Abstract
Muscle damage and systemic stress responses are common consequences of intense physical exertion. However, the extent to which these responses vary according to the severity of muscular strain remains unclear. We conducted a field-based study involving 24 active-duty elite military personnel who participated in five distinct operational missions in Brazil. Circulating levels of 36 plasma biomarkers were assessed and compared between individuals presenting moderate or exacerbated muscular response, defined by serum creatine kinase (CK) concentrations below or above 1000 U/L, respectively. Statistical comparisons and multivariate logistic regression were performed to evaluate associations between biomarker levels and muscular response severity. Our analysis revealed that individuals with exacerbated response exhibited distinct systemic profiles, marked by elevated tissue injury markers, reduced eosinophil and lymphocyte counts, and lower adrenal steroid hormone concentrations. Furthermore, regression models identified creatine kinase muscle-brain (CKMB) and alanine aminotransferase (ALT) as independently associated with higher CK levels, while heightened eosinophil counts showed a potential protective trend. These findings suggest that greater degrees of muscular stress are associated with broader systemic dysregulation, and that specific circulating biomarkers may serve as indicators of individual susceptibility to acute stress in high-demand physical environments.
Similar content being viewed by others
Introduction
Intense physical exertion, such as that encountered during military operations, elicits a cascade of systemic stress that triggers a physiological response encompassing musculoskeletal injury, metabolic strain, neuroendocrine activation and immune modulation. The exercise-induced muscle damage (EIMD) is frequently monitored via serum creatine kinase (CK) levels to indicate muscle injury with clinical relevance1,2. While these elevations are typically transitory in trained individuals, extremely high CK responses may reflect excessive strain that exceeds the body’s reparative capacity, posing potential life risks such as rhabdomyolysis3,4,5.
Intense physical exertion is consistently associated with elevations in biomarkers of tissue injury and metabolic strain - including creatine kinase muscle-brain (CKMB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and urea - suggesting simultaneous hepatic, renal and musculoskeletal involvement6,7. Immunological alterations also emerge post-exercise, notably lymphocytopenia and eosinopenia, consistent with transient immune redistribution during the so-called “open window” of reduced host defense8. Though often short-lived, these shifts may compromise recovery or increase infection risk when stress is sustained. Endocrine responses are equally relevant. Reductions in adrenal and gonadal steroids such as testosterone and its derivatives, have been linked to high training loads and energy deficits, reflecting hypothalamic-pituitary-adrenal axis modulation under cumulative stress9,10.
Few studies have explored how these biomarkers interact under real-world conditions of operational stress, nor how their patterns may differ according to the severity of muscle strain, especially after cumulative stress. This represents a critical gap in tactical human performance science and the broader field of sports physiology, where identifying individuals at risk for exacerbated stress response is essential for guiding early interventions, optimizing recovery strategies and personalizing training load management.
Here, we investigated whether systemic biochemical, immune, and hormonal profiles differed according to the severity of muscular stress following intense operational activity in a prospective cohort of active-duty elite military personnel in Brazil. We utilized a panel of 36 circulating biomarkers measured after five consecutive tactical missions distributed across 259 days of training and explored associations that could reflect broader physiological adaptations to cumulative exertion, aiming to characterize systemic signatures related to different levels of muscular strain. We hypothesized that individuals exhibiting more exacerbated muscle damage would also present an increased systemic cellular, immunological and hormonal disturbance across multiple physiological axes.
Materials and methods
Ethics statement
All clinical procedures were conducted in accordance with the principles expressed in the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of the Marcílio Dias Naval Hospital (approval no. 67381317.7.00000.5256). All participants were fully informed about the objectives and procedures of the study and provided written informed consent prior to enrolment.
Study design and participants
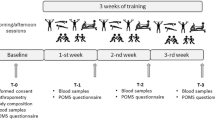
This was a prospective, observational cohort study conducted over a 259-day period and structured around five sequential high-intensity field missions. All procedures were carried out under operational conditions simulating real combat scenarios. The course is designed to prepare officers and non-commissioned officers for amphibious tactical operations and rapid-response missions under hostile and variable environmental conditions. Inclusion criteria consisted of active enrolment in the SACC. All participants were male, active-duty military personnel. Exclusion criteria included failure to complete any phase of data collection, medical leave requiring hospitalization, or reported use of anabolic steroids. Participants provided written informed consent prior to enrolment.
