Abstract
Ethiopia faces challenges of food insecurity, malnutrition, and biodiversity loss. The predominantly rural population relies on staple cereals, leading to deficiencies in essential micronutrients. Ethiopia’s Eastern Afromontane biodiversity hotspot hosts a variety of wild edible fruits (WEFs) that could help alleviate these deficiencies. However, WEFs are stigmatised as “food-for-the-poor” and remain underutilised partly due to limited data on their nutritional value. This study is the first to systematically assess the elemental composition of 23 wild and four cultivated fruit species from Oromia and Southern Nations Nationalities and Peoples (SNNP) regions of Ethiopia. Using inductively coupled plasma mass spectrometry, we found several WEFs to be rich in Ca, Fe, Mg, and Se, often surpassing levels in cultivated fruits. A 100 g serving of these fresh fruits could provide up to 40% of recommended nutrient intakes for adolescent boys. Analysis of soil samples collected from fruit harvesting sites revealed significant correlations between soil and fruit elemental concentrations for several minerals, highlighting the importance of soil properties in determining the nutritional quality of WEFs. Species distribution modelling for 11 selected WEF species identified suitable habitats across southern Ethiopia, with significant spatial variation, suggesting opportunities for targeted promotion and conservation. Integrating WEFs into diets and agroforestry systems could enhance nutrition and biodiversity. Further research on bioavailability, domestication, and policy engagement is recommended.
Similar content being viewed by others
Introduction
Ethiopia, home to over 120 million people1, is facing critical challenges in terms of food and nutritional security as well as biodiversity conservation. The country’s predominantly rural population depends on small-scale agriculture for their livelihoods2,3,4, which has implications for both dietary diversity and environmental sustainability. The reliance on staple crops5,6, due to limited land and agricultural resources2,3,4, contributes to dietary deficiencies in essential minerals7, exacerbating the burden of disease8.
Ethiopia harbours a significant portion of the Eastern Afromontane biodiversity hotspot, including thousands of plant species9. However, the pressure of an increasing population has led to deforestation and biodiversity loss10,11, with implications for both the environment11,12 and food security. Despite the global recommendation for a diet rich in fruits and vegetables13 to ensure nutritional adequacy, access to and affordability of such nutritious foods remain a global challenge in general and in Ethiopia in particular14, where diets are predominantly cereal-based and often lack diversity7.
Ethiopia’s forests and woodlands are sources of wild edible fruits15,16, which could play a crucial role in alleviating dietary micronutrient (mineral and vitamin) deficiencies17,18. However, WEFs are frequently stigmatised as “food-for-the-poor” and are generally consumed by the broader community only during periods of food scarcity. Consequently, these fruits have been underutilised and overlooked in agricultural, environmental, and health policies, partly due to insufficient data on their nutritional value.
The nutritional value of fruits, particularly their mineral element composition, is strongly influenced by the soil in which the plants grow. Soil properties such as pH, organic matter content, and mineral availability directly affect plant uptake of essential elements19,20. This soil–plant relationship is particularly important in the diverse agroecological zones of Ethiopia, where soil types and properties vary considerably6. Understanding these relationships is crucial for identifying areas where WEFs might naturally accumulate higher concentrations of nutritionally important elements, as well as for developing potential cultivation strategies for these species.
The distribution of wild fruit species across Ethiopia’s landscape is determined by complex interactions between biotic and abiotic factors, including climate and soil conditions21,22. Climate change and land-use modifications are altering these distributions11,23,24,25, potentially threatening the availability of these nutritional resources26. Species distribution modelling can identify suitable habitats for these fruit species27,28, which is essential for conservation planning and for identifying potential areas for domestication or in-situ management. Furthermore, understanding the relationship between species distribution, soil conditions, and fruit nutritional quality can help identify priority areas where conservation efforts might yield the greatest nutritional benefits for local communities.
Addressing these interconnected challenges, our study presents the first systematic assessment of the elemental composition of 23 wild, and four cultivated edible fruit species found across Ethiopia’s diverse agroecosystems. We specifically aimed to:
-
1.
Determine the mineral element composition of selected wild and cultivated fruit species to assess their potential contribution to addressing nutritional deficiencies,
-
2.
Investigate the relationship between soil mineral content and fruit elemental concentrations to understand how environmental factors influence the nutritional quality of these fruits, and
-
3.
Model the potential distribution of selected wild fruit species across southern Ethiopia to identify areas suitable for conservation, sustainable harvesting, or potential domestication.
By documenting the mineral nutritional content of these edible fruits and relating it to soil conditions, and predicting their potential spatial distribution, this research aims to highlight their dietary value, encourage their domestication and conservation, and support their inclusion in dietary recommendations. This integrated approach not only aims to improve dietary diversity and nutritional status among Ethiopians but also to foster a greater appreciation for the country’s rich plant biodiversity. Engaging local communities and policy makers in the sustainable use and management of these resources could ensure their availability and nutritional benefits for future generations, contributing to the dual goals of improving food security and as non-timber forest products which maintains the vegetation for conserving biodiversity in Ethiopia.
