Abstract
Manganese oxide (MnOx)-based nanozymes have attracted growing interest due to their low cost, high catalytic robustness, and adjustable oxidation states that influence enzyme-mimicking performance. In this study, we report a systematic approach to synthesize MnOx nanocomposites with tunable Mn valence states via a two-step pyrolysis of Mn-BTC precursors, enabling us to explore their valence-dependent peroxidase-like activities. Among the synthesized forms, Mn3O4 exhibited the highest catalytic efficiency by facilitating the decomposition of H₂O₂ to generate hydroxyl radicals (•OH), which oxidize tetramethylbenzidine (TMB) to produce a blue-colored product. The catalytic mechanism was supported by UV–vis spectroscopy and fluorescence assays. Kinetic studies revealed low Km values, indicating high substrate affinity. Leveraging these properties, we further developed a disposable paper-based colorimetric bioassay using Mn3O4, which demonstrated excellent selectivity, sensitivity, and a low detection limit (0.25 μM in solution and 6.77 μM on paper). This work not only elucidates the correlation between Mn valence state and catalytic behavior but also provides a promising strategy for designing efficient nanozymes for low-cost, portable biosensing platforms.
Similar content being viewed by others
Introduction
Nanozymes have the merits of low cost, high chemical stability, adjustable affinity for the substrate and good catalytic activity, therefore have been extensively investigated for substituting natural enzymes in diverse applications such as sensing and detection1,2, energy storage and battery engineering3, dye biodegradation4,5, point-of-care monitoring6, and cancer treatment7. Numerous studies on manganese oxide-based nanozymes have been reported both in vitro and in silico8,9,10,11. These works help frame the context of MnOx nanozyme research and support the scientific foundation of our study. Beyond single nanozyme systems, multinanozyme configurations have recently emerged to enhance sensitivity and selectivity in colorimetric sensing by integrating different nanozymes with complementary catalytic functions that act synergistically to overcome the limitations of individual systems. For instance, Hormozi Jangi et al. developed a hybrid system combining Au- and MnO₂-based nanozymes for glutathione detection, in which Au components enhanced selectivity while MnO₂ improved sensitivity. This synergistic design achieved superior analytical performance over single nanozyme counterparts12. Despite these valuable contributions, few studies have systematically explored the valence-dependent catalytic activity of different MnOx phases (MnO, Mn3O4, Mn2O3), especially in the context of their integration with low-cost, disposable, paper-based colorimetric platforms. Our study fills this gap by correlating manganese valence states with peroxidase-like activity and demonstrating the practical application of Mn3O4 nanozymes in a one-time-use bioassay with high selectivity and sensitivity toward H₂O₂. Peroxidase-mimicking properties of nanozymes have been widely studied for detecting reactive oxygen species (e.g., H2O2) through a colorimetric reaction, which has great potential for practical clinical diagnosis and food analysis13,14,15,16,17,18.
Ever since the first report on Fe3O4-based peroxidase nanozyme in 2007, enormous efforts have been paid to design various nanostructures, tailor their compositions, and make hybridizations19,20,21, however, only a few studies have been focused on identifying the active sites of nanozymes and investigating the key parameters that determine their catalytic activities. For instance, Sun et al. developed a fast co-precipitation method for the synthesis of ultrathin NiCo layered double hydroxide (LDH) at room temperature22. The obtained nanosheets possessed an average lateral size of 26 nm and an average thickness of 2 nm, so can be well dispersed in water. The elemental composition of NiCo LDH was tunable and the active sites were identified to be Co owing to the low redox potential of Co3+/Co2+ and the strong Lewis acidity of Co3+. Recently, the same group prepared ultrathin NiMn LDH. The nanosheets exhibited peroxidase mimicking property with the catalytic performance even superior to that of natural horseradish peroxidase due to the presence of Mn23.Typically, Mn shows multiple oxidation states in its compounds, which severely determines the final peroxidase-like properties; nevertheless, the in-depth research on the valence-dependent activities of Mn remain inadequate24,25,26,27.