VO2 max estimated by the 12 min running test11. Body composition was done using the seven skin folds protocol: abdominal, pectoral, middle axillary, supra iliac, subscapular, tricipital, and thigh12. Next, the Siri equation was applied to calculate the fat percentage13. Total Body Mass (TBM): electronic scale (Ecoline - Tech Line, Brazil), to the nearest 0.1 Kg; Height: portable stadiometer (Personal Capirce, Brazil), to the nearest 1.0 (one) centimeter; Skin folds: scientific adipometer (Sanny, Brazil), to the nearest 0.1 mm. Lean body mass was determined by subtracting fat mass, assessed through skinfold-based adipometry, from total body mass.
Special amphibious command course (SACC)
The SACC includes multistage operational training across distinct Brazilian biomes, including cold mountainous terrain, high-altitude humid forests, and coastal tropical environments. Each mission lasted between 6 and 12 days and involved sustained physical and cognitive demands, including continuous loaded marches (2–40 km), concentric and eccentric resistance tasks, sleep restriction, and ad libitum hydration. The specific durations of each mission were: introductory training (10 days), tropical mission (10 days), cold mission (6 days), jungle mission (7 days), and high altitude mission (12 days). Food intake followed a controlled caloric plan, aligned with operational rations provided during field missions. Participants returned to a centralized military base (MO2) between missions for recovery and pre-mission preparation. The final mission was conducted in a high-altitude setting and involved an extended tactical escape simulation with unrestricted physical demand and no scheduled sleep or feeding. This scenario was developed to replicate sustained combat exposure and evaluate cumulative physiological strain. Biological samples were collected at MO2 before and after each mission to assess systemic biomarker responses to physical stress exposure under standardized conditions.
Study groups
Participants were stratified in exacerbated muscular response, defined by post-mission creatine kinase (CK) levels exceeding 1000 U/L and moderate muscular response defined by post-mission CK < 1000 U/L. The threshold was selected in alignment with previous studies that suggest this value as a conservative lower limit for exertional rhabdomyolysis, marking a clinically relevant threshold for abnormal muscle damage responses in healthy populations undergoing intense physical exertion14,15,16.
Blood sample collection and biochemical analyses
Peripheral blood was collected in the morning, with participants seated and their right arm positioned for venipuncture at the antecubital fossa. Two 5 mL tubes (Vacuette, Greiner Bio-One), containing separating gel and no anticoagulant, were obtained per participant. Collections were performed at baseline—prior to the Introductory Training phase—and immediately after each operational mission. All samples were processed and analyzed at the Marine referral clinical laboratory using standardized protocols. All biochemical measurements were performed on the Vitros 5600 Integrated System (Ortho-Clinical Diagnostics, Johnson & Johnson, Rochester, NY) by certified laboratory staff. Serum levels of creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine were measured using the dry chemistry method17. Lactate dehydrogenase (LDH) was assessed via the pyruvate-lactate enzymatic method18. Gamma-glutamyl transferase (GGT), albumin, urea, and electrolytes (sodium, potassium, calcium, and magnesium) were quantified using standard clinical chemistry protocols. GGT was determined using a modified kinetic method. The estimated glomerular filtration rate (eGFR) was calculated using serum creatinine and the 2022 KDIGO formula, which excludes racial adjustment19. The formula applied was:
Where: k = 0.7 for women and 0.9 for men, and α = −0.241 for women and − 0.302 for men.
Data analysis
In this study, the dependent variable was the presence of exacerbated muscular response (CK ≥ 1000 U/L), defined as a binary categorical variable. Independent variables included continuous numerical variables (VO₂ max, body composition, and biochemical markers) and categorical variables representing operational context (e.g., mission type, altitude, temperature, and humidity). These were detailed in Tables 1 and 2, and 3. Categorical variables were presented as count and percentage and numerical variables with medians and interquartile ranges (IQR). Continuous variables were compared using the Mann-Whitney U test. Correlations between the different parameters were evaluated with the Spearman test. Biomarker concentrations were log10-transformed for data normalization. P-values < 0.05 were considered statistically significant.
A logistic regression model was used to investigate associations between circulating biomarkers and the presence of an exacerbated muscular response (CK ≥ 1000 U/L). The modeling process followed a backward stepwise selection approach, performed automatically based on the Akaike Information Criterion (AIC). Variables were removed iteratively when their exclusion improved model parsimony without compromising fit, with AIC used as the primary metric for model comparison at each step.