Materials and methods
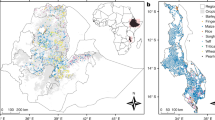
The research was carried out in selected areas of the Oromia and Southern Nations Nationalities and Peoples (SNNP) regions of Ethiopia (Fig. 1) where WEFs grow. These two regions have a combined total population of around 58 million people29. They are renowned for their extensive forest cover, practicing various agroforestry systems, and have diverse agroclimatic zones ranging from low-lying arid lands to high mountain peaks.
Sampling sites of edible fruits (coloured circles) across the Oromia and SNNP regions of Ethiopia. Elevation (meters above sea level; m.a.s.l.) is shown in greyscale. The elevation data was obtained from the digital elevation model of the Shuttle Radar Topography Mission53. The shape files were acquired from the Global Administrative District Map (GADM) Version 4.154. Refer to Table 1 for the scientific names corresponding to the species’ acronyms.
Edible fruit species selection
Important wild edible fruits (WEFs) growing in Ethiopia were identified and selected through ethnobotanical literature reviews16,17,18,30,31,32,33,34. From these reviews, about 200 indigenous woody plant species that bear edible fruits were identified and out of which 138 were selected as priority and used to guide fruit sampling. After determining the priority edible fruit-bearing plant species and the fruiting season, subsequent field sampling was determined by analysing literature data35 and personal communication with forestry and agriculture development workers, and researchers living and working in the areas where these plants grow.
Field sampling of fruits
The planning of field sampling involved using the Global Biodiversity Information Facility (GBIF) data, which serves as a repository for species taxonomic and occurrence data36. To create a comprehensive plan, we combined the occurrence geographic coordinates of native plants in Ethiopia that bear edible fruits, obtained from GBIF, with the zonal thematic geolocation information provided in a study by Teketay, et al.35 that focused on wild edible plants. Teketay, et al.35 had compiled the zonal WEF occurrence and fruiting phenology data using an outdated administrative map of Ethiopia, which was converted into a digital format using QGIS37. By overlaying this digitized map with the WEF point occurrence geolocation data from GBIF, we were able to generate a fruiting calendar for the various WEFs. This calendar was instrumental in planning field sampling, allowing us to target a time when numerous species were fruiting simultaneously, thereby reducing the need for several field trips to Ethiopia. In total, fruits from 23 wild, and four cultivated plant species representing 11 orders and 15 families (see Table 1 and Fig. 2) and growing in various parts of Ethiopia (Fig. 1) were sampled during October–November 2022 and March–April 2023. Fruit samples from cultivated species were obtained from farmers’ agricultural fields, whereas wild species fruit samples were gathered from their native environments. The identification of the plants providing the edible fruits was conducted using digital images of the plant parts by Mr. Melaku Wondafrash from the National Herbarium at Addis Ababa University, and Dr. Feyera Senbeta, a coauthor of this paper. No voucher specimens of the plants bearing the edible fruits were preserved.
The research complied with relevant institutional, national, and international guidelines and legislation. Before conducting surveys and field sampling of edible fruits, pre-informed consent was obtained from the Ethiopian Biodiversity Institute in May 2022. Following the completion of field sampling, a material transfer agreement was signed among the lead researcher (Dr. Diriba B. Kumssa), the University of Nottingham, and the Ethiopian Biodiversity Institute, in accordance with the Nagoya Protocol on Access and Benefit Sharing of biodiversity resources. This agreement covered the export of two rounds of freeze-dried and milled fruit samples, as well as air-dried soil samples. Prior to exporting these samples from Ethiopia to the United Kingdom for biochemical analysis, a phytosanitary certificate was secured from the Ethiopian Agriculture Authority and the Ethiopian National Soil Laboratory.
Wild edible fruits ethnobotanical data and metadata
Ethnobotanical information regarding the use of the wild fruits for human consumption, including who usually consumes them, their taste and colour when ripe, and modes of fruit consumption (see Table 2) were collected using a structured KoBoToolbox electronic questionnaire (see Appendix 1). Prior to conducting the survey with local adults, the study received an ethical approval from the University of Nottingham, School of Biosciences Research Ethics Committee (SBREC), approval number SBREC202122024FEO and the research was performed in accordance with the relevant guidelines and regulations. Survey participants were provided with information about the study and their informed consent was sought prior to interview. Furthermore, fruit and soil georeferenced metadata were collected using an electronic questionnaire implemented in KoBoToolbox (Appendix 2).