Recent advancements in portable nanozyme-based sensing platforms have revolutionized analytical applications by combining low cost, ease of use, and real-time detection. Particularly, systems integrated with smartphone cameras and color-recognition software provide simple and cost-effective alternatives to traditional laboratory instruments, expanding the scope of on-site diagnostics and monitoring28,29,30. Alongside smartphone-assisted systems, paper-based sensing platforms have also gained attention due to their low-cost fabrication, flexibility, and disposability. When integrated with functional materials such as MOFs or nanozymes, these paper-based sensors exhibit enhanced sensitivity and selectivity for colorimetric and fluorescence-based assays. These attributes make them promising for environmental monitoring and point-of-care diagnostics31,32,33.Herein, we develop a systematic strategy to synthesize MnOx with controllable valance states through a two-step pyrolysis of Mn-based metal organic frameworks (MOFs). The peroxidase-like activity of the obtained MnOx was found to highly depend on the valance state of the Mn sites. The optimized MnOx was further utilized as nanozyme for single-use colorimetric bioassays for H2O2 detection, showing good selectivity, high sensitivity and a low detection limit.
Experimental section
Materials and reagents
Manganese acetate tetrahydrate (Mn(CH3COO)2•4H2O, AR grade, 99.9%), trimesic acid (BTC, AR grade, 99.9%), polyvinylpyrrolidonee (PVP, AR grade, 99.9%) were obtained from Shanghai Ling Feng Chemical Reagent Co., Ltd. 3,3’,5,5’-Tetramethylbenzidine (TMB), H2O2 (30%), dopamine (DA, AR grade) , ascorbic acid (AA, AR grade), sucrose (AR grade), and glucose (AR grade) were purchased from Shanghai Energy Chemical Co., Ltd.
Synthesis of Mn-BTC and MnOx
In a typical reaction to synthesize Mn-BTC, 0.22 g of trimesic acid was dissolved in 20 mL of ethanol, while 0.18 g of manganese acetate tetrahydrate and 2.50 g of polyvinylpyrrolidone were dissolved in a mixture of 10 mL of ethanol and 10 mL of deionized (DI) water. The two solutions were subsequently mixed and stirred for 30 min, then aged at 200 °C for 3 h. The resulting white precipitates (Mn-BTC) were collected, washed, and dried in an oven at 60 °C.
The MnOx/C nanocomposites were obtained by thermal treatment of the above obtained Mn-BTC precursors in a N2 atmosphere at 650 °C for 2 h with a ramping rate of 5 °C min−1. Then, the MnOx powder was obtained by heating the composites in air at 200, 300 and 400 °C for 2 h by setting the ramping rate at 1 °C min−1. To evaluate the potential interference from common coexisting substances in food systems, several representative molecules were tested, including ascorbic acid (AA), dopamine (DA), glucose, sucrose, and potassium chloride (KCl), each at a final concentration of 0.1 mM in the reaction system.
Characterization
UV-Vis spectroscopy (UV-1600, Mapada), X-ray diffraction (XRD, Rigaku-Ultima III, λ = 1.5418 Å), field emission scanning electron microscopy (FESEM, JSM-7800F, JEOL) equipped with an energy dispersive spectroscopy (EDS) detector, transmission electron microscopy (TEM, JEM-1400F, JEOL), and X-ray photoelectron spectroscopy (XPS, PHI Quantera spectrometer) were used to characterize the absorption properties, crystal structures, morphologies, and bonding states of the MnOx/C nanocomposites.
To evaluate the peroxidase-like activity of the nanozymes, the MnOx/C nanocomposites were first dispersed in deionized water by ultrasonication to obtain a homogeneous suspension at a concentration of 1 mg·mL⁻1. In a typical reaction system, 10 μL of the Mn3O4 nanozyme suspension was added into 1.0 mL of phosphate buffer solution (PBS, 10 mM, pH = 5.35), which contained 0.2 mL of TMB (1 × 10⁻3 M) and an appropriate concentration of H₂O₂. This system was used to assess the catalytic efficiency by monitoring the colorimetric reaction. The apparent kinetic parameters of the nanozyme can be calculated according to the Michaelis–Menten equation:
where the Michaelis–Menten constant Km describes the affinity between nanozyme and substrate; v and vmax are the initial reaction velocity and the maximal velocity, respectively; and [S] represents the concentration of substrate.
Evaluation of nanozyme activity
The oxidase-like activity of the Mn3O4 nanozyme was further evaluated based on the colorimetric reaction system described above. The catalytic performance was investigated under various experimental parameters to determine the optimal operating conditions, including TMB concentration, H₂O₂ dosage, pH (2.5–10), and temperature (25–60 °C). UV–Vis spectroscopy at 652 nm was employed to monitor the oxidation of TMB, and the catalytic efficiency was quantitatively analyzed. The apparent kinetic parameters were subsequently calculated using the Michaelis–Menten equation and Lineweaver–Burk plots, confirming typical enzyme-like kinetics. The results demonstrated strong substrate affinity and high catalytic activity, consistent with previously reported temperature-resilient Mn–MOF nanozymes34,35.