Results
Characteristics of study participants
The baseline characteristics of the military participants are summarized in (Table 1). At enrollment, participants (n = 24) exhibited a median age of 28 years (IQR: 27.2–30.7), with median body weight of 79.6 kg (73.3–85.8), height of 178 cm (172.5–182.6), and lean mass of 70.9 kg (66–74.4). Estimated aerobic capacity was relatively homogeneous, with a median VO₂ max of 51.3 ml/kg/min (49.6–52.4).
When stratified by mission site (Table 2), median demographic and physiological parameters remained largely consistent across groups. However, substantial differences were observed in the proportion of individuals exhibiting exacerbated muscular response, defined as post-mission CK levels ≥ 1000 U/L. While tropical mission and high-altitude mission showed notably high frequencies (94.4% and 93.7%, respectively).
Systemic biomarkers disruption characterizes individuals with exacerbated muscular stress post-mission
We examined the circulating profile of 36 plasma mediators, including biochemical, hematological, and hormonal markers, in military personnel following operational missions. This approach was employed to investigate whether individuals presenting with exacerbated muscular response (CK ≥ 1000 U/L) exhibit a distinct systemic profile when compared to those with moderate response (concentration values are described in Table 3). Individuals with exacerbated responses exhibited higher concentrations of classic muscle damage biomarkers, such as CKMB, LDH, AST, and ALT, indicating greater cellular injury and hepatic stress. In addition, elevations in urea and serum phosphorus suggested increased protein catabolism and metabolic demand. Curiously, markers of renal function (creatinine), glucose, and fluid-electrolyte homeostasis (sodium, potassium, calcium, osmolality) showed no significant differences between groups, suggesting that these systems remained relatively stable regardless of muscular response severity.
Notably, the exacerbated group demonstrated lower counts of circulating lymphocytes and eosinophils, potentially reflecting immune suppression or redistribution under physiological stress. Among hormonal mediators, reductions in androsterone, etiocholanolone, and epitestosterone were observed, supporting a pattern of suppressed adrenal steroidogenesis in those with greater muscular stress. Testosterone showed a trend toward lower values in the exacerbated group, although without statistical significance.
Differential associations between CK and systemic biomarkers according to muscular response severity
To explore potential mechanistic links underlying the systemic alterations observed in individuals with exacerbated muscular response, Spearman correlation analyses were performed to assess the associations between CK levels and selected plasma mediators. For this analysis, only the biochemical, hematological, and hormonal markers that showed statistically significant differences between groups were included (rho values 6). Strong positive associations were observed between CK and markers of tissue damage, including CKMB (r = 0.84; p < 0.001), LDH (r = 0.89; p < 0.001), AST (r = 0.89; p < 0.001), and ALT (r = 0.69; p < 0.001), as well as with urea (r = 0.41; p = 0.02), consistent with muscle injury and increased protein catabolism (Fig. 1A). In contrast, negative correlations were found with lymphocyte count (r = −0.52; p = 0.001), eosinophil count (r = −0.52; p = 0.0001), serum phosphorus (r = −0.43; p = 0.02), and adrenal steroid hormones levels including androsterone (r = −0.47; p = 0.003), etiocholanolone (r = −0.38; p = 0.01), and epitestosterone (r = −0.50; p = 0.0007).
Biochemical Correlation Patterns Post-Mission Differentiate Exacerbated and Moderate Muscular Response Groups. (A) Spearman correlation between CK and biochemical parameters post-mission; bars represent the Spearman rank (rho) values. Colored bar indicated statistically significant correlation (P < 0.05). Red bar represents positive correlation and blue bar negative correlation. (B) Spearman correlation between CK levels with biochemical markers in military personnel with Exacerbated Muscular Response (CK ≥ 1000 U/L) and Moderate Muscular Response (CK < 1000 U/L); the values of r and p shown in the figure correspond to exacerbated muscular response (yellow box) and moderate muscular response (blue box). Abbreviations: ALT = Alanine Aminotransferase, AST = Aspartate Aminotransferase, CK = Creatine Kinase, CKMB = Creatine Kinase Muscle-Brain, LDH = Lactate Dehydrogenase.