Fruit sample pre-processing for biochemical analyses
Representative, ripe fruits in good health, suitable for human consumption, were gathered from various locations on the plants bearing edible fruits. Upon collection, the fruit samples were washed using bottled potable water. The edible portions for each fruit were extracted and placed in one or more 600 mL aluminium foil food containers with lids (Venture Team Ltd, Dunstable, UK). To maintain their freshness while in the field, these containers carrying the edible parts of the fruits were stored in a portable car battery operated Alpicool T60 freezer (Foshan Alpicool Electric Appliance Co. Ltd, Foshan, Guangdong, China), which maintained a temperature of − 10 to − 20 °C.
In order to render samples stable at ambient temperature, frozen fruit samples were blended, their fresh weights recorded and stored at − 40 °C. These samples were then subjected to freeze-drying (FreeZone 12 Litre − 84 °C Console Freeze Dryer with Stoppering Tray Dryer, Labconco Corporation, Kansas City, USA) in a set of 18 containers over a seven-day period until a constant weight was achieved. After the freeze-drying process, the weights of the dried fruit samples were recorded, and the dried fruits were vacuum-sealed in bisphenol-A free food bags using the Homeasy vacuum sealer (Homeasy Ltd, Chester, UK). Finally, the sealed, freeze-dried fruit samples were exported to the University of Nottingham, United Kingdom, for biochemical analyses. Prior to biochemical analyses, the freeze-dried fruit samples were milled using an ultra-centrifugal mill (ZM 200, Retsch GmbH, Haan, Germany) until they could pass through a 2 mm screen.
Elemental analyses
The analyses of the elemental concentrations in the fruits and corresponding soils sampled from beneath the canopy of the fruit species was performed through the application of Inductively Coupled Plasma Mass Spectrometry (ICPMS) using a Thermo Fisher Scientific iCAP Q instrument (Thermo Fisher Scientific, Bremen, Germany). Detailed descriptions of the methodological approaches, including the analytical procedures, are provided in the subsequent sections briefly and used the standard procedures described by6,38.
Fruit samples elemental analysis
Fruit samples were prepared for ionomic analysis via a microwave digestion process, utilizing a Multiwave 5000 platform equipped with a 41HVT56 rotor with 41 vessels (Anton Paar Gmbh, Graz, Austria). Perfluoroalkoxy (PFA) digestion vessels were employed for this purpose. Initially, a finely ground fruit sample weighing 0.2 g was introduced into each PFA digestion vessel. Subsequently, 3 mL of > 68% Trace Analysis Grade (TAG) nitric acid (HNO3), 3 mL of Milli-Q water (18.2 MΩ cm; Fisher Scientific UK Ltd, Loughborough, UK), and 2 mL of Primar TAG hydrogen peroxide (H2O2) (Fisher Scientific UK Ltd, Loughborough, UK) were pipetted into the vessels containing the fruit samples. The digestion process was conducted under the following microwave conditions: power = 1400 W, temperature = 150 °C, pressure = 2 MPa, and a total duration of 45 min. As part of each digestion run, three operational blanks were included to account for any background contamination. The limit of detection (LOD) was determined as three times the standard deviation of analyte concentrations measured in blank samples. Elemental concentrations in the fruit samples that fell below the established LOD were excluded. Moreover, duplicate samples of a certified reference material (CRM: Wheat flour SRM 1567b, National Institute of Standards and Technology, Gaithersburg, MD, USA) were incorporated in every digestion run to ensure analytical accuracy, hence enabling determination of the percentage recovery (see Supplementary Table 2).
After completion of the digestion process, each digestion vessel was adjusted to a final volume of 20 mL by adding 12 mL of Milli-Q water. Subsequently, the contents were transferred to 25 mL universal tubes (Sarstedt Ltd., Nümbrecht, Germany) and stored at room temperature. Prior to analysis, the samples were further diluted at a 1:10 ratio with Milli-Q water in 13 mL tubes (Sarstedt Ltd., Nümbrecht, Germany). Elemental concentrations, including Ag, Al, As, B, Ba, Be, Ca, Cd, Cr, Co, Cs, Cu, Fe, K, Li, Mg, Mn, Na, Ni, P, Pb, Rb, S, Se, Sr, Ti, Tl, U, and V, were quantified using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (Thermo Fisher Scientific iCAPQ, Thermo Fisher Scientific, Bremen, Germany).
Soil samples elemental analysis
Soil samples, collected from beneath the canopies of fruit-bearing plants and reaching a depth of 50 cm, were composited. In Ethiopia, these soil samples underwent an initial process of air-drying and sieving to pass through a 2 mm mesh screen. Upon their arrival in the UK, the samples were further desiccated by placing them in an oven dryer at a temperature of 40 °C for a duration of three days to ensure complete dryness. Subsequently, 0.4 g subsamples of soil were placed into aqua regia digestion tubes (Sarstedt Ltd., Nümbrecht, Germany), into which 3 mL of Trace Analysis Grade (TAG) nitric acid (HNO3) and 2 mL of hydrogen peroxide (H2O2) (Fisher Scientific UK Ltd, Loughborough, UK) were pipetted. Glass watches were positioned on top of the digestion tubes, allowing the soil samples to soak overnight.