Sensing assay and design
Based on the catalytic oxidation reaction, a paper-based colorimetric platform was developed for portable sensing. A defined volume of Mn3O4 nanozyme suspension was drop-cast onto cellulose pads and dried at room temperature. After adding a TMB/H₂O₂ mixture, the resulting color change was recorded using a smartphone camera. The color intensity was quantitatively analyzed via RGB extraction using ImageJ software, enabling low-cost and on-site detection applications. The proposed paper-based platform is designed for single use and therefore not suitable for reproducibility testing36.
Catalytic mechanism and proposed pathway
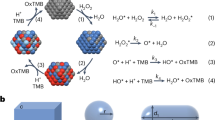
The peroxidase-mimicking mechanism of Mn3O4 nanozyme was investigated to elucidate its redox behavior and reactive species generation during the colorimetric oxidation process. The catalytic activity originates from the intrinsic multivalent states of manganese ions (Mn2⁺/Mn3⁺), which enable rapid electron transfer and continuous redox cycling analogous to natural peroxidases. Upon interaction with hydrogen peroxide (H₂O₂), Mn2⁺ species are oxidized to Mn3⁺, generating highly reactive hydroxyl radicals (•OH) through a Fenton-like reaction. These radicals subsequently oxidize chromogenic substrates such as TMB, producing the characteristic blue oxidized product (oxTMB) that confirms the peroxidase-like pathway.
The catalytic mechanism can be described by the following reactions:
This redox cycle ensures sustained radical generation and continuous catalytic turnover. The coexistence of Mn ions with different oxidation states and abundant surface oxygen vacancies facilitates electron delocalization and enhances H₂O₂ adsorption and activation. Moreover, the structural stability and high surface-to-volume ratio of Mn3O4 contribute to efficient ROS production under mild conditions37,38.
Results and discussion
Despite the extensive research on Mn-based nanostructures for catalytic and sensing applications, most prior studies have concentrated on a single oxidation state and have not systematically elucidated the relationship between Mn valence and catalytic performance. In addition, the integration of Mn-based nanozymes into low-cost, portable, and disposable sensing platforms has received relatively little attention. To overcome these limitations, we developed a controllable two-step pyrolysis strategy that enables precise tuning of Mn valence states and evaluation of their influence on peroxidase-like activity. This approach focuses on the valence-dependent catalytic behavior of Mn-based nanozymes.
The two-step calcination process resulted in a hybrid structure composed of MnOx nanocrystals homogeneously embedded in carbon frameworks (MnOx/C) due to the decomposition of organic ligands and the formation of Mn–O bonds (Fig. 1).
The derivatives obtained at a calcination temperature of 200, 300, and 400 °C are respectively presented in Fig. 2a–h, which inherited the spherical morphology of the Mn-BTC precursor. The diameter distribution statistics in Fig. 2i reveal that the diameter of Mn-BTC, MnO, Mn3O4, and Mn2O3 nanoparticles are 1.9 μm, 1.05 μm, 0.95 μm, and 0.65 μm, respectively, which demonstrates an inverse correlation between the diameter of the derivatives and the calcination temperature. The corresponding XRD patterns in Fig. 2j indicate the successful conversion of the Mn-BTC precursors into MnO (JCPDS 07–0230), Mn3O4 (JCPDS 24–0734), and Mn2O3 (JCPDS 41–1442) when the calcination temperature was set at 200, 300, 400 °C, respectively. Moreover, the EDS mappings further confirm the homogenous elemental distribution of MnOx and carbon frameworks in the nanocomposites (Fig. S1). TEM image in Fig. S2 suggests that the hierarchical morphology of MnOx maintains. The valence state distribution of Mn in the derivatives can be determined from the high-resolution XPS spectra of Mn 2p (Fig. 2k). Deconvolution of the Mn 2p₃/₂ peak reveals contributions from Mn2⁺ (~ 640.9 eV), Mn3⁺ (~ 642.1 eV), and Mn4⁺ (~ 643.8 eV). These three oxidation states coexist in the material, with Mn2⁺ dominating at lower temperatures. As the calcination temperature increases, the content of Mn2⁺ decreases while that of Mn3⁺ gradually increases, which agrees with the phase evolution observed in the XRD results. These findings further confirm the progressive oxidation of Mn during thermal treatment and the tunability of Mn valence through calcination. The oxidation state of Mn plays a crucial role in determining the peroxidase-like catalytic activity of MnOx materials. Previous reports have demonstrated that Mn3⁺, owing to its moderate redox potential and strong Lewis acidity, can effectively decompose H₂O₂ into hydroxyl radicals (•OH), which in turn oxidize TMB to its colored product39. As depicted in Fig. 2l, the content of Mn2+ decreases with increasing calcination temperature, while the content of Mn3+ gradually increases. These results demonstrate that the valence states of Mn in MnOx can be regulated by controlling the calcination temperature in the two-step synthesis process, which is crucial for further investigations into the relationship between Mn valence states and the peroxidase-like catalytic activity of MnOx.
