significant difference (P < 0.05). * * indicates that the difference is high significant (P < 0.01).
Concentration of gonadal related hormones at different time points after injection of siRNA in male Procambarus clarkii. (a) Gonad-inhibiting Hormone, (b) Gonadotropic hormone, (c) Serotonin, (d) Melatonin, (e) Testosterone. The data were presented as mean ± SD (n = 3). * indicates significant difference (P < 0.05). * * indicates that the difference is high significant (P < 0.01).
Changes of GIH and GTH contents in circulation after MTRI interference
The contents of GIH and GTH in the hemolymph of female crayfish were significantly lower than those in the control group before 96 h after MTRI silencing (P < 0.05) (Fig. 9a,b). The contents of GIH and GTH in the hemolymph of male crayfish were significantly higher than those in the control group (P < 0.05) before 96 h (Fig. 9c,d). After 120 h, the contents of these two hormones in female and male crayfish returned to normal levels. There was no significant difference compared with the control group (P > 0.05).
The content of GIH and GTH in hemolymph of Procambarus clarkii after injection of siRNA (a) GIH content of female crayfish, (b) GTH content of female crayfish, (c) GIH content of male crayfish, (d) GTH content of male crayfish. The data were presented as mean ± SD (n = 3). * indicates significant difference (P < 0.05). * * indicates that the difference is high significant (P < 0.01).
Changes of related genes in gonads after MTRI interference
The RNA interference efficiency of MTRI in P. clarkii persisted for 96 h, with complete recovery of gene expression observed by 120 h post-injection (Figs. 10a and 11a). The relative expression of Pcna, Dmc and Cyclin (Fig. 10b–d) was significantly lower (P < 0.05) in female crayfish until 96 h inject compared to negative controls. At 120 h injection, the expression of Pcna and Dmc was not significantly different from that of the control group (P > 0.05), and the expression of Cyclin was significantly higher than that of the control group (P < 0.05). The relative expression of Pcna, Dmc and Cyclin in male crayfish was significantly higher than that of the negative control until 96 h (P > 0.05) (Fig. 11b–d).
Changes in the relative expression of gonad-related genes at different time points after injection of siRNA in female Procambarus clarkii. (a) MTRI, (b) Pcna, (c) Dmc, (d) Cyclin. The data were presented as mean ± SD (n = 3). * indicates significant difference (P < 0.05). * * indicates that the difference is high significant (P < 0.01).
Changes in the relative expression of gonad-related genes at different time points after injection of siRNA in male Procambarus clarkii. (a) MTRI, (b) Pcna, (c) Dmc, (d) Cyclin. The data were presented as mean ± SD (n = 3). * indicates significant difference (P < 0.05). * * indicates that the difference is high significant (P < 0.01).
Discussion
Current research on MTR in crustaceans has mainly focuses on growth, immune and antioxidant related aspects4,5,6, with limited investigation of their gonadal functions. A large number of studies have shown that melatonin is an important reproductive hormone25, so it is necessary to study the effect of melatonin on the gonad of P. clarkii. Gene cloning is helpful to understand the structure and function of genes. Therefore, this study mainly cloned the cDNA sequence of MTRI from P. clarkii, and analyzed the physicochemical properties, secondary structure, tertiary structure and hydrophobicity of the deduced amino acid sequence by bioinformatics analysis. Domain analysis revealed that MTRI possesses a conserved rhodopsin-like domain characteristic of GPCR family members. The GPCR family is involved in reproduction, nutrition and other physiological regulation26. Its mediated signaling pathway stimulates the production of gonadal-associated steroid hormones27. In mammals, GPCR inactivation can cause complete reproductive failure28. In this study, the results of spatiotemporal expression analysis demonstrated that MTRI was highly expressed in gonadal tissues. The results of in situ hybridization showed that MTRI was revealed stage-specific subcellular localization patterns. It shows that MTRI are involved in different functions during gonadal development. In the early stage, MTRI is expressed in the nucleus, and the nucleus is the main place for gene transcription in the cell. At this time, MTRI regulates genes related to reproductive development in the nucleus. Late MTRI is transported to the cell membrane by translation modification in the nucleus to regulate cell maturation.
