Abstract
Overweight/Obesity, characterized by body mass index (BMI) ≥ 25 kg/m2, is recognized as a significant prognostic factor in HER2-positive breast cancer, yet its precise influence on neoadjuvant targeted therapy response remains incompletely understood. To address this critical knowledge gap, we conducted a multicentre retrospective cohort study investigating the impact of BMI on pathological complete response (pCR) among HER2-positive breast cancer patients undergoing neoadjuvant targeted therapy. The study comprised 826 Chinese patients from January 2013 to June 2024. Patients were stratified into into two cohorts: underweight/normal weight (UW/NW: BMI < 25 kg/m2) and overweight/obese (OW/OB: BMI ≥ 25 kg/m2). Our analysis revealed a statistically significant disparity in pCR rates, with OW/OB patients demonstrating markedly lower response rates compared to UW/NW patients (36.87% vs. 44.56%; adjusted odds ratio 0.71, 95% CI 0.51–0.99; p = 0.025). Sophisticated restricted cubic spline (RCS) analysis uncovered a nuanced non-linear negative correlation between BMI and pCR rate. Exploratory subgroup analysis further elucidated that OW/OB patients, particularly those aged > 50 years, postmenopausal, with lymph node involvement, hormone receptor negativity, or HER2 3 + status, exhibited substantially compromised pCR rates. Collectively, these findings substantiate the potential of BMI as a robust predictive biomarker for neoadjuvant therapy response in Chinese HER2-positive breast cancer patients.
Similar content being viewed by others
Introduction
Breast cancer has emerged as the most predominant malignancy among women globally. According to the comprehensive GLOBOCAN 2022 epidemiological data, approximately 2.31 million new breast cancer cases were diagnosed worldwide, representing 11.6% of all female malignant neoplasms1. Notably, China contributed substantially to this global burden, with approximately 416,371 new breast cancer cases in 2020, accounting for 18% of the worldwide total2. Within this landscape, HER2-positive breast cancer represents a particularly critical subtype, comprising approximately 15% of all breast cancers and characterized by its notably aggressive clinical behavior3. Pathological complete response (pCR) has been established as a pivotal surrogate endpoint for evaluating the efficacy of neoadjuvant therapeutic interventions, with reported pCR rates in HER2-positive breast cancer ranging between 57.6% and 64.4% across diverse clinical studies4,5. Critically, patients achieving pCR in HER2-positive breast cancer demonstrate significantly superior survival outcomes compared to non-responders6. Body mass index (BMI), a fundamental anthropometric measure of nutritional status, has been consistently demonstrated to exhibit a complex relationship with breast cancer incidence and prognosis7. The potential mechanisms through which obesity modulates breast cancer treatment response are multifaceted, including perturbations in estrogen metabolism, insulin signaling pathways, and systemic inflammatory responses8,9.
The impact of BMI on treatment response may exhibit substantial heterogeneity across different molecular breast cancer subtypes. Holm et al. found that patients who are overweight (BMI = 25–30 kg/m²) and obese (BMI ≥ 30 kg/m²) had 22% and 27% lower odds of achieving pCR, respectively10. However, an exploratory analysis by Wang et al. based on I-SPY2 trial data showed no significant difference in pCR rates across different BMI groups within the biologically high-risk breast cancer population11. In HER2-positive breast cancer, BMI potentially modulates pCR rates of neoadjuvant therapy through complex mechanisms involving tumor microenvironment alterations, drug metabolism, and immune function regulation. Existing literature presents conflicting evidence regarding BMI’s influence on neoadjuvant therapy efficacy in HER2-positive breast cancer. A comprehensive subgroup analysis integrating eight prospective clinical trials reported no significant correlation between BMI and pCR in HER2-positive patients12. Conversely, a recent study and meta-analysis by Chen and colleagues demonstrated that overweight/obese HER2-positive patients exhibited significantly reduced pCR rates13. Given these contradictory findings, our study aims to comprehensively investigate the impact of BMI on neoadjuvant therapy response in HER2-positive breast cancer patients through a robust multi-center retrospective cohort analysis.