The following morning, an additional 9 mL of TAG hydrochloric acid (HCl) (Fisher Scientific UK Ltd, Loughborough, UK) was introduced to the solution, and the tubes were heated to a temperature of 108 °C for a duration of two hours on hot plates. Subsequently, the samples were allowed to cool for one hour. After cooling, the digestion tubes were adjusted to a final volume of 50 mL by adding 36 mL of Milli-Q water, and they were stored at room temperature. Prior to analysis, the samples were further diluted at a ratio of 1:10 with Milli-Q water in 13 mL tubes (Sarstedt Ltd., Nümbrecht, Germany). Quantification of elemental concentrations, encompassing Al, B, Ba, Ca, Cd, Co, Cr, Cs, Cu, Fe, K, Li, Mg, Mn, Na, Ni, P, Pb, Rb, S, Se, Sr, Ti, Tl, V and Zn, was conducted using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) employing the Thermo Fisher Scientific iCAPQ (Thermo Fisher Scientific, Bremen, Germany).
Assessing elemental concentration against reference nutrient intake
For essential minerals with established recommended nutrient intake (RNI), that is for calcium (Ca), iron (Fe), selenium (Se), magnesium (Mg) and zinc (Zn) we assessed the proportion of the RNI fulfilled by consuming the fruits. We used the RNI data from the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO)39. The RNI is a daily nutrient intake that meets the nutrient requirements of 97.5% of apparently healthy individuals within a given age and sex group39. Our assessment focused on determining the percentage of a specific element’s RNI met by consuming 100 g of edible parts of fresh fruits of a particular species. This analysis was based on a reference group of healthy adolescent male children, aged 10–18 years. This choice reflects the common scenario in rural Ethiopia, where male adolescents, often cattle herders, are the primary consumers of wild edible fruits. The RNIs (mg day−1) for this demographic are 1300 for Ca, 14.6 for Fe, 230 for Mg, 0.032 for Se, and 17.1 for Zn. We used these RNI values considering a bioavailability of 10% for Fe and the lowest bioavailability for Zn39.
Statistical analysis and visualisation
To analyse the differences in elemental concentrations across different fruit species, Welch’s analysis of variance (ANOVA) test was employed. This test was used when there were three or more replicate samples for a given fruit species. The Python pingouin package was used to run Welch’s ANOVA as well as the Game-Howell post-hoc test, which allows for multiple pairwise comparisons while controlling for unequal variances and sample sizes between groups40.
Furthermore, to investigate the relationship between the elemental levels found in the fruits and those present in the corresponding soil samples, Pearson’s correlation analysis was conducted. The scipy package in Python was used to compute these correlation coefficients, which measure the strength and direction of the linear association between the two variables (fruit and soil elemental concentrations) across the samples. Additionally, various graphical visualizations, including bar charts, violin plots, box plots, and scatter plots, were created to represent the data using the matplotlib and seaborn packages in the Python programming language.
Species distribution modelling
Species occurrence data sourced from the Global Biodiversity Information Facility (GBIF)36 and the data collected by the project “Wild Edible Fruits (WEF) for Food and Environmental Security in Ethiopia” were employed for modelling the distribution of 11 selected WEF across the study area based on sufficient occurrence data availability. Predictive variables for species distribution encompassed raster environmental variables obtained from Google Earth Engine (GEE)41 , climatic data from WorldClim42, and soil property data provided by the International Soil Reference and Information Centre (ISRIC)43.
Point presence data of wild edible fruits
Data on the occurrence of Wild Edible Fruit (WEF) species in Ethiopia was acquired via the GBIF Occurrences plugin in Quantum GIS (QGIS)37. Searches for each WEF species were conducted individually, and data were extracted specifically within the study regions of Oromia and SNNP. The downloaded data was then refined to eliminate duplicated records at identical geographic points using QGIS’s “delete duplicate geometries” feature. Field sampling records from the WEF project were also merged with the curated GBIF WEF data. In cases where merged occurrence points were less than 1500 m apart, they were displaced by 3000 m to avoid clustering. Eleven species with over 40 unique occurrence records were selected for subsequent species distribution modelling (see Fig. Sup. 1).
Environmental variables
For the modelling of species presence, a range of predictor variables were used, which included:
-
Tree cover percentage data were obtained from the Moderate Resolution Imaging Spectroradiometer (MODIS) Terra’s Vegetation Continuous Fields (VCF) product, providing annual global coverage at 250 m resolution for 2019/20.
-
Data on the predominant land cover type (LC) were sourced from the MODIS annual global land cover type product at 500 m resolution for 2021/22.
-
The Enhanced Vegetation Index (EVI) was extracted from MODIS’s Combined 16-day global product at 500 m resolution for October 2022.