In aquaculture, the gonadal development cycle of crustaceans affects meat quality and reproduction. In order to study the role of MTR in the gonad of P. clarkii, we employed RNAi to assess its effects on sex hormone regulation and gene expression. The results of this study showed that GIH in the gonads of female P. clarkii increased after MTRI silencing, while other hormones: GTH, 5-HT and MT showed a downward trend. GIH is a kind of neuropeptide that inhibits the gonadal maturation of crustaceans, which can directly or indirectly affect the gonadal development and reproduction of crustaceans29. The living environment of P. clarkii is complex and changeable, and its physiological state needs to be flexibly adjusted according to the environment and its own conditions30,31. Han et al. found that under the stress of harmful substances, GIH in Litopenaeus vannamei was significantly up-regulated, and endocrine regulation was affected32. We hypothesize that after MTRI silencing, the P. clarkii perceives that the current environment or physiological state is not suitable for the normal development of the gonads. Since gonadal development and reproduction require a lot of energy, thus increasing the secretion of GIH to inhibit gonadal development to ensure that individuals in this mutation situation, more energy is used to maintain other important physiological functions such as survival, and other hormones that promote ovarian development also show a decreasing trend. This possibility is supported by a large number of experimental results. After changing the original eating habits of P. clarkii, it can affect its gonadal development and reproductive status33. The expression level of related genes in the gonad of P. clarkii after drought stress was significantly reduced, which was consistent with the gene expression level of female P. clarkii in this experiment34. The freshwater prawn under the stress of microplastics not only has a significant effect on the expression of steroid genes in the gonads of parental shrimp, but also has cross-generational toxicity35. Collectively, these results suggest that MTRI plays a pivotal role in mediating the trade-off between reproduction and stress adaptation in P. clarkii.
GTH primarily mediates its effects through upstream gonadotropin-releasing hormone (GnRH) signaling, a key pathway promoting ovarian development in crustaceans36. Some scholars have reported that MT is an important factor in regulating GnIH and GnRH in fish, and found that the expression of MT will decrease after the increase of GnRH in Amphiprion melanopus, with elevated GnRH expression correlating negatively with MT levels37. This is consistent with the results of our study on male P. clarkii. After reducing the expression of MTRI by siRNA, GTH increased. As the precursor substance of MT synthesis, 5-HT regulates endogenous melatonin levels in organisms. The results of this study showed that 5-HT showed an upward trend after the MTRI of male P. clarkii was interfered. Elevated 5-HT levels lead to increased melatonin synthesis. However, MTRI interference prevents melatonin from binding to its receptors, thereby disrupting gonadal development in P. clarkii. In the study of Mystus cavasius, it was also confirmed that 5-HT and melatonin can interfere with gonadal development38.
The changes of GIH and GTH contents in hemolymph were dynamically correlated with the hormone levels in gonads. In female crayfish, hemolymph GIH decreased significantly before 96 h. However, the gonadal GIH is temporarily increased due to receptor-mediated endocytosis retention effect, forming a reverse gradient of reduced hemolymph supply and local accumulation of gonads. At the same time, the synchronous decrease of hemolymph GTH directly leads to the decrease of gonad GTH and inhibits ovarian development. So E2 goes down. On the contrary, in male crayfish, incomplete MTR silencing may trigger a compensatory mechanism to increase the level of GTH in the hemolymph, while the transient increase of GIH in the hemolymph accelerates the receptor binding and degradation of GIH in the gonad, showing an increased hemolymph and gonad consumption pattern. This difference between hemolymph and gonadal hormones indicates that MTR plays a pivotal role in coordinating endocrine and target organ communication—not only regulating the systemic transport of hormones, but also affecting the local response of gonads to hemolymph signals, eventually leading to differential regulation of sexual reproduction strategies. Female conservative inhibition, male stress activation.