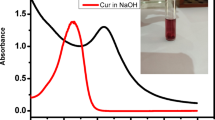
The peroxidase-like properties of MnOx were probed using TMB as the allochronic substrate in the presence of H2O2. As shown in Fig. 3a, a strong absorption peak at 652 nm is observed upon adding MnOx (herein we use Mn3O4 as an example) in the mixture of H2O2 and TMB; on the contrary, no absorption peaks were found in the absence of Mn3O4 or H2O2 in the system. In addition, Mn3O4 has a higher catalytic activity by exhibiting a stronger absorption peak at 652 nm, as compared to MnO and Mn2O3 (Fig. 3b).
(a) UV–Vis absorption spectra of TMB + H2O2, TMB + Mn3O4, and TMB + H2O2 + Mn3O4 solutions. (b) Catalytic performance comparison with MnO, Mn3O4 and Mn2O3 as POD-like nanozyme. (c) Fluorescence emission spectra revealing the generation of •OH through the catalytic reaction using TA as a fluorescent probe. (d) The calalytic mechanism of Mn3O4. POD-like activity of Mn3O4 nanozyme against (e) pH value, (f) reaction temperature, and (g) storage time.
Fluorescence spectroscopy was employed to elucidate the catalytic mechanism by examining the generation of hydroxyl radicals (•OH) with the assistance of terephthalic acid (TA) as the capture agent40,41,42. TA can act as a capture molecule and react with ·OH to generate fluorescent 2-hydroxyterephthalic acid (TAOH)43. As shown in Fig. 3c, a strong fluorescence peak is observed at 430 nm upon the addition of H2O2 into TA, suggesting the generation of •OH by the decomposition of H2O2. To better visualize the catalytic mechanism, a schematic illustration is shown in Fig. 3d. Mn3O4 catalyzes the decomposition of H₂O₂ into hydroxyl radicals (•OH), which subsequently oxidize the colorless TMB into a blue-colored product (oxTMB). The color change correlates with H₂O₂ concentration and is used for quantitative analysis. This mechanism underpins both the solution-based and paper-based bioassays developed in our work. The fluorescence resonance peak becomes stronger after adding Mn3O4 into the system, which indicates that the catalyst promoted the generation of •OH. Therefore, it can be concluded that the colorless TMB is oxidized by •OH to produce blue color. The concentration of H2O2 can be indirectly evaluated based on the color change. The catalytic performance of Mn3O4 was further examined in PBS buffer with varied pH values from 2.5 to 10 at an increasing reaction temperature from 25 to 60 °C. Mn3O4 exhibits the highest peroxidase-like activity at a pH of 5.35 (Fig. 3e). The activity initially increases with the rising reaction temperature and peaks at 35 °C, then gradually decreases when the reaction temperature is further increased, with a ~ 60% retention at 60 °C (Fig. 3f). Besides, Mn3O4 shows excellent long-term catalytic stability without negligible degradation after being stored for 10 days (~ 96% retention of the activity as shown in Fig. 3g), which is superior to that of natural enzyme. All of the above results demonstrate that Mn3O4 can function as peroxidase-mimicking nanozyme with high activity and chemical stability.
To strengthen the catalytic assessment, a comparative analysis was performed with reference to recent studies on Mn-based oxidase-like nanozymes. The catalytic behavior of Mn3O4 was consistent with temperature-resilient Mn–MOF systems34,35, in which robust frameworks and redox-active Mn centers sustain high catalytic efficiency under diverse environmental conditions. These findings demonstrate the excellent oxidase-like activity and environmental adaptability of Mn3O4, underscoring its potential for reliable and versatile sensing applications.