Results
Patients characteristics
The study enrolled a total of 826 patients with confirmed HER2-positive breast cancer. Patients were stratified into two groups: underweight/normal weight (UW/NW, n = 487) and overweight/obese (OW/OB, n = 339). Baseline characteristics were comprehensively detailed in Table 1. The OW/OB group exhibited statistically significant differences compared to the UW/NW group, notably characterized by a higher median age and a substantially greater proportion of hormone receptor (HR)-positive patients. Patients in the OW/OB cohort demonstrated a higher likelihood of receiving docetaxel and carboplatin (TCb) as their neoadjuvant chemotherapy regimen. A significant association was observed between BMI category and pCR (p = 0.027). Notably, no statistically significant correlations were detected between BMI category and other clinicopathological factors, underscoring the nuanced relationship between BMI and treatment response in HER2-positive breast cancer.
BMI and neoadjuvant therapy response
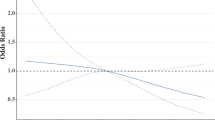
A total of 342 patients (41.4%) achieved pCR following neoadjuvant targeted therapy. Restricted cubic spline (RCS) analysis with three knots demonstrated a progressive decline in pCR probability with increasing BMI, even after adjusting for potential confounders (Fig. 1). When stratified by BMI categories, the pCR rates were 44.56% in underweight/normal weight (UW/NW) and 36.87% in OW/OB groups. Multivariate analysis revealed that patients in the OW/OB groups had significantly lower odds of achieving pCR compared to the UW/NW groups (odds ratio [OR] = 0.71, 95% confidence interval [CI]: 0.51–0.99, p = 0.025). Additionally, our study uncovered that HER2-positive breast cancers with HR-positive status or HER2 staining intensity of 3 + exhibited higher pCR rates following neoadjuvant therapy. Notably, patients receiving TCb chemotherapy regimen or trastuzumab plus pertuzumab combination therapy demonstrated a higher likelihood of achieving pCR (Table 2).
Restricted cubic splines describing the non-linear association between body mass index (BMI) and pathological complete response (pCR) in HER2-positive breast cancer patients receiving neoadjuvant targeted therapy. Odds ratios (OR) are based on logistic regression adjusted for age, clinical T stage, node status, hormone receptor status, HER2 staining intensity, Ki-67, neoadjuvant chemotherapy regimen and targeted therapy.
Exploratory subgroup analysis of BMI and pCR
Exploratory subgroup analysis revealed potential associations between BMI and neoadjuvant therapy response in HER2-positive breast cancer patients. Notably, OW/OB patients exhibited significantly compromised pCR rates across clinically critical subgroups, including patients aged > 50 years, postmenopausal individuals, those with lymph node involvement, HR-negative patients, and individuals with HER2 3 + status (Fig. 2). Moreover, OW/OB patients treated with TCb chemotherapy or trastuzumab demonstrated markedly reduced pCR rates compared to their UW/NW counterparts.
Odds ratio (OR) of pathological complete response (pCR) for HER2-positive breast cancer patients stratified by body mass index (BMI) category across different subgroups. underweight (UW, BMI < 18.5 kg/m²), normal weight (NW, 18.5 ≤ BMI < 25 kg/m²), overweight (OW, 25 ≤ BMI < 30 kg/m²), and obese (OB, BMI ≥ 30 kg/m²).
Discussion
In this multicenter retrospective study involving 826 consecutive HER2-positive breast cancer patients, we investigated the association between BMI and pCR rate after neoadjuvant targeted therapy. The strengths of this study include its large sample size and data collection from 42 centers across China, enhancing the representativeness and reliability of our findings. Our key results demonstrate that overweight/obese (BMI ≥ 25.
kg/m²) HER2-positive breast cancer patients had significantly lower odds of achieving pCR compared to normal/underweight patients. Specifically, the pCR rate was 44.56% in the NW/UW group versus 36.87% in the OW/OB group. This finding supports the value of BMI as a potential biomarker for predicting neoadjuvant treatment efficacy in HER2-positive breast cancer patients.