Pcna and Cyclin are critical regulators of cell proliferation and differentiation. After melatonin binds to the melatonin receptor, the transcriptional activity of the Pcna is enhanced by activating the protein kinase C (PKC) pathway, thereby upregulating the expression of Pcna39. However, the expression of Pcna was inhibited under stress conditions40. Cyclin functions by binding to its cognate cyclin-dependent kinase (CDK) to form an active complex. Due to the large amount of free radicals produced in the ovary during ovulation, the increase of melatonin level in the follicle before ovulation may protect the oocyte from the influence of free radicals. Melatonin, as a free radical scavenger, plays an important role in the physiological process of oocyte maturation by maintaining a suitable redox state and ensuring that the Cyclin-CDK complex can play a normal role41.
This study revealed sexually dimorphic responses to MTRI knockdown in P. clarkii. While female crayfish exhibited decreased hormone levels and gene expression, males showed opposite trends. However, the sex hormones E2 and T showed a downward trend. This may be due to the interference efficiency of siRNA. In this study, the interference efficiency of female crayfish was higher than that of male crayfish. Oocyte development requires a large amount of material exchange, which enhances the endocytosis efficiency of siRNA42. Male germ cells are wrapped by dense accessory cells, hindering the delivery of siRNA43. After female crayfish were interfered, the secretion of central GTH decreased, resulting in a decrease in sex hormone synthesis. The decrease of sex hormone relieved the negative feedback regulation of GIH, leading to elevated GIH. In male crayfish, the interference efficiency is lower than that of female crayfish, and this low-dose interference activates the compensatory mechanism in male crayfish, so it has the opposite trend with female crayfish. Despite the opposite trend in central hormones, sex hormones declined in both sexes. In summary, MTRI silencing can hinder gonadal development of P. clarkii.
Material and methods
Animals
Healthy and vigorous P. clarkii (body weight 18 ± 1.5 g, body length 7.5 ± 0.5 cm) were purchased from Xuyi Crayfish Industry Group (Huai’an, China). They were acclimation in pump oxygen aquarium (0.7 × 0.35 × 0.25 m) of the Aquatic Breeding Laboratory of Huaiyin Normal University. During acclimation, the water temperature, DO and pH were 24.5 ± 0.3 °C, 6.5 ± 0.5 mg/L and 7.5 ± 0.2 respectively, and the photoperiod was 12:12 (light: dark). Special compound feed (crude protein 42%, crude lipid 3.5%, crude fiber 4.6%, crude ash 10.8%) for crayfish at 10 a.m. and 6 p.m. every day. After 2 h of feeding every afternoon, feces are siphoned off to clean and replace half of the water.
Sample collection, total RNA extraction and cDNA synthesis
After 2 weeks acclimation, randomly selected crayfish and placed on ice for anesthesia. Extraction of eyestalk, hepatopancreas, gill, muscle, ovary, testis and mandibular tissues. In addition, P. clarkii at different developmental stages were selected, and ovary tissues at different stages were extracted44. Placed tissues in1.5 mL centrifuge tube stored at −80 °C for subsequent analysis. Hemolymph was extracted from the base of the third appendage of the crayfish with a 1 ml sterile syringe and anticoagulant was added at 1:1 (EDTA-Na2/K2). For elisa analysis.
The total RNA of P. clarkii was extracted using Trizol reagent (Sangon Biotech Shanghai Co., Ltd.) Purity of RNA was detected by micro-ultraviolet spectrophotometer (Gene Company Limi ed.), the purity of all samples was between 1.8 and 2.0. Double-stranded cDNA was synthesized by reverse transcription using the ToloScript ALL-in-one RT EasyMix for qPCR kit (Tolo Harbour Biotechnology Co., Ltd. Shanghai, China). CDNA was stored at −20 °C for subsequent analysis.
MTRI gene cloning
The 3′- and 5′- ends of the MTRI cDNA were cloned by rapid amplification of cDNA ends (RACE), the primers used in the experiment were provided by Wuhan Aikangjian Biotechnology Co., Ltd (Table 1). The amplification system is as follows: Icongene PCR Mix 16 uL, Primer-F (10 um) 1 uL, Primer-R (10 um) 1 uL, cDNA sample (25 ng/uL) 2 uL. The amplification procedure was as follows: predenaturation at 95 °C for 10 min, amplification at 95 °C for 15 s, amplification at 55 °C for 15 s, amplification at 72 °C for 90 s, 35 cycles of amplification, final 72 °C for 2 min, termination at 4 °C. The PCR products were detected by agarose gel electrophoresis. The PCR sample purification kit was used to purify the sample for sequencing.