-
Gross primary productivity (GPP) data was derived from the MODIS Aqua’s net primary productivity gap-filled annual global dataset at 500 m resolution for 2022/23.
-
Climatic variables including mean annual temperature (Temp) and precipitation (Ptn), seasonality of precipitation (Ptn_SN), and precipitation during the driest (Ptn_Dry) and warmest quarters (Ptn_Warm), along with a digital elevation model (Elev), were all at a 1 km resolution. The climate data averages were from 1970 to 2000.
-
Soil organic carbon (SoC) and nitrogen (N) content at various depths (15–30, 30–60, 60–100, and 100–200 cm) with a spatial resolution of 1 km.
To maintain consistency in spatial resolution, all environmental raster variables were reprojected to 1 km resolution. The resampling employed the nearest neighbour method for categorical data and the cubic spline interpolation for continuous data, using the rasterio package in Python.
Wild edible fruits species distribution model
The Maxent software version 3.4.444 was used to model45,46 the distribution of the 11 WEFs. All geographic data were projected to the EPSG:32637 coordinate reference system. The point occurrence data were organised to include the geographic coordinates, and the species name so that the modelling was conducted for all species in one run. Eighty percent of the occurrence data was used for training while 20% was used for testing the model. Raster environmental predictor variables were converted from GeoTIFF to Environmental Systems Research Institute’s (ESRI) American Standard Code for Information Interchange (ASCII) grid which was the format supported by Maxent.
One of the outcomes from using the Maxent model for species distribution was a raster map indicating the presence probabilities for each of the 11 species studied, considering current environmental variables. To synthesize this information, a composite map was created that showed the areas with the highest presence probability for any of the 11 species. This was done by overlaying the individual raster maps of presence probability for each species and assigning the highest value from these layers to the consolidated map. The rasterio package in Python was employed to manage and merge the raster data for this purpose. The summary area statistics with high likelihood of presence, i.e., > = 0.8, for both individual WEF species and the 11 merged WEF species raster was calculated using the rasterio package. All map visualisations were produced using QGIS version 3.34.
Results
Data on ethnobotany and elemental composition, along with the implications on dietary nutrition from fruits collected from 23 wild, and four cultivated plant species spanning 11 orders and 15 families, are presented in the subsequent sections. Additionally, the modelled likelihood of the 11 wild edible fruit species presence across the study area is presented. These species, detailed in Table 1, thrive across diverse Ethiopian landscapes, with altitudinal range from 1120 to 2750 m above sea level (Fig. 1 and Supplementary Table 1). Regarding their growth forms, the investigated edible fruit species comprised trees (41%), shrubs or trees (11%), shrubs (22%), lianas or shrubs (15%), and lianas or herbs (11%) as indicated in Table 1.
Ethnobotanical data
Within the scope of this paper, the term ‘fruits’ denotes the edible, fleshy tissues derived from mature ovaries gathered from the 27 plant species included in this study. Consumption demographics revealed that the fruits were universally eaten by all age groups (93%), while 7% were being particularly favoured by children. The flavour profiles of the ripe fruits varied, with 70% described as sweet, 15% as astringent, and the remaining 15% possessing combinations of sweet, astringent, or sour tastes. The consumption methods of the fruits included eating whole and fresh (30%), whole and fresh but deseeded (30%), peeled fresh (14%), and consuming fresh after deseeding (26%), as specified in Table 2.
Elemental concentration in edible fruits
The top five edible fruits for Ca content (mean ± std, mg per 100 g fresh weight) were R. abyssinica (228 ± 64), R. glutinosa (146 ± 54), Z. spina-christi (128 ± 48), R. pinnatus (116 ± 2), and M. kummel (107 ± 6). For Fe, the top five were R. glutinosa (3.57 ± 1.49), S. guineense ssp. guineense (2.41 ± 3.31), R. steudneri (1.95 ± 1.32), C. africana (1.68 ± 1.15), and R. volkensii (1.65 ± 0.88). Magnesium concentration was highest in R. pinnatus (76 ± 2), T. mauritianum (72 ± 0), R. steudneri (64 ± 8), R. abyssinica (60 ± 7), and R. apetalus (57 ± 7). Selenium concentration peaked with S. mitis (0.014 ± 0), with C. monoica and C. myxa (both 0.006 ± 0), M. kummel (0.004 ± 0.004) and P. guajava (0.003 ± 0.004) followed closely. While T. mauritianum (1.132 ± 0), R. pinnatus (0.489 ± 0.01), R. rosifolius (0.453 ± 0.105), C. myxa (0.449 ± 0.004), and C. spinarum (0.433 ± 0.236) were the top five for zinc content. More detailed statistics for these and other species can be found in Supplementary Table 3. Fig. Sup. 2 presents box plots of the individual elemental concentrations for the 27 edible fruit species, with comparisons to the concentrations in strawberries where data is available.