Our findings that higher BMI is associated with reduced pCR rates in HER2-positive breast cancer patients receiving neoadjuvant targeted therapy are consistent with recent evidence. Chen et al. conducted a retrospective study of 186 HER2-positive breast cancer patients and found that overweight/obese patients had significantly lower pCR rates compared to normal-weight patients (35.1% vs. 53.7%), which is highly consistent with our observed difference (36.87% vs. 44.56%)14. Their study employed similar BMI categorization criteria and adjusted for confounding factors comparable to ours, including age, HR status, and clinical stage. Crozier et al. reported similar findings in a study of 278 HER2-positive breast cancer patients receiving trastuzumab-based neoadjuvant therapy, demonstrating that for every 5 kg/m² increase in BMI, the likelihood of achieving pCR decreased by 21%15. This aligns with our RCS analysis showing a declining trend in pCR rates with increasing BMI. In addition, the NeoALTTO trial demonstrated that overweight/obese patients with HER2-positive luminal-like breast cancer had reduced pCR rates following neoadjuvant treatment, suggesting that obesity may interfere with therapeutic response, particularly via crosstalk with hormonal metabolic pathways16. However, the observed results differ markedly from those of Mazzarella et al. In their pooled analysis of eight prospective European clinical trials (n = 1695), no significant association was found between body mass index (BMI) and pathological complete response (pCR) rates in HER2-positive breast cancer patients17. This discrepancy may be attributed to multiple factors: first, our study focused on Chinese patients, while their research primarily included Caucasian populations, with potential racial differences in metabolic patterns and treatment responses; second, we employed standardized trastuzumab-based chemotherapy approaches, whereas their study encompassed varied treatment regimens; third, their analysis was derived from randomized controlled trials, while our study was based on real-world data with inherently different patient characteristics and clinical practices. Collectively, our study not only corroborates prior evidence but also provides large-scale, multicenter real-world data from Chinese patients, thereby enriching the global evidence base.
The potential mechanisms by which overweight/obesity affects neoadjuvant therapy efficacy in HER2-positive breast cancer are multifaceted. First, adipose tissue functions as an endocrine-active organ that secretes various pro-inflammatory cytokines, including TNF-α, IL-6, and leptin, which can activate NF-κB and STAT3 signaling pathways, promoting tumor growth and inhibiting drug sensitivity18. Second, obesity-associated insulin resistance and hyperinsulinemia can enhance tumor cell survival through activation of the PI3K/Akt/mTOR pathway, which is also a key downstream component of HER2 signaling and may contribute to resistance to targeted therapy19. Third, increased volume of distribution in obese patients may affect the pharmacokinetic properties of anti-HER2 agents and chemotherapeutic drugs, potentially reducing effective drug concentrations at tumor sites20. Additionally, obesity is associated with alterations in the tumor immune microenvironment, such as decreased CD8 + T-cell infiltration and increased regulatory T cells, which may impair the immune system’s ability to recognize and eliminate tumor cells, thereby affecting treatment response21. Our subgroup analysis further revealed significantly lower pCR rates in overweight/obese patients who were older than 50 years, postmenopausal, lymph node-positive, HR-negative, or HER2 3+. These findings highlight the importance of BMI as a potential predictive biomarker in specific high-risk populations, providing valuable information for clinical decision-making.