MTRI bioinformatics analysis
Use the NCBI (https://www.ncbi.nlm.nih.gov/orffinder/) online website to find the MTRI open reading frame. The MTRI sequence was translated into protein sequences using DNAMAN9.0. The physical and chemical properties, isoelectric point and molecular weight of MTRI protein were analyzed by ExPasy (https://www.expasy.org/). The online tool NovoPro (https://www.novopro.cn/tools/protein-hydrophilicity-plot.html) was used to predict the hydrophilicity or hydrophobicity. The secondary structure of MTRI protein was analyzed using Prabi (https://predictprotein.org/). Domain analysis was performed using InterPro (https://www.ebi.ac.uk/interpro/). NCBI Blast was used to search the amino acid sequence of MTRI homology, and Genedoc software was used to analyze the multiple sequence of amino acids. The phylogenetic tree was constructed using MEGA11 software.
In situ hybridization
In situ hybridization probes were designed according to the ORF region of MTRI, the probe was synthesized by Thermo Fisher Scientific (Table 1). Some gonadal tissues were fixed in in situ hybridization solution at room temperature for 24 h for sectioning. After dehydration, transparency, embedding, cut into 5 μm tissue sections and dewaxing pre-hybridization. After being repaired, digested, hybridized, washed, reconstituted, dehydrated and sealed, the sections of gonadal in situ hybridization with sense and antisense probes were observed under microscope, and the images were collected and analyzed.
MTRI interference experiment
According to the cloned complete sequence of MTRI, siRNA was designed and synthesized for Wuhan Jinkairui Bioengineering Co., Ltd (Table 1). According to the instructions, DEPC water was used to configure siRNA. Two combinations of female and male P. clarkii were set up, each combination was repeated three times, each repeated 20 P. clarkii, 120 P. clarkii in total (60 female crayfish, 60 male crayfish). The configured siRNA was injected from the pericardium and placed in a pump oxygen aquarium (0.7 × 0.35 × 0.25 m). The breeding environment and feeding time were consistent with domestication. At 24 h, 48 h, 72 h, 96 h and 120 h, three P clarkii were randomly selected and placed on ice for anesthesia. The gonadal tissue was dissected, placed in 1.5 mL centrifuge tube and stored at −80 °C for subsequent analysis.
Estimation of hormones using ELISA
Tissue samples were processed according to the instructions in the ELISA kit. The hemolymph samples were centrifuged at 4 °C, 2000 rpm for 20 min, and the supernatant was taken for determination. The crayfish gonad tissue was accurately weighed (g), and 9 times the volume of PBS (ml) was added and ground on a homogenizer to obtain tissue homogenate. The tissue homogenate was centrifuged at 4 °C, 1200 rpm for 20 min, and the supernatant was taken for hormone determination. GTH (gonadotropic hormone), GIH (gonad-inhibiting hormone), MT (melatonin), 5-HT (serotonin) were determined by ELISA kit (Shanghai Yuanju Biological Co., Ltd. China. Product numbers are YJ872210, YJ8447223, YJ884185, YJ847228). E2 (estradiol) and T (testosterone) were determined by ELISA kit (Shanghai Enzyme linked Biological Co., Ltd. China. Product numbers are ml855268L, ml103518). Samples and standard solutions were accurately added to the monoclonal antibody-coated microplate. Then sealed with plate-sealing film and incubated at 37 °C for 30 min. After incubation, the plate was washed with prepared washing buffer for 5 times. The enzyme-linked reagent (HRP) was added and incubated at 37 °C for 30 min. Repeat the washing operation. Add chromogenic substrate (TMB) to each well, mix well and avoid light for 10 min. Finally, the stop solution was added and the absorbance of each hole was measured at 450 nm45,46,47.