Subsequently, the steady-state kinetic of Mn3O4 was evaluated using the Michaelis–Menten model under the optimized testing conditions (Fig. 4). The Michaelis–Menten curves were experimentally obtained by changing the contents of H2O2 and TMB; the corresponding Lineweaver Burk diagrams were calculated to determine the kinetic parameters. The Km and vmax values for the substrates of H2O2 and TMB were respectively calculated to be 0.043 mM, 19.22 × 10−8, and 0.054 mM, 8.15 × 10−8.
(a) The dependence of the reaction velocity on the H2O2 concentration (the concentration of TMB was fixed at 166 μM). (b) The corresponding Lineweaver–Burk plot. (c) The dependence of the reaction velocity on the TMB concentration (the concentration of H2O2 was fixed at 314 μM). (d) The corresponding Lineweaver–Burk plot. The test conditions: 10 μL of 1 mg mL−1 Mn3O4 nanozymes in 1 mL of 10 mM PBS (pH = 5.35) at 35 °C. The error bars were obtained based on three sets of measurement.
These results confirm that the Mn3O4 nanozyme follows typical Michaelis–Menten kinetics similar to natural peroxidases. The relatively low Km values indicate a strong substrate-binding affinity, superior to most reported metal-oxide nanozymes. Moreover, the observed kinetics agree with recent findings on Mn- and Ag-based oxidase- or peroxidase-like nanozymes, where multivalent metal centers and surface oxygen vacancies jointly promote substrate activation and electron transfer during catalysis44,45,46.
Food safety is of significance to human health. Although H2O2 is a widely adopted agent for food preservation, its content should be strictly controlled to avoid any harm to the consumers47,48,49,50,51. In this work, Mn3O4 were utilized for colorimetric detection of H2O2 in the mixture of TMB and H2O2. As exemplified by Mn3O4 shown in Fig. 5a, the intensity of the absorption peak increases as the concentration of H2O2 rises. Figure 5b displays the corresponding absorbance as a function of the concentration of H2O2, which has a good linearity (R2 = 0.9992) in the range of 0.01–0.20 mM. The limit of detection (LOD) can be calculated to be 0.25 μM based on the formula:
where σ represents the standard deviation of background signal, the slope is the linear calibration in Fig. 5b. Benefiting from the excellent peroxidase-like activity of Mn3O4, a colorimetric bioassay was fabricated by coating nanozyme particles on cellulose paper, as a proof-of-concept disposable biosensor for the detection of H2O2. The color change of the bioassay induced by the mixture of TMB and H2O2 was recorded by photographs; the relative intensity of green color in the broadband color (G/R + G + B) was analyzed as a function of the H2O2 content (Fig. 5c). A low LOD of 6.77 μM were obtained in the first linear range (0.16–1.26 mM) for the paper bioassay, which is lower than the allowance level of US FDA regulations (15 μM). Furthermore, the detection of H2O2 can be interfered by other components such as ascorbic acid (AA), dopamine (DA), sucrose, glucose and K+ in food, it is critical for the biosensor to have a high selectivity. In our experiment, even in the presence of these substances, the content of H2O2 can be selectively determined (Fig. 5d), indicating the paper bioassay is applicable for fast and low-cost food analysis.
Although this study demonstrates the promising oxidase-like activity and sensing potential of the Mn3O4 nanozyme, several limitations remain. The catalytic evaluation and sensing performance were primarily conducted for H₂O₂ detection under controlled laboratory conditions; therefore, further validation involving diverse analytes and complex real samples is necessary to assess its practical applicability. In future studies, efforts should focus on optimizing the nanozyme synthesis and surface modification to improve selectivity and sensitivity, integrating it into portable or microfluidic sensing systems, and expanding its applications in biomedical diagnostics, food safety, and environmental monitoring.