We then stratified the correlation analysis according to muscular response classification (moderate vs. exacerbated) to determine whether the associations observed in the overall population were consistent within each subgroup (Fig. 1B). In individuals with exacerbated response, CK levels displayed strong positive correlations with markers of tissue damage, including CKMB (r = 0.63; p = 0.003), LDH (r = 0.88; p < 0.0001), AST (r = 0.87; p < 0.0001), ALT (r = 0.71; p < 0.001) and urea (r = 0.56; p = 0.001), as well as negative correlations with lymphocyte count (r = −0.51; p = 0.005), eosinophil count (r = −0.46; p = 0.01), serum phosphorus (r = −0.37; p = 0.04), and adrenal steroid hormones, including androsterone (r = −0.47; p = 0.01), etiocholanolone (r = −0.39; p = 0.03), and epitestosterone (r = −0;36; p = 0.05). In the moderate response group, several of these correlations persisted but were weaker in magnitude. Interestingly, urea showed an inverse association with CK in the moderate group (r = −0.06; p = 0.70), contrasting with the positive correlation seen in the exacerbated group. Also of note, eosinophil counts were positively correlated with CK levels in individuals with a moderate muscular response (r = 0.13; p = 0.52).
ALT, CKMB and eosinophils independently associate with exacerbated muscular response in adjusted models
To further investigate which markers were independently associated with an exacerbated muscular response, a stepwise logistic regression analysis was conducted, including all variables previously correlated with CK levels. In the final adjusted model, CKMB (β = 0.157; 95% CI: 0.045 to 0.268) and ALT (β = 0.206; 95% CI: 0.021 to 0.390) remained significantly associated with the likelihood of presenting CK levels above 1000 U/L (Fig. 2). Interestingly, while eosinophil count did not reach statistical significance, it was retained in the stepwise model and exhibited a negative β coefficient, suggesting a possible protective effect in this physiological context. Another relevant observation is that ALT emerged as an independent variable, whereas AST—despite its biological relevance and correlation with CK—was not retained.
Factors associated with exercise intensity. Forest plot presents the adjusted Odds Ratios (OR) and corresponding 95% confidence intervals (CI) for variables retained in the final model following a backwards stepwise regression analysis. The vertical dashed line at OR = 1 represents the null effect. The model was adjusted for CKMB, LDH, AST, ALT, Urea, Phosphorus, Lymphocyte and Eosinophil count, Androsterone, Etiocholanolone, Epitestoterone. Only parameters that persisted in the last step are shown. P-value in bold font are statistically significant. Participants were classified as Moderate Muscular Response (CK < 1000 U/L) or Exacerbated Muscular Response (CK ≥ 1000 U/L) Abbreviations: ALT = Alanine Aminotransferase, AST = Aspartate Aminotransferase, CK = Creatine Kinase, CKMB = Creatine Kinase Muscle-Brain, LDH = Lactate Dehydrogenase.
Discussion
Muscle damage resulting from high-intensity physical exertion is a well-documented phenomenon in exercise physiology20,21,22. However, the extent to which this response is accompanied by broader systemic alterations - particularly in immune and endocrine pathways - remains incompletely characterized, especially under real-world operation conditions. In this study, we investigated a cohort of active-duty military personnel exposed to cumulative physical stress during five distinct field missions, and demonstrated that individuals with exacerbated muscular response (defined by post-mission CK ≥ 1000 U/L) exhibited distinct systemic biomarkers profiles. These included elevations in classical markers of tissue injury, reductions in circulating immune cells, and suppression of adrenal steroid hormones. To our knowledge, this is the first study to describe this multisystemic physiological pattern in physically healthy individuals submitted to extreme field conditions and its association with stratified levels of muscular stress. Although derived from military tactical settings, these findings may also have relevance for high-performance athletic environments, where cumulative training loads and insufficient recovery periods can similarly lead to physiological dysregulation. Future studies are necessary to test such hypotheses.