Edible fruits were categorized into two groups for comparison of their essential mineral concentrations: cultivated (CEF) and wild (WEF). The CEF was comprised of A. cherimola, C. edulis, D. caffra and P. guajava, while WEF consisted of 23 species (refer to Table 1 for the lists). It is important to highlight that while D. caffra is primarily cultivated for use as a live fence or hedge, the fruits are sometimes consumed on an occasional basis by certain individuals, despite not being the main purpose for growing this plant species. Results demonstrated that the median concentrations of essential minerals were greater in WEFs versus CEFs for all elements analysed except Cr (Fig. 3). Furthermore, the mineral content was larger among WEFs compared to concentrations reported for commercially grown mango (Fig. 3). The comparison of mean elemental contents of the different fruit species for selected essential elements is presented in Supplementary Table 4.
Elemental concentrations (log10 scale y-axis) across 27 edible fruit bearing species categorised as cultivated (CEF, n = 36), and wild (WEF, n = 64). Each panel depicts the elements. Medians (turquoise lines in the overlaid box plots) for each category are benchmarked against mango elemental concentration values (red dashed lines47).
Association between fruit and soil elemental concentrations
The range of plant-essential elements concentrations found in soil samples taken from beneath the canopies of edible fruit-bearing plants are presented in Fig. 4. The levels of these elements varied widely, with Mo displaying the smallest concentration (mean ± std) at 0.08 ± 0.05, and Fe exhibiting the largest at 49,012 ± 16,960 mg kg−1 of dry matter. Detailed data can be found in Supplementary Table 1 and descriptive statistics can be seen in Supplementary Table 3.
The relationship between elemental concentrations in fruits and their corresponding soils was characterized by generally weak correlations. The correlation coefficients varied from a low of -0.26 for Mg to a high of 0.5 for Ag as detailed in Fig. 5. Notably, significant correlations were observed for elements essential to human health, including Ca (r = 0.16, p = 0.042), Cu (r = − 0.24, p = 0.002), Fe (r = 0.3, p = 0.000), Mn (r = 0.21, p = 0.010), Se (r = 0.38, p = 0.000), and Zn (r = 0.37, p = 0.000) (see Supplementary Table 3).
Contribution to dietary mineral intake by wild edible fruits
The mineral intake contributed by the fruits shows variability contingent on the type of element and the specific fruit species, as indicated in Fig. 6. For boys aged 10–18 years, a 100-g serving of fresh fruits from R. abyssinica (RAb), R. glutinosa (RG), R. pinnatus (RP), S. mitis (SM), and T. mauritianum (TM) has the potential to provide up to 20%, 43%, 33%, 44%, and 7% of their daily recommended nutrient intake for Ca, Fe, Mg, Se, and Zn, respectively (Fig. 6).
Percentage of Recommended Nutrient Intake (RNI) for boys aged 10–18 provided by 100 g of different fresh fruits. Minerals include Ca, Fe, Mg, Se, and Zn shown in separate panels. Error bars show the 95% confidence interval. Refer to Table 1 for scientific names of the species acronyms (y-axis).
Wild edible fruits species distribution
The Maxent model indicated substantial spatial variation in the presence of wild edible fruit species across the 433,950 km2 study area (Figs. 7 and 8). The results indicate that 42.47% of the study area had a high likelihood of presence (probability ≥ 0.8) for at least one of the 11 species considered. When considering the maximum probability of presence across all species, 26.64% of the study area had high suitability indicating the presence of more than one species at a given locality. Among the individual species, C. spinarum, S. guineense, and M. kummel exhibited widespread presence (Fig. 7), with 7.91%, 6.11%, and 5.42% of the study area, respectively, having high suitability for these species. The log of Maxent model prediction and parameters, measures of model performance can be found in the Supplementary file 1.
Discussion
This study provides the first comprehensive assessment of the elemental composition of 23 wild and four cultivated edible fruits found across diverse agroecological zones in southern parts of Ethiopia. The findings reveal that several wild edible fruit (WEF) species are good sources of essential minerals, often surpassing the levels found in cultivated edible fruits (CEFs) and commercially grown fruits like avocados, blackberries, mangoes, oranges, papayas, and strawberries. When comparing our elemental concentration findings with the USDA FoodData Central database47, readers should note that wild edible fruits and commercial varieties differ fundamentally. These differences include genetic variations, growth in distinct soil and environmental conditions across different geographic regions, and varying fruit moisture contents between the two categories. Notably, C. africana, C. myxa, C. spinarum, R. abyssinica, R. apetalus, R. glutinosa, R. pinnatus, R. rosifolius, R. steudneri, R. volkensii, S. mitis, T. mauritianum, and S. guineense ssp. guineense emerged as good sources of Ca, Fe, Mg or Se. They have the potential to contribute significantly to the daily recommended nutrient intake for these minerals, especially for vulnerable groups like adolescent boys. The high Se content in S. mitis fruits, reaching 0.31 mg kg−1 dry matter, surpasses the levels reported for baobab fruits (0.169 mg kg−1 dry matter) and lower than that of Moringa oleifera immature pods (1.99 mg kg−1 dry matter)48, suggesting that this species may possess exceptionally efficient mechanisms for accumulating and storing Se from its environment.