Conclusion
In summary, we have developed a valence-controlled synthesis method for MnOx nanozymes and systematically investigated their peroxidase-like catalytic activities. Among them, Mn3O4 exhibited the best catalytic performance due to its optimal Mn2⁺/Mn3⁺ ratio, promoting efficient •OH generation from H₂O₂. Spectroscopic and kinetic analyses confirmed the mechanism. Based on this, we constructed a disposable paper-based colorimetric bioassay using Mn3O4, which showed high sensitivity, good selectivity, and a low detection limit for H₂O₂. Importantly, this sensing mechanism is highly compatible with portable platforms. The Mn3O4 nanozyme can be stably coated on low-cost cellulose paper and integrated into smartphone-readable colorimetric strips. Due to its visual signal output, low power requirement, and simple fabrication, the platform is suitable for on-site, user-friendly detection without complex instruments. Therefore, our work not only deepens the mechanistic understanding of MnOx nanozymes, but also demonstrates a practical pathway to translate nanozyme catalysis into portable, low-cost diagnostic tools for food safety, clinical analysis, and environmental monitoring.
Data availability
The data that support the findings of this study are available on reasonable request.
References
Akhond, M., Hormozi Jangi, S. R., Barzegar, S. & Absalan, G. Introducing a nanozyme-based sensor for selective and sensitive detection of mercury(II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chem. Pap. 74, 1321–1330 (2020).
Hormozi Jangi, S. R. & Dehghani, Z. A novel dual-function biomimetic approach for high-throughput organic dye biodegradation and hydrogen peroxide sensing using a nanosized artificial peroxidase with ultra-improved substrate affinity and superb catalytic efficiency. Process. Biochem. 150, 1–20 (2025).
Hormozi Jangi, S. R. Lithium-electroactive peroxidase-like MnO₂ nanomaterials as an ultrasensitive and selective sensing platform for carcinogenic 3,3′-diaminobenzidine and high-capacity lithium-ion battery cathode materials. Chem. Pap. 78, 5367–5379 (2024).
Ahmadi-Leilakouhi, B., Hormozi Jangi, S. R. & Khorshidi, A. Introducing a novel photo-induced nanozymatic method for high-throughput reusable biodegradation of organic dyes. Chem. Pap. 77, 1033–1046 (2023).
Hormozi Jangi, S. R., Khoshalhan Davoudli, H., Delshad, Y., Hormozi Jangi, M. R. & Hormozi Jangi, A. R. A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surf. Interfaces 21, 100771 (2020).
Hormozi Jangi, S. R., Akhond, M. & Absalan, G. A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3,3′-diaminobenzidine. Microchim. Acta 187, 431 (2020).
Zhang, X., Chen, X. & Zhao, Y. Nanozymes: Versatile platforms for cancer diagnosis and therapy. Nano-Micro Lett. 14, 95 (2022).
Jiang, M. et al. A bimetallic core–shell Cu@MnO nanozyme with enhanced oxygen activation for efficient oxidase-like activity. Talanta 293, 128059 (2025).
Shahraki, S., Vaziri, E., Saboury, A. A. & Fan, K. Biomedical potential of nanozymes: Harnessing redox enzyme mimicry for theranostic applications. Coord. Chem. Rev. 517, 215937 (2024).
Poon, K., Gupta, A., Price, W. S., Zreiqat, H. & Singh, G. Manganese oxide nanoplatforms for disease diagnosis and treatment: Progress, challenges and opportunities. Coord. Chem. Rev. 500, 215548 (2024).
Qin, J., Guo, N., Yang, J. & Wei, J. Recent advances in metal oxide nanozyme-based optical biosensors for food safety assays. Food Chem. 447, 139019 (2024).
Hormozi Jangi, S. R., Akhond, M. & Absalan, G. A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Anal. Chim. Acta 1127, 1–8 (2020).
Liang, M. & Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 52, 2190–2200 (2019).
Zhao, Y. et al. Nanozyme-reinforced hydrogel as a H₂O₂-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nat. Commun. 13, 6758 (2022).
Amalraj, A., Narayanan, M. & Perumal, P. Highly efficient peroxidase-like activity of a metal–oxide-incorporated CeO₂–MIL(Fe) metal–organic framework and its application in the colorimetric detection of melamine and mercury ions via induced hydrogen and covalent bonds. Analyst 147, 3234–3247 (2022).
Chen, J. et al. Glucose-oxidase-like catalytic mechanism of noble metal nanozymes. Nat. Commun. 12, 3375 (2021).
Jesuraj, R., Amalraj, A., Vaidyanathan, V. K. & Perumal, P. Exceptional peroxidase-like activity of an iron- and copper-based organic framework nanosheet for consecutive colorimetric biosensing of glucose and kanamycin in real food samples. Analyst 148, 5157–5171 (2023).