Quantitation real-time PCR
Primers were designed by Primer Premier 5.0 and synthesized by Shenggong Bioengineering Co., Ltd. (China, Shanghai) (Table 1). Real-time PCR was performed using NovoStart SYBR Qpcr SuperMix Plus kit (Vazyme Biotechnology Co., Ltd., Nanjing, China) two-step amplification method. The reaction procedure is as follows: reaction at 95 °C for 1 min, cycles at 95 °C 20 s and 60 °C for 1 min for 36 times. The data were processed and analyzed with 2−ΔΔCt, and three replicates were set for each group.
Statistics
The experimental data were expressed as mean ± standard deviation (mean ± SD). One-way ANOVA and independent sample t-test were performed using SPSS 27.0 statistical software. Duncan’s was used for multiple comparison, and Leven’s was used to test the homogeneity of variance. Significance level was set to P < 0.05. Graphpad Prism 9.5 was used to draw figures.
Conclusion
In summary, this study cloned the full-length cDNA sequence of the MTRI of P. clarkii, analyzed its structure and characteristics using related bioinformatics. Through the temporal and spatial distribution of expression, it was found that the relative expression of MTRI was the highest in gonadal tissue. It mainly exists in the nucleus and cell membrane of gonadal tissue. And verified that MTRI had a certain effect on the gonads of P. clarkii by RNA interference. The results of this study showed that the interference efficiency was different due to the specificity of the reproductive system of female and male crayfish, and the opposite trend was observed. However, the interference of MTRI hinders the development of gonads. Therefore, the results of this study promote our understanding of MTR and provide a theoretical basis for future research on the effects of melatonin on crustaceans.
Data availability
All data generated or analysed during this study are included in this article and its Supplementary Information files. The sequence information used in this article has been uploaded to the GenBank with the accession number PV159334.1. The datasets generated and analyzed during the current study are available in the [NCBI] repository, (https://www.ncbi.nlm.nih.gov/nuccore/PV159334.1/).
References
Liu, J. J. et al. Research progress in the regulation of follicle development by melatonin. Acta Academiae Medicinae Sinicae. 45(6), 19971004. https://doi.org/10.3881/J.ISSN.1000-503X.15473 (2023).
Kim, P. et al. Melatonin’s role in the timing of sleep onset is conserved in nocturnal mice. Sci. Rep-UK 1(1), 13–13. https://doi.org/10.1038/S44323-024-00013-1 (2024).
Clyne, M. et al. Male factor infertility: Melatonin could offer protection against testicular damage caused by a high-fat diet. Nat. Rev. Urol. 9(3), 1121. https://doi.org/10.1038/nrurol.2012.17 (2012).
Márcio, A. G. et al. Effect of melatonin in the antioxidant defense system in the locomotor muscles of the estuarine crab Neohelice granulata (Decapoda, Brachyura). Gen Comp. Endocrinol. 166, 72–82. https://doi.org/10.1016/j.ygcen.2009.09.018 (2009).
Fábio, E. M. et al. Melatonin as a signaling molecule for metabolism regulation in response to hypoxia in the crab Neohelice granulata. Int. J. Mol. Sci. 15(12), 22405–22420. https://doi.org/10.3390/ijms151222405 (2014).
Han, Z. B., Li, X. D., Li, X., Xu, W. B. & Li, Y. D. Circadian rhythms of melatonin in haemolymph and optic lobes of Chinese mitten crab (Eriocheir sinensis) and Chinese grass shrimp (Palaemonetes sinensis). Biol. Rhythm Res. 50(3), 400–407. https://doi.org/10.1080/09291016.2018.1452592 (2019).
Hiroyuki, H. O., Erika, C., Osamu, N., Slivia, R. & Ralf, J. Melatonin receptor structure and signaling. J. Pineal Res. 76(3), 12952–12952. https://doi.org/10.1111/jpi.12952 (2024).
Hattori, A. & Suzuki, N. Receptor and receptor-independent actions of melatonin in vertebrates. Zool. Sci. 41(1), 105–116. https://doi.org/10.2108/zs230057 (2024).