The clinical value of this study lies in its systematic revelation of the association between BMI and neoadjuvant therapy efficacy in a large Chinese cohort of HER2-positive breast cancer patients, providing crucial evidence for precision medicine. Compared to previous research, our unique contribution includes the incorporation of multi-center real-world data, exploration of the non-linear relationship between BMI and pCR using restricted cubic spline modeling, and identification of high-risk populations where BMI impact is more pronounced through comprehensive subgroup analyses. These findings have multiple implications for clinical practice: first, for overweight/obese HER2-positive breast cancer patients, particularly those who are > 50 years old, postmenopausal, lymph node-positive, HR-negative, or HER2 3+, more intensified treatment regimens should be considered, such as adding pertuzumab for dual HER2-targeted therapy; second, for patients with BMI ≥ 25 kg/m², clinicians should enhance monitoring during treatment, evaluate therapeutic response, and adjust treatment strategies accordingly; third, BMI can serve as a risk stratification tool early in diagnosis to identify patients who might benefit from more intensive therapy, facilitating individualized treatment decisions; fourth, lifestyle interventions and weight management should be emphasized for obese patients as potential adjunctive strategies to improve treatment efficacy.
Despite the robust findings of our investigation, several methodological limitations necessitate cautious interpretation. As a retrospective observational study, we can exclusively demonstrate an association between BMI and pCR rates, falling short of establishing definitive causal relationships. The study’s population, confined to Chinese HER2-positive breast cancer patients receiving trastuzumab-based neoadjuvant therapy, introduces significant generalizability constraints. While our multi-center approach involving 42 medical institutions enhances internal validity, the ethnic specificity limits broader extrapolation, particularly given the well-documented metabolic and adipose tissue distribution variations across racial populations. Our analytical approach, though rigorously controlling for age, hormone receptor status, and clinical stage, cannot comprehensively eliminate potential unmeasured confounding factors. Critical data limitations prevented incorporation of nuanced parameters such as detailed body composition analysis (e.g., DEXA/CT-based assessments), molecular characteristics like PIK3CA mutations, precise diagnostic timelines, medication dose modifications, and comprehensive lifestyle factors including dietary patterns and physical activity levels. Methodologically, relying exclusively on BMI as an obesity indicator represents a significant constraint, as this metric fails to capture complex parameters such as fat distribution patterns, central obesity characteristics, and muscle-to-fat ratio—parameters potentially more predictive of treatment response. Furthermore, the retrospective design’s inherent limitations precluded obtaining long-term follow-up data, rendering impossible a comprehensive evaluation of BMI’s potential impact on disease-free and overall survival trajectories. Notwithstanding these substantive methodological constraints, our research provides valuable preliminary insights into BMI’s potential influence on neoadjuvant therapy efficacy. Future research directions include exploring how obesity-related molecular mechanisms affect anti-HER2 treatment response, investigating the impact of drug dose-weight relationships on treatment outcomes, evaluating whether weight management interventions can improve prognosis in obese patients, and validating the predictive value of BMI across different treatment regimens. Additionally, integrating analyses with genomic and immunological biomarkers may further optimize BMI-based risk stratification models. In conclusion, this study not only deepens our understanding of BMI’s role in HER2-positive breast cancer treatment but also provides valuable insights for clinical decision-making and future research.
Methods
Data acquisition
All data for this study were obtained from the Shanghai Jiaotong University Breast Cancer Database (SJTU-BCDB) (http://47.100.125.104:8080/), which integrates breast cancer information from 42 medical centers across China. Breast cancer diagnoses were confirmed with core needle biopsy prior to initiating neoadjuvant therapy. This study included patients diagnosed with HER2-positive breast cancer who underwent neoadjuvant therapy followed by radical breast surgery between January 2013 and June 2024. The neoadjuvant chemotherapy regimens utilized in this study included: 1.EC-T regimen: Epirubicin (100 mg/m²) and cyclophosphamide (600 mg/m²) administered every three weeks for four cycles, followed by docetaxel (80 mg/m²) every three weeks for four cycles. 2. TCb regimen: Docetaxel (75 mg/m²) and carboplatin (area under the curve [AUC] = 6) administered every three weeks for six cycles. All patients received trastuzumab, administered as a loading dose of 8 mg/kg followed by a maintenance dose of 6 mg/kg, or along with pertuzumab (loading dose of 840 mg on cycle 1 and 420 mg thereafter every three weeks). Chemotherapy and Anti-HER2 targeted therapy are administered simultaneously, except when combined with anthracyclines. The study protocol was approved by the independent ethics committee of all participating hospitals, adhering to the principles of the Declaration of Helsinki. Given the retrospective design of the study, the requirement for informed consent was waived by the Ethics Committee of Quanzhou First Hospital. Patients with metastatic disease and those with missing baseline BMI data were excluded from the analysis.