Yang, J. et al. 2D material-based peroxidase-mimicking nanozymes: Catalytic mechanisms and bioapplications. Anal. Bioanal. Chem. 414, 2971–2989 (2022).
Ai, Y. et al. Recent advances in nanozymes: from matters to bioapplications. Adv. Funct. Mater. 32, 2110432 (2022).
Gao, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583 (2007).
Wang, L. et al. Fe,N-doped carbon as peroxidase mimics for single-use colorimetric bioassays. J. Mater. Sci. 56, 13579–13589 (2021).
Sun, Y. et al. Identifying the active site of ultrathin NiCo LDH as an efficient peroxidase mimic with superior substrate affinity for sensitive detection of hydrogen peroxide. J. Mater. Chem. B 7, 6232–6237 (2019).
Sun, Y. et al. Ultrathin NiMn layered double hydroxide nanosheets with superior peroxidase-mimicking performance to natural HRP for disposable paper-based bioassays. J. Mater. Chem. B 9, 983–991 (2021).
Wu, J., Yang, Q., Li, Q., Li, H. & Li, F. Two-dimensional MnO₂ nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H₂O₂ and color. Anal. Chem. 93, 4084–4091 (2021).
Zhu, Y. et al. Stimuli-responsive manganese single-atom nanozyme for tumor therapy via integrated cascade reactions. Angew. Chem. Int. Ed. 60, 9480–9488 (2021).
Tang, M., Zhang, Z., Sun, T., Li, B. & Wu, Z. Manganese-based nanozymes: Preparation, catalytic mechanisms, and biomedical applications. Adv. Healthc. Mater. 11, 2201733 (2022).
Baral, A., Satish, L., Zhang, G., Ju, S. & Ghosh, M. K. A review of recent progress on nano MnO₂: Synthesis, surface modification, and applications. J. Inorg. Organomet. Polym. 31, 899–922 (2021).
Ameen, S. S., Bedair, A., Hamed, M., Mansour, F. R. & Omer, K. M. Recent advances in metal–organic frameworks as oxidase mimics: A comprehensive review on rational design and modification for enhanced sensing applications. ACS Appl. Mater. Interfaces 17, 110–129 (2025).
Ameen, S. S. M., Algethami, F. & Omer, K. M. Magnetic rod-shaped Mn-based MOF as a multifunctional and recyclable platform for dual-mode ratiometric nitrite detection. Microchim. Acta 192, 194 (2025).
Ameen, S. S. M., Algethami, F. K. & Omer, K. M. Flower-like Ag–ZIF nanoparticles with petal-like structures as effective hot/coldadapted oxidase mimic: Visual color tonality nitrite detection. Food Chem. 478, 143615 (2025).
Ameen, S. S. & Omer, K. M. Recent advances of bimetallic metal–organic frameworks: Preparation, properties, and fluorescence-based biochemical sensing applications. ACS Appl. Mater. Interfaces 16, 31895–31921 (2024).
Ameen, S. S. & Omer, K. M. Lanthanide- and functionalization-free dual-state emitting zinc-based MOFs followed by dual-state detection: ratiometric and color-tonality visual detection of tetracycline in solution and on paper in food and environmental samples. Microchim. Acta 192, 22 (2024).
Ameen, S. S. & Omer, K. M. Dual-state red-emitting zinc-based MOF accompanied by dual-mode and dual-state detection: Color-tonality visual mode for the detection of tetracycline. ACS Appl. Mater. Interfaces 16, 51376–51383 (2024).
Ameen, S. S. & Omer, K. M. Temperature-resilient and sustainable Mn-MOF oxidase-like nanozyme (UoZ-4) for total antioxidant-capacity sensing in citrus fruits: Breaking the temperature barrier. Food Chem. 448, 139170 (2024).
Ameen, S. S. & Omer, K. M. Multifunctional MOF: Cold/hot-adapted sustainable oxidase-like MOF nanozyme with ratiometric and color-tonality response for nitrite-ion detection. Food Chem. 462, 141027 (2025).
Hormozi Jangi, S. R. & Gholamhosseinzadeh, E. Developing an ultra-reproducible and ultrasensitive label-free nanoassay for L-methionine quantification in biological samples toward application in homocystinuria diagnosis. Chem. Pap. 77, 6505–6517 (2023).