Dong, B. Y., Da, F. F., Chen, Y. L. & Ding, X. C. Melatonin treatment maintains the quality of fresh-cut Gastrodia elata under low-temperature conditions by regulating reactive oxygen species metabolism and phenylpropanoid pathway. Int. J. Mol. Sci. 24(18), 14284. https://doi.org/10.3390/ijms241814284 (2023).
Heward, C. B. & Hadley, M. E. Structure-activity relationships of melatonin and related indoleamines. Life Sci. 17(7), 1167–1177. https://doi.org/10.1016/0024-3205(75)90340-9 (1975).
Cai, P. F. et al. Insulin-like androgenic gland hormone induced sex reversal and molecular pathways in Macrobrachium nipponense: Insights into reproduction, growth, and sex differentiation. Int. J. Mol. Sci. 24(18), 14306. https://doi.org/10.3390/ijms241814306 (2023).
Ngernsoungnern, A. et al. The existence of gonadotropin-releasing hormone (GnRH) immunoreactivity in the ovary and the effects of GnRHs on the ovarian maturation in the black tiger shrimp Penaeus monodon. Aquaculture 279(1–4), 197–203. https://doi.org/10.1016/j.aquaculture.2008.04.018 (2008).
Ngernsoungnern, A. et al. The identification and distribution of gonadotropin-releasing hormone-like peptides in the central nervous system and ovary of the giant freshwater prawn Macrobrachium rosenbergii. Invertebr. Neurosci. 8(1), 49–57. https://doi.org/10.1007/s10158-008-0067-5 (2008).
Sarojini, R. et al. Bihormonalcontrol of oogenesis in the freshwater prawn Macrobrachium kistnensis. Acta Physiol. Hung. 61, 5–10. https://doi.org/10.1007/BF02464415 (1983).
Kulkarni, G. et al. Effect of progesterone on ovarian maturation in a marine penaeid prawn Parapenaeopsis hardwickii (Miers, 1878). Indian J. Exp. Biol. 17, 986–987 (1979).
Otsu, T. Bihormonal control of sexual cycle in the freshwater crab Potamon dehaani. Embryologia. 8, 1–20 (1963).
Treerattrakool, S. et al. Molecular characterization of gonad-inhib-iting hormone of Penaeus monodon and elucidation of itsinhibitory role in vitellogenin expression by RNA interference. Febs. J. 275, 970–980. https://doi.org/10.1111/j.1742-4658.2008.06266.x (2008).
Wang, X. M. et al. Melatonin stimulates STAR expression and progesterone production via activation of the PI3K/AKT pathway in bovine theca cells. Int. J. Biol. Sci. 15(2), 404–415. https://doi.org/10.7150/ijbs.27912 (2019).
Ciani, E. et al. Melatonin receptors in Atlantic salmon stimulate cAMP levels in heterologous cell lines and show season-dependent daily variations in pituitary expression levels. J. Pineal Res. 67(3), e12590. https://doi.org/10.1111/jpi.12590 (2019).
Khan, I. A. & Thomas, P. Melatonin influences gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus). Gen. Comp. Endocrinol. 104(2), 231–242. https://doi.org/10.1006/gcen.1996.0166 (1996).
Yang, Y. et al. Effects of dietary melatonin on growth performance, antioxidant capacity, and nonspecific immunity in crayfish Cherax destructor. Fish Shellfish Immunol. 138, 108846. https://doi.org/10.1016/j.fsi.2023.108846 (2023).
Fu, C. P. et al. Comparative transcriptome analysis reveals related regulatory mechanisms of androgenic gland in Eriocheir sinensis. Biomed Res. Int. https://doi.org/10.1155/2017/4956216 (2017).
Lutterschmidt, D. I. & Wilczynski, W. Sexually dimorphic effects of melatonin on brain arginine vasotocin immunoreactivity in green treefrogs (Hyla cinerea). Brain Behav. Evol. 80(3), 222–232. https://doi.org/10.1159/000341238 (2012).
Xu, D. L. et al. Functional Involvement of Melatonin and Its Receptors in Reproductive Regulation of the Marine Teleost, Large Yellow Croaker (Larimichthys crocea). Fishes. 10(1), 28–28. https://doi.org/10.3390/FISHES10010028 (2025).