Among commercial fruits, oranges have the largest Ca content (42 mg per 100 g fresh fruit), but 15 of the wild edible fruit (WEF) species contain even higher levels of Ca than oranges. Blackberries have the highest Fe content (0.62 mg per 100 g fresh fruit) among commercial fruits, but 17 WEF species surpass blackberries in Fe concentration. Avocados have the highest Mg content (29 mg per 100 g fresh fruit) in commercial fruits, but 15 WEF species exceed avocados in Mg levels. Papayas have the highest Se content (0.6 μg per 100 g fresh fruit) among commercial fruits, while 16 WEF species contain more Se than papayas. Avocados have the highest Zn content (0.64 mg per 100 g fresh fruit) among commercial fruits, but the WEF species T. mauritianum has twice the Zn concentration of avocados (refer to Table 347, and Supplementary Table 3).
The concentrations of some essential minerals in the WEFs were correlated with elemental levels in the soils where they grow, suggesting soil fertility could be leveraged to increase the micronutrient content in these species. However, the generally weak correlation indicates that factors beyond soil composition, such as plant genetic traits and other environmental conditions, play a role in determining mineral uptake and accumulation in edible fruits, corroborating previous research49,50,51. Further research should explore the relative influences of genotype and environment to better understand fruit nutrient biofortification potential.
Our data demonstrate that reasonable serving sizes of WEF species like R. abyssinica and R. glutinosa could supply up to 40% of daily recommended intakes of essential minerals like calcium and iron for Ethiopian adolescent boys. Complementing staple cereal intake with these fruits can help address widespread mineral nutritional deficiencies7,8,52 in rural communities where access to commercial fruits are very low. As wild harvests are unlikely to meet national-scale demand, integrating native fruit trees into smallholder farms via agroforestry could boost both access and conservation. The diverse growth forms of the studied WEFs, ranging from trees and shrubs to lianas and herbs, present opportunities for their integration into various agroforestry systems and traditional home gardens, promoting both nutritional security and biodiversity conservation. The universal consumption of these fruits across age groups and their varied flavour profiles indicate their potential to be embraced by local communities as part of their dietary traditions. However, further research is needed on propagation, cultivation requirements, and fruit production potential.
The substantial spatial variation observed in the distribution of WEF species across Ethiopia’s landscapes highlights the need for location-specific strategies to promote their utilization and conservation. The extensive prevalence of species like C. spinarum, S. guineense, and M. kummel suggests that these fruits could be prioritized in consultation with relevant stakeholders for incorporation into local diets and agricultural systems in areas where they are abundant. Interventions should include training programs for Health Extension Workers and Agriculture Extension Workers regarding indigenous fruit availability and nutritional composition profiles.
This study reinforces the need to valorise and conserve these underutilized resources by highlighting their nutritional value. Integrating WEFs into dietary recommendations, agricultural practices, and environmental policies could contribute to addressing dietary mineral deficiencies, improving food and nutritional security, and preserving Ethiopia’s rich plant biodiversity.
However, further research is warranted to investigate factors influencing the bioavailability of these essential minerals from WEF species, as well as to assess their other potential nutritional, anti-nutritional and bioactive components. This study lays the foundation to promote the use and conservation of little-known but nutritionally and ecologically important native fruits. Our documentation of the ethnobotanical knowledge, uses and chemistry of these species can guide utilization campaigns, inform dietary recommendations, and support biodiversity policy in Ethiopia. Public education, engagement with local communities and policy makers will be key next steps to realize the potential of these overlooked resources to enhance food and nutrition security. Overall, this research supports an integrated socioecological approach leveraging biodiversity and traditional knowledge to sustainably address malnutrition while protecting invaluable genetic resources for future generations.
In conclusion, this study underscores the potential of WEF species as nutritious and readily available resources that could play a vital role in enhancing dietary diversity and alleviating mineral deficiencies in Ethiopia. By fostering the sustainable utilization and conservation of these underutilized plant resources, Ethiopia can simultaneously advance its goals of improving food and nutritional security while preserving its rich biodiversity.
Data availability
Data is provided within the manuscript or supplementary information files. Additionally, the data is publicly available at the following link on Figshare. https://doi.org/10.6084/m9.figshare.25869115.
References
World Bank. World development indicators, https://databank.worldbank.org/source/world-development-indicators/ (2023).
Sintayehu, D. W. Impact of climate change on biodiversity and associated key ecosystem services in Africa: a systematic review. Ecosyst. Health Sustain. 4, 225–239. https://doi.org/10.1080/20964129.2018.1530054 (2018).