Definitions
Women were considered postmenopausal if they had no menstruation for the past 12 consecutive months. Clinical tumor size and axillary lymph node status before neoadjuvant therapy were evaluated by color Doppler ultrasound and staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) Breast Cancer Staging Manual. Height and weight were recorded at patients’ first hospitalization prior to initiating neoadjuvant therapy. BMI was calculated as weight (kg) divided by height squared (m²). Patients were categorized into four groups: underweight (UW, BMI < 18.5 kg/m²), normal weight (NW, 18.5 ≤ BMI < 25 kg/m²), overweight (OW, 25 ≤ BMI < 30 kg/m²), and obese (OB, BMI ≥ 30 kg/m²)10. Estrogen receptor (ER), progesterone receptor (PR), and HER2 were assessed by immunohistochemistry (IHC). ER and PR were considered positive if ≥ 1% of tumor nuclei showed positive staining, and patients were classified as hormone receptor (HR) negative when both ER and PR were negative. HER2 positivity was defined as IHC score of 3 + or IHC score of 2 + with HER2 amplification confirmed by fluorescence in situ hybridization (FISH). Patients were stratified into low and high Ki67 expression groups using a 30% cutoff value. Pathological complete response (pCR) was defined as the absence of residual invasive disease in both breast and axillary lymph nodes (ypT0/is, ypN0).
Statistical analysis
Categorical variables were analyzed using chi-square tests to examine associations between BMI categories and clinicopathological characteristics. The impact of BMI on neoadjuvant therapeutic response was evaluated both as a continuous and categorical variable. Restricted cubic spline (RCS) regression with three knots was employed to explore potential non-linear relationships between BMI and pCR. Multivariate logistic regression analysis was conducted to identify factors independently associated with pCR. All statistical analyses were performed using R statistical software (version 4.3.3). A two-sided p-value < 0.05 was considered statistically significant.
Data availability
The data supporting all tables and figures in this published article are not publicly available to protect patient privacy but can be accessed from the corresponding author on request.
References
Bray, F. et al. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 74, 229–263 (2024).
Tao, X. et al. Mortality, Survival, and disease burden of breast cancer in China compared to other developed countries. Asia-Pac J. Clin. Oncol. 19, 645–654 (2023).
Agostinetto, E., Curigliano, G. & Piccart, M. Emerging treatments in Her2-Positive advanced breast cancer: keep Raising the bar. Cell. Rep. Med. 5, 101575 (2024).
Chen, X. C. et al. De-Escalated neoadjuvant weekly Nab-Paclitaxel with trastuzumab and Pertuzumab versus Docetaxel, Carboplatin, trastuzumab, and Pertuzumab in patients with Her2-Positive early breast cancer (Helen-006): A Multicentre, Randomised, phase 3 trial. Lancet Oncol. 26, 27–36 (2025).
Li, J. J. et al. Efficacy and safety of neoadjuvant Shr-a1811 with or without Pyrotinib in women with locally advanced or early Her2-Positive breast cancer: A Randomized, Open-Label, phase II trial. Ann. Oncol. 36, 651–659 (2025).
van Mackelenbergh, M. T. et al. Pathologic complete response and individual patient prognosis after neoadjuvant chemotherapy plus Anti-Human epidermal growth factor receptor 2 therapy of human epidermal growth factor receptor 2-Positive early breast cancer. J. Clin. Oncol. 41, 2998–3008 (2023).