The study population was composed of young, physically active military personnel with relatively homogeneous anthropometric and physiological characteristics, and who were part of an elite team. Baseline comparisons across mission sites revealed a similar physical profile in terms of age, weight, height, lean mass, and estimated aerobic capacity, suggesting comparable characteristics at the beginning of the training course. The estimated VO₂ max values are indicative of elevated aerobic capacity in a young, physically active population23,24 which is consistent with what would be expected for individuals undergoing advanced military training. These findings are relevant because they indicate that the systemic differences observed post-mission are unlikely to be attributable to pre-existing physiological variability. Interestingly, despite the similar baseline profile, the proportion of individuals with exacerbated muscular response varied substantially across tropical and high-altitude missions. This variation raises the possibility that environmental or contextual factors specific to each mission, such as altitude, climate, terrain, or operational intensity, may modulate the magnitude of physiological stress independently of baseline fitness. These findings are consistent with previous studies showing that external load and adverse environmental factors serve as independent drivers of muscle damage and systemic dysregulation25,26,27,28,29. Although such differences were not directly measured in the present study, this finding suggests the complexity of systemic responses to physical exertion in real-world operational settings, where the interaction between external load and physiological reserve remains poorly understood.
Despite this high level of conditioning, the pronounced elevation in CK observed in specific missions suggests that aerobic fitness alone may not prevent exacerbated muscular stress under extreme and prolonged exertion. This is aligned with findings from exercise physiology studies showing that well-trained individuals can still exhibit significant muscle damage markers following repeated eccentric loading, especially under conditions of limited recovery30,31,32,33. Similarly, the narrow distribution of lean mass across participants minimizes the likelihood that differences in muscle volume contributed to the variability in post-mission biomarker responses. Taken together, these observations reinforce the hypothesis that environmental and operational stressors—rather than baseline physiological variability—played a central role in shaping the systemic response profiles observed in this study.
The physiological stress induced by intense and repeated physical exertion extends far beyond the musculoskeletal system. While the elevation of CK is commonly used as a marker of muscle damage, the systemic repercussions of such muscular stress remain underappreciated. Exercise-induced increases in CK are typically regarded as localized responses to eccentric or prolonged loading; however, emerging literature suggests that CK elevation may be a sentinel for broader perturbations in immune and metabolic homeostasis, especially under conditions of cumulative strain or incomplete recovery1,20,22,34,35. One intriguing aspect of this physiological cascade lies in the coordinated changes between CK and other biomarkers. Studies in endurance athletes and tactical populations have shown that muscle stress may be accompanied by shifts in hepatic enzymes, circulating immune cells, and even hormonal mediators involved in recovery and adaptation8,20,36,37. This aligns with our observations, where individuals with higher CK levels also displayed alterations in markers commonly associated with systemic stress. Among these, enzymes such as alanine ALT and CKMB, although traditionally linked to hepatic or cardiac injury, have been increasingly recognized in the context of high-volume physical stress as reflecting broader cellular disruption. Their elevation, in the absence of clinical pathology, may signal a systemic spillover effect from skeletal muscle breakdown or secondary tissue involvement.
The immune system appears equally responsive to this overload. Reductions in eosinophil and lymphocyte counts, as documented following intense physical activity, are often interpreted as part of a transient immunosuppressive state - an adaptive, yet potentially risk, phase following acute exertion20,38,39 These alterations, mediated in part by cortisol and catecholamines, suggest a redistribution of immune activity toward damaged tissues, but may also expose the organism to increased susceptibility to infections or delayed recovery. While transient leukopenia is a well-recognized phenomenon post-exercise, few studies have distinguished the specific behavior of different immune cell subsets. The selective reduction of eosinophils and lymphocytes observed in our cohort suggests that cellular responses to acute stress may be more nuanced than previously appreciated, potentially reflecting differential mobilization mechanisms. The inverse relationship between these immune markers and CK in our findings supports the notion that immune modulation is intricately tied to the magnitude or muscular strain. In parallel, the hormonal axis reflects another dimension of this systemic adaptation. Androsterone, etiocholanolone, and epitestosterone were consistently lower in individuals with greater muscular response. This suppression may represent a compensatory adjustment to cumulative physiological load, consistent with studies showing reduced steroidogenic responses in overreached or overtrained states40,41,42. The convergence of muscular, immune, and endocrine alterations delineates a systemic pattern of dysregulation in response to physical overload where CK is not an isolated marker, but part of broader physiological perturbation.