Cheol, Y. C. et al. Time-related effects of various LED light spectra on reproductive hormones in the brain of the goldfish Carassius auratus. Biol. Rhythm Res. 46(5), 671–682. https://doi.org/10.1080/09291016.2015.1046247 (2015).
Artigas, G. Q., Lapébie, P., Leclère, L., Bauknecht, P. & Houliston, E. A G protein–coupled receptor mediates neuropeptide-induced oocyte maturation in the jellyfish Clytia. PLos Biol. 10, 801225. https://doi.org/10.1371/journal.pbio.3000614 (2020).
Dilip, M. et al. Membrane receptor cross talk in gonadotropin-, IGF-I-, and insulin-mediated steroidogenesis in fish ovary: An overview. Gen. Comp. Endocrinol. 240(1), 10–18. https://doi.org/10.1016/j.ygcen.2016.09.002 (2017).
Tarryn, R., Ross, C. A., Robert, P. M. & Claire, L. N. Restoring function to inactivating G protein-coupled receptor variants in the hypothalamic-pituitary-gonadal axis. J. Neuroendocrinol. 36(9), 13418–13418. https://doi.org/10.1111/jne.13418 (2024).
Tong, C. et al. Characterization of gonadal development phases and maturation mechanisms in male Pacific whiteleg shrimp (Litopenaeus vannamei). Aquaculture 584(15), 669–740. https://doi.org/10.1016/J.AQUACULTURE.2024.740669 (2024).
Chen, Q. L. et al. Differential effect of waterborne cadmium exposure on lipid metabolism in liver and muscle of yellow catfish Pelteobagrus fulvidraco. Aquat. Toxicol. 142–143, 380–386. https://doi.org/10.1016/j.aquatox.2013.09.011 (2013).
Liu, Z. et al. Effects of cadmium on lipid metabolism in female estuarine crab, Chiromantes dehaani. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 188, 9–16. https://doi.org/10.1016/j.cbpc.2016.06.002 (2016).
Han, Y. et al. Microplastics and bisphenol A hamper gonadal development of whiteleg shrimp (Litopenaeus vannamei) by interfering with metabolism and disrupting hormone regulation. Sci. Total Environ. 810, 152354. https://doi.org/10.1016/j.scitotenv.2021.152354 (2021).
Tian, Y. K. et al. Effects of intermittent fasting on growth, metabolism and stress resistance in freshwater crayfish (Procambarus clarkii). Aquacult. Rep. 38, 102275. https://doi.org/10.1016/J.AQREP.2024.102275 (2024).
Su, Y. et al. Effect of drought stress on the growth, ovary development, antioxidant capacity, sex hormone content, and reproductive-related gene expression of red swamp crayfish (Procambarus clarkii). Aquacult. Rep. 38, 102352. https://doi.org/10.1016/J.AQREP.2024.102352 (2024).
Sun, S. G., Jin, Y. T., Luo, P. H. & Shi, X. T. Polystyrene microplastics induced male reproductive toxicity and transgenerational effects in freshwater prawn. Sci. Total Environ. 842(10), 156820. https://doi.org/10.1016/j.scitotenv.2022.156820 (2022).
Guan, Z. B., Shui, Y., Liao, X. R., Xu, Z. H. & Zhou, X. Primary structure of a novel gonadotropin-releasing hormone (GnRH) in the ovary of red swamp crayfish Procambarus clarkii. Aquaculture 418–419, 67–71. https://doi.org/10.1016/j.aquaculture.2013.10.010 (2014).
Young, J. C., Na, N. K., Hamid, R. H. & Cheol, Y. C. Effects of gonadotropin inhibitory hormone or gonadotropin-releasing hormone on reproduction-related genes in the protandrous cinnamon clownfish Amphiprion melanopus. Gen. Comp. Endocr. 235, 89–99. https://doi.org/10.1016/j.ygcen.2016.06.010 (2016).
Muhammad, B., Taro, I., Rohul, A. & Md, S. Melatonin inhibits reproductive activity through changes of serotonergic activity in the brain of freshwater catfish (Mystus cavasius). Aquaculture 526(15), 735378. https://doi.org/10.1016/j.aquaculture.2020.735378 (2020).