Alshatteri, A. H., Ameen, S. S., Latif, D., Mohammad, Y. O. & Omer, K. M. Nanoscale mineral as a novel class of enzyme mimic (mineralzyme) with total antioxidant-capacity detection: Colorimetric and smartphone-based approaches. Mater. Today Chem. 40, 102262 (2024).
Ameen, S. S., Alshatteri, A. H., Latif, D. A., Mohammad, Y. O. & Omer, K. M. Nanomineralzyme as a novel sustainable class of nanozyme: Chalcopyrite-based nanozyme for the visual detection of total antioxidant capacity in citrus fruit. Food Chem. 471, 142769 (2025).
Ilton, E. S., Post, J. E., Heaney, P. J., Ling, F. T. & Kerisit, S. N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 366, 475–485 (2016).
Gan, Z. et al. Dual enzyme-mimicking fluorescent amino terephthalic acid/CuFe/adenosine triphosphate nanoparticles for determination of H₂O₂ and ascorbic acid. Microchem. J. 182, 107939 (2022).
Hou, J.-T. et al. Fluorescent detectors for hydroxyl radical and their applications in bioimaging: A review. Coord. Chem. Rev. 421, 213457 (2020).
Liu, T. et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 11, 2788 (2020).
Hormozi Jangi, S. R. Developing a novel ultraselective and ultrasensitive label-free direct spectrofluorimetric nanobiosensor for direct highly fast field detection of explosive triacetone triperoxide. Anal. Chim. Acta 1320, 343016 (2024).
Mohammed Ameen, SSh. & Omer, K. M. Pushing boundaries: Introducing silver-based metal–organic framework oxidase-like nanozyme over a wide-range temperature. ACS Appl. Nano Mater. 7, 20793–20803 (2024).
Mohammed Ameen, SSh., Algethami, F. & Omer, K. M. Pine needle-derived oxidase-like Mn nanozymes: sustainable nanozyme, scalable synthesis, and visual and colorimetric nitrite detection. Microchim. Acta 192, 146 (2025).
Karrat, A., Benssbihe, J., Mohammed Ameen, SSh., Omer, K. M. & Amine, A. Development of a silver-based MOF oxidase-like nanozyme modified with molecularly imprinted polymer for sensitive and selective colorimetric detection of quercetin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 330, 125735 (2025).
Huang, Y. et al. The recent development of nanozymes for food quality and safety detection. J. Mater. Chem. B 10, 1359–1368 (2022).
Ivanova, A. S., Merkuleva, A. D., Andreev, S. V. & Sakharov, K. A. Method for determination of hydrogen peroxide in adulterated milk using high-performance liquid chromatography. Food Chem. 283, 431–436 (2019).
Song, W., Zhao, B., Wang, C., Ozaki, Y. & Lu, X. Functional nanomaterials with unique enzyme-like characteristics for sensing applications. J. Mater. Chem. B 7, 850–875 (2019).
Payal, A., Krishnamoorthy, S., Elumalai, A., Moses, J. A. & Anandharamakrishnan, C. A review on recent developments and applications of nanozymes in food safety and quality analysis. Food Anal. Methods 14, 1537–1558 (2021).
Uematsu, K. Detection of hydrogen peroxide. Anal. Sci. 38, 457–458 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22375092), Pearl River Talent Program of Guangdong Province (Youth Top-Notch Talent, 2021QN02X046), the Guangdong Basic and Applied Basic Research Fund (2023A1515012820), the Science and Technology Planning Project of Guangzhou (2024A04J6316), the Natural Science Foundation of Jiangsu Province (BK20241846), the scientific research foundation for high-level personnel in Jinling Institute of Technology(jit-b-201811) and the Industry-University-Research Cooperation Project of Jiangsu Province (BY2021300).
Author information
Authors and Affiliations
Contributions
Zhao Zhang contributed to the methodology, investigation, formal analysis, writing—original draft. Tian Zhang, Henghan Dai, Zengyu Hui, Lumin Wang, Xin Liu, Shan Hong and Haoran Zheng assisted in the writing—review and editing, Jianing An and Gengzhi Sun supervised the study and polished the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Z., Zhang, T., Dai, H. et al. Manganese oxide (MnOx) as peroxidase-mimicking nanozymes with valence-dependent activity for single-use colorimetric bioassays. Sci Rep 15, 42163 (2025). https://doi.org/10.1038/s41598-025-26164-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26164-0