Although the elevation of CK is a well-established marker of muscle damage following strenuous exercise, the broader systemic disturbances that accompany exacerbated muscular response remain less clearly defined. Emerging evidence suggests that muscle injury severity extends beyond mechanical stress, encompassing a complex physiological disruption involving tissue metabolism, immune regulation, and endocrine balance20,43,44. In this context, our findings suggest that markers traditionally associated with muscle injury, such as CKMB and ALT, may serve as indicators of systemic stress adaptation, rather than reflect isolated damage. While CKMB is linked to myocardial injury, its elevation following no-cardiac exertion has been recognized in exercise-related studies1,45, suggesting a generalized cellular disruption as opposed to isolated cardiac injury. Interestingly, despite both ALT and AST being traditionally used as markers of muscle and liver integrity, only ALT was independently associated with exacerbated muscular response in our cohort. Given that AST is widely distributed across multiple tissues and typically considered a marker of generalized cellular injury46,47,48, its absence from the multivariable model raises the possibility that systemic stress under extreme operational load may engage metabolic pathways or selective membrane permeability mechanisms rather than inducing widespread structural disruption. This distinction suggests that ALT may capture aspects of systemic physiological strain that extend beyond only mechanical muscle damage, offering potential as a more sensitive biomarker of systemic stress adaptation in high-demand environments.
Our multivariable modeling revealed that eosinophil counts exhibited a negative association with exacerbated muscular response, independently of other biochemical, hematological, and hormonal variables included in the model. Although the statistical significance was marginal, the independent association strengthens previous observations suggesting a potential modulatory or protective role of eosinophils under cumulative stress conditions. The role of eosinophils under exercise-induced systemic stress remains poorly characterized; however, experimental data suggest that eosinophils may contribute to tissue repair and inflammation resolution49,50,51, processes critically activated following muscle damage. The persistence of this association underscores the need for further investigation, particularly in tactical and athletic populations subjected to repeated cycles of intense physical overload and recovery.
This study has limitations. The relatively small and specialized sample may have influenced the strength and generalizability of the associations detected. The absence of an external control group limits direct comparisons, and we did not perform longitudinal follow-up to assess the persistence of marker changes. Additionally, due to operational constraints, detailed data on carried load, number and distance of marches, resistance task load, sleep duration and caloric intake were not recorded. Prior environmental exposure as time spent at altitude or in extreme climates was also not assessed. These factors may influence individual physiological responses and should be considered in future studies aiming for greater granularity in real-world field settings. Despite these limitations, our findings offer valuable insights into systemic adaptations to extreme physical stress and underscore the potential of integrated biomarker approaches for monitoring physiological strain in high-demand tactical environments.
Conclusion
This study describes a multisystemic physiological pattern associated with exacerbated muscular response in military personnel exposed to cumulative physical overload. By integrating biochemical, immunological, and endocrine biomarkers, we demonstrated that extreme physical stress induces coordinated systemic alterations beyond isolated muscle injury. These findings underscore the relevance of integrated interpretations of traditional clinical markers in high-demand physiological contexts and support future strategies for individualized monitoring and recovery in both tactical and athletic settings.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Brancaccio, P., Maffulli, N. & Limongelli, F. M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 81–82, 209–230 (2007).
Bragg, R. W. et al. Failure and fatigue characteristics of adhesive athletic tape. Med. Sci. Sports Exerc. 34, 403–410 (2002).
Robson-Ansley, P. J., Gleeson, M. & Ansley, L. Fatigue management in the Preparation of olympic athletes. J. Sports Sci. 27, 1409–1420 (2009).
Kim, J. et al. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 5, 324–333 (2015).
Scalco, R. S. et al. Exertional rhabdomyolysis: physiological response or manifestation of an underlying myopathy? BMJ Open. Sport Exerc. Med. 2, e000151 (2016).
Nindl, B. C. et al. Physiological consequences of U.S. Army ranger training. Med. Sci. Sports Exerc. 39, 1380–1387 (2007).
Wei, C. et al. Effect of multiple-nutrient supplement on muscle damage, liver, and kidney function after exercising under heat: based on a pilot study and a randomised controlled trial. Front. Nutr. 8, 740741 (2021).
Nieman, D. C. & Wentz, L. M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 8, 201–217 (2019).
Hackney, A. C. Hypogonadism in exercising males: dysfunction or adaptive-regulatory adjustment? Front. Endocrinol. (Lausanne). 11, 11 (2020).
Cadegiani, F. A. & Kater, C. E. Hypothalamic-pituitary-adrenal (HPA) axis functioning in overtraining syndrome: findings from endocrine and metabolic responses on overtraining syndrome (EROS)—EROS-HPA axis. Sports Med. Open 3, 45 (2017).