Gallol, L. E., Busolini, F. I. & Mohamed, F. H. Influence of melatonin and sexual hormones on the expression of proliferating cell nuclear antigen in the adrenal cortex of a seasonal breeder (Lagostomus maximus). Anat. Rec. (Hoboken) 303(12), 3052–3067. https://doi.org/10.1002/ar.24457 (2007).
Teng, Z. et al. Melatonin alleviates oxidative damage in mouse spermatogenesis and sperm quality parameters induced by exposure to Bisphenol A. Ecotox. Environ. Safe. 253, 114709. https://doi.org/10.1016/J.ECOENV.2023.114709 (2023).
Tamura, H. et al. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 92(1), 328–43. https://doi.org/10.1016/j.fertnstert.2008.05.016 (2009).
Yang, H. et al. Selenium mitigated cadmium-induced ovarian retardation in female Procambarus clarkii by regulating vitellogenin synthesis and transfer in the hepatopancreas. Ecotox. Environ. Safe. 288(2024), 117339. https://doi.org/10.1016/J.ECOENV.2024.117339 (2024).
Federica, P. et al. Managing of Procambarus clarkii by X-ray sterilisation of male: Cytological damage to gonads. Micron 77(2015), 32–40. https://doi.org/10.1016/j.micron.2015.05.016 (2015).
David, M. H. Biology of Freshwater Crayfish (School of life and Environmental Sciences, University of Nottingham, UK, 2002).
Salwa, A. H. et al. Biological, biochemical, hormonal effect, and histological perturbations of Procambarus clarkii (Crustacea: Cambaridae) hemolymph on Biomphalaria alexandrina snails. Aquacult. Int. 33(5), 328–328. https://doi.org/10.1016/10.1007/S10499-025-01956-3 (2025).
Merlin, J. R. et al. Vertebrate-type steroid profile in different tissues of wild and endocrinologically manipulated female brood stocks of Penaeus monodon. Curr. Sci. India. 111(7), 1194–1200. https://doi.org/10.1016/10.18520/cs/v111/i7/1194-1200 (2016).
Epro, B. et al. Impact of photoperiod and temperature on melatonin, growth hormone, estradiol, and vitellogenin levels in female African catfish (Clarias gariepinus) reproduction cycle. Reprod. Breed. 5(1), 54–66. https://doi.org/10.1016/J.REPBRE.2025.01.002 (2025).
Acknowledgements
This study was funded by Jiangsu Provincial Agricultural Science and Technology Independent Innovation Fund (CX223082), Jiangsu Collaborative Innovation Center of Regional Modern Agriculture & Environmental Protection, Huaiyin Normal University (HSXT30312) and Science Technology Program of Huaiyin Normal University (31WH000).
Funding
The project was funded by the Jiangsu Provincial Agricultural Science and Technology Independent Innovation Fund (Grant No. CX223082), Jiangsu Collaborative Innovation Center of Regional Modern Agriculture & Environmental Protection, Huaiyin Normal University (Grant No. HSXT30312), Science Technology Program of Huaiyin Normal University (Grant No. 31WH000).
Author information
Authors and Affiliations
Contributions
M.H.: investigation, data analysis, methodology, writing-original draft. L.W.: conceptualization, supervision. Q.Z: data curation, writing-review. T.G.: data curation, writing-review. Y.W.: data analysis. L.C.: methodology. H.W.: conceptualization, funding acquisition, software, supervision. J.L.: supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study is approved by the Research Ethics Committee of Huaiyin Normal University (20240107001A).
Consent to participate
The authors declare that they consent to participate in the work.
Consent for publication
The authors declare that they consent to publish this paper.
Authorship
I have read the Nature Portfolio journal policies on author responsibilities and submit this manuscript in accordance with those policies.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, M., Wang, L., Zhu, Q. et al. Gender-specific function of melatonin receptor (MTRI) in gonads of red swamp crayfish, Procambarus clarkii. Sci Rep 15, 43426 (2025). https://doi.org/10.1038/s41598-025-25664-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25664-3