Chan, D. et al. Postdiagnosis body Fatness, weight change and breast cancer prognosis: global cancer update program (Cup global) systematic literature review and Meta-Analysis. Int. J. Cancer. 152, 572–599 (2023).
Javed, S. R., Skolariki, A., Zameer, M. Z. & Lord, S. R. Implications of obesity and insulin resistance for the treatment of oestrogen Receptor-Positive breast cancer. Br. J. Cancer. 131, 1724–1736 (2024).
Kolb, R. & Zhang, W. Obesity and breast cancer: a case of inflamed adipose tissue. Cancers 12, (2020).
Holm, J. B. et al. The association between body mass index and neoadjuvant chemotherapy response in patients with breast cancer. Breast Cancer Res. 27, 130 (2025).
Wang, H. et al. Impact of body mass index on pathological response after neoadjuvant chemotherapy: results from the I-Spy 2 trial. Breast Cancer Res. Treat. 204, 589–597 (2024).
Fontanella, C. et al. Impact of body mass index on neoadjuvant treatment outcome: A pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res. Treat. 150, 127–139 (2015).
Chen, L. et al. Impact of body mass index in therapeutic response for Her2 positive breast cancer treated with neoadjuvant targeted therapy: A Multi-Center study and Meta-Analysis. Npj Breast Cancer. 9, 46 (2023).
Chen, S. et al. Obesity or overweight is associated with worse pathological response to neoadjuvant chemotherapy among Chinese women with breast cancer. Plos One. 7, e41380 (2012).
Crozier, J. A. et al. Effect of body mass index on tumor characteristics and Disease-Free survival in patients from the Her2-Positive adjuvant trastuzumab trial N9831. Cancer 119, 2447–2454 (2013).
Di Cosimo, S. et al. Effect of body mass index on response to Neo-Adjuvant therapy in Her2-Positive breast cancer: an exploratory analysis of the Neoaltto trial. Breast Cancer Res. 22, 115 (2020).
Mazzarella, L. et al. Obesity increases the incidence of distant metastases in oestrogen receptor-Negative human epidermal growth factor receptor 2-Positive breast cancer patients. Eur. J. Cancer. 49, 3588–3597 (2013).
Iyengar, N. M., Gucalp, A., Dannenberg, A. J. & Hudis, C. A. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J. Clin. Oncol. 34, 4270–4276 (2016).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. Ca-Cancer J. Clin. 67, 378–397 (2017).
de Azambuja, E. et al. The effect of body mass index on overall and Disease-Free survival in Node-Positive breast cancer patients treated with docetaxel and Doxorubicin-Containing adjuvant chemotherapy: the experience of the big 02–98 trial. Breast Cancer Res. Treat. 119, 145–153 (2010).
Koru-Sengul, T. et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of Crown-Like structures associated with lower survival compared to Non-Black Latinas and Caucasians. Breast Cancer Res. Treat. 158, 113–126 (2016).
Acknowledgements
We appreciate the data support provided by the Shanghai Jiaotong University-Breast cancer Database (SJTU-BCDB) (http://47.100.125.104:8080/).
Funding
This work was supported by the Fujian Provincial Health Technology Project (Grant No.2024QNA087).
Author information
Authors and Affiliations
Contributions
WL, CH and DC contributed to the study conception and design, analysis of data was contributed by CH and PY, CH and PY prepared all the figures and tables, CH drafted the manuscript, WL, PY, CH and DC discussed and edited the paper. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by Ethics Committee of Quanzhou First Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hong, C., Yang, P., Chen, D. et al. Association between body mass index and efficacy of neoadjuvant targeted therapy in HER2-positive breast cancer in a Chinese multicenter cohort. Sci Rep 15, 42130 (2025). https://doi.org/10.1038/s41598-025-26197-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26197-5