Cooper, K. H. A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA 203, 201–204 (1968).
Jackson, A. S. & Pollock, M. L. Practical assessment of body composition. Phys. Sportsmed. 13, 76–90 (1985).
Siri, W. E. The gross composition of the body. Adv. Biol. Med. Phys. 4, 239–280 (1956).
Lee, J. & Clarkson, P. M. Plasma creatine kinase activity and glutathione after eccentric exercise. Med. Sci. Sports Exerc. 35, 930–936 (2003).
Isaacs, A. W., Macaluso, F., Smith, C. & Myburgh, K. H. C-reactive protein is elevated only in high creatine kinase responders to muscle damaging exercise. Front. Physiol. 10, 86 (2019).
Bäcker, H. C. et al. Exertional rhabdomyolysis and causes of elevation of creatine kinase. Phys. Sportsmed. 48, 179–185 (2020).
Walter, B. Dry reagent chemistries in clinical analysis. Anal. Chem. 55, 498A–514A (1983).
Minniti, G., Cerone, R. & De Toni, E. Determination of lactic acid, pyruvic acid, and ketone bodies in serum and cerebrospinal fluid by HPLC. Am. Clin. Lab. 20, 21–23 (2001).
Kirsztajn, G. M. et al. Estimated glomerular filtration rate in clinical practice: consensus positioning of the Brazilian society of nephrology (SBN) and Brazilian society of clinical pathology and laboratory medicine (SBPC/ML). J. Bras. Nefrol. 46, e20230193 (2024).
Peake, J. M., Neubauer, O., Della Gatta, P. A. & Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 122, 559–570 (2017).
Owens, D. J., Twist, C., Cobley, J. N., Howatson, G. & Close, G. L. Exercise-induced muscle damage: what is it, what causes it and what are the nutritional solutions? EJSS (Champaign). 19, 71–85 (2019).
Clarkson, P. M. & Hubal, M. J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 81, S52–69 (2002).
Kaminsky, L. A., Arena, R. & Myers, J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise National database. Mayo Clin. Proc. 90, 1515–1523 (2015).
ACSM & & Cemal, O. ACSM’s Guidelines for Exercise Testing and Prescription (Wolters Kluwer Health, 2025).
Mazzeo, R. S. Altitude, exercise and immune function. Exerc. Immunol. Rev. 11, 6–16 (2005).
Di Domenico, I., Hoffmann, S. M. & Collins, P. K. The role of sports clothing in thermoregulation, comfort, and performance during exercise in the heat: A narrative review. Sports Med. Open. 8, 58 (2022).
De Blois, J. et al. The effects of climate change on cardiac health. Cardiology 131, 209–217 (2015).
Nieman, D. C. Current perspective on exercise immunology. Curr. Sports Med. Rep. 2, 239–242 (2003).
Hassan, E. S. Muscle damage and immune responses to prolonged exercise in environmental extreme conditions. J. Sports Med. Phys. Fit. 56, 1206–1213 (2016).
Paulsen, G., Mikkelsen, U. R., Raastad, T. & Peake, J. M. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 18, 42–97 (2012).
Lin, C. H., Lin, Y. A., Chen, S. L., Hsu, M. C. & Hsu, C. C. American ginseng attenuates eccentric exercise-induced muscle damage via the modulation of lipid peroxidation and inflammatory adaptation in males. Nutrients 14, 78 (2021).
Sayers, S. P., Clarkson, P. M. & Lee, J. Activity and immobilization after eccentric exercise: II. Serum CK. Med. Sci. Sports Exerc. 32, 1593–1597 (2000).
McHugh, M. P., Connolly, D. A., Eston, R. G. & Gleim, G. W. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med. 27, 157–170 (1999).
Lin, A. C. M., Lin, C. M., Wang, T. L. & Leu, J. G. Rhabdomyolysis in 119 students after repetitive exercise. Br. J. Sports Med. 39, e3 (2005).
Brancaccio, P., Limongelli, F. M. & Maffulli, N. Monitoring of serum enzymes in sport. Br. J. Sports Med. 40, 96–97 (2006).
Lee, E. C. et al. Biomarkers in sports and exercise: tracking health, performance, and recovery in athletes. J. Strength. Cond Res. 31, 2920–2937 (2017).
Koch, A. J., Pereira, R. & Machado, M. The creatine kinase response to resistance exercise. J. Musculoskelet. Neuronal Interact. 14, 68–77 (2014).
Campbell, J. P. & Turner, J. E. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front. Immunol. 9, 648 (2018).
Walsh, N. P. et al. Position statement. Part one: immune function and exercise. Exerc. Immunol. Rev. 17, 6–63 (2011).
Cadegiani, F. A. & Kater, C. E. Hormonal aspects of overtraining syndrome: a systematic review. BMC Sports Sci. Med. Rehabil. 9, 14 (2017).
Tanskanen, M. M. et al. Serum sex hormone-binding Globulin and cortisol concentrations are associated with overreaching during strenuous military training. J. Strength. Cond Res. 25, 787–797 (2011).
Lehmann, M. et al. Training-overtraining: performance, and hormone levels, after a defined increase in training volume versus intensity in experienced middle- and long-distance runners. Br. J. Sports Med. 26, 233–242 (1992).
Chazaud, B. Inflammation during skeletal muscle regeneration and tissue remodeling: application to exercise-induced muscle damage management. Immunol. Cell. Biol. 94, 140–145 (2016).
Hicks, K. M., Onambélé, G. L., Winwood, K. & Morse, C. I. Muscle damage following maximal eccentric knee extensions in males and females. PLoS One. 11, e0150848 (2016).
Miles, M. P. & Schneider, C. M. Creatine kinase isoenzyme MB May be elevated in healthy young women after submaximal eccentric exercise. J. Lab. Clin. Med. 122, 197–201 (1993).
Giannini, E. G., Testa, R. & Savarino, V. Liver enzyme alteration: a guide for clinicians. CMAJ 172, 367–379 (2005).
Karmen, A., Wroblewski, F. & Ladue, J. S. Transaminase activity in human blood. J. Clin. Invest. 34, 126–131 (1955).
Suzuki, K. et al. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 87, 1360–1367 (1999).
Day, K. S., Rempel, L., Rossi, F. M. V. & Theret, M. Origins and functions of eosinophils in two non-mucosal tissues. Front. Immunol. 15, 1368142 (2024).
Coden, M. E. & Berdnikovs, S. Eosinophils in wound healing and epithelial remodeling: is coagulation a missing link? J. Leukoc. Biol. 108, 93–103 (2020).
Lokwani, R. et al. Eosinophils respond to extracellular matrix treated muscle injuries but are not required for macrophage polarization. Adv. Healthc. Mater. 14, e2400134 (2025).
Acknowledgements
The authors would like to thank the Brazilian Navy – Marinha do Brasil (Comando do Material do Corpo de Fuzileiros Navais, Hospital Naval Marcílio Dias, Instituto de Pesquisas Biomédicas da Marinha, and Centro de Instrução Almirante Sylvio de Camargo) for research support and for providing access to facilities. We are also grateful to Dr. Eduardo Pernambuco for sharing laboratory space and equipment. Special thanks to Greiner Bio-One Brasil Produtos Médicos Hospitalares and Anil Lab 1288 Comércio e Representações for providing materials and reagents for biochemical analyses. Financial support for this study was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant numbers 2018/18257-1, 2018/15549-1, 2020/04923-0, and 2014/27198-8). This work was also supported by the Intramural Research Program of the Fundação Oswaldo Cruz (B.B.A.), Intramural Research Program of the Fundação José Silveira (B.B.A). B.B.D received a fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Finance code: 001). B.B.A is senior investigators and fellows from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
B.B.A., A.C, D.V.G., B.B.D., D.O.S. designed the study and mentored the work; J.B.P., F.R.A.N., R.L. performed the experiments and data collection; B.B.A., D.O.S., B.B.D. performed data analyses; D.O.S. wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclaimer
The views expressed in this manuscript are solely those of the authors and do not necessarily reflect the official policies or positions of the Brazilian Navy, the Department of Defense, or the Brazilian Government.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oliveira-de-Souza, D., Gomes, D.V., Pesquero, J.B. et al. Biochemical, immune, and endocrine biomarkers associated with exacerbated muscular response: insights from a field-based operational cohort study. Sci Rep 15, 42041 (2025). https://doi.org/10.1038/s41598-025-26196-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26196-6