Nega, B., Adenew, B. & Gebre Sellasie, S. Current land policy issues in Ethiopia. In Land Reform, Land Settlement, and Cooperatives 103–124 (Food and Agriculture Organisation, 2003).
Abay, K., Hirvonen, K. & Minten, B. Farm size, food security, and welfare. in Ethiopia’s agrifood system: Past trends, present challenges, and future scenarios 147–173 (International Food Policy Research Institute, 2020).
Zerfu, T. A., Umeta, M. & Baye, K. Dietary habits, food taboos, and perceptions towards weight gain during pregnancy in Arsi, rural central Ethiopia: a qualitative cross-sectional study. J. Health, Popul. Nutr. 35, https://doi.org/10.1186/s41043-016-0059-8 (2016).
Gashu, D. et al. The nutritional quality of cereals varies geospatially in Ethiopia and Malawi. Nature 594, 71–76. https://doi.org/10.1038/s41586-021-03559-3 (2021).
Kumssa, D. B. et al. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 5, 10974. https://doi.org/10.1038/srep10974 (2015).
Muthayya, S. et al. The global hidden hunger indices and maps: an advocacy tool for action. PLoS ONE 8, e67860. https://doi.org/10.1371/journal.pone.0067860 (2013).
Mittermeier, R. A., Turner, W. R., Larsen, F. W., Brooks, T. M. & Gascon, C. Global biodiversity conservation: the critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas 3–22 (Springer, 2011).
Mengist, W., Soromessa, T. & Feyisa, G. L. Estimating the total ecosystem services value of Eastern Afromontane Biodiversity Hotspots in response to landscape dynamics. Environ. Sustain. Indic. 14, 100178. https://doi.org/10.1016/j.indic.2022.100178 (2022).
Tolessa, T., Senbeta, F. & Kidane, M. The impact of land use/land cover change on ecosystem services in the central highlands of Ethiopia. Ecosyst. Serv. 23, 47–54. https://doi.org/10.1016/j.ecoser.2016.11.010 (2017).
FAO. Global Forest Resources Assessment 2020 Report, Ethiopia. (Food and Agriculture Organisation of the United Nations (FAO), Rome, 2020).
Willett, W. et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 393, 447–492. https://doi.org/10.1016/S0140-6736(18)31788-4 (2019).
World Bank. ATLAS of Sustainable Development Goals 2023, https://datatopics.worldbank.org/sdgatlas/goal-2-zero-hunger#c2s1 (2023).
Ickowitz, A., Powell, B., Salim, M. A. & Sunderland, T. C. H. Dietary quality and tree cover in Africa. Glob. Environ. Chang. 24, 287–294. https://doi.org/10.1016/j.gloenvcha.2013.12.001 (2014).
Asfaw, Z. & Tadesse, M. Prospects for sustainable use and development of wild food plants in Ethiopia. Econ. Bot. 55, 47–62. https://doi.org/10.1007/BF02864545 (2001).
Berihun, T. & Molla, E. Study on the diversity and use of wild edible plants in Bullen District Northwest Ethiopia. J. Bot. 1–10, 2017. https://doi.org/10.1155/2017/8383468 (2017).
Duguma, H. T. Wild edible plant nutritional contribution and consumer perception in Ethiopia. Int. J. Food Sci. 2020, 2958623. https://doi.org/10.1155/2020/2958623 (2020).
Wang, X. et al. Multi-nutrient fertilization-based analysis of fruit quality and mineral element composition during fruit development in Merlot wine grapevines. J. Integr. Agric. 24, 1503–1514. https://doi.org/10.1016/j.jia.2024.04.032 (2025).
Donhouede, J. C. F., Salako, K. V., Assogbadjo, A. E., Ribeiro-Barros, A. I. & Ribeiro, N. The relative role of soil, climate, and genotype in the variation of nutritional value of Annona senegalensis fruits and leaves. Heliyon 9, e19012. https://doi.org/10.1016/j.heliyon.2023.e19012 (2023).
Fischer, G., Parra-Coronado, A. & Balaguera-López, H. E. Altitude as a determinant of fruit quality with emphasis on the Andean tropics of Colombia: A review. Agron. Colomb. 40, 212–227. https://doi.org/10.15446/agron.colomb.v40n2.101854 (2022).
Fischer, G., Balaguera-López, H. E., Parra-Coronado, A. & Magnitskiy, S. Adaptation of fruit trees to different elevations in the tropical Andes. In Ecophysiology of Tropical Plants 193–208 (CRC Press, 2023).
Bacelar, E. et al. Impacts of climate change and mitigation strategies for some abiotic and biotic constraints influencing fruit growth and quality. Plants 13, 1942. https://doi.org/10.3390/plants13141942 (2024).
Sanchez, A. C., Osborne, P. E. & Haq, N. Climate change and the African baobab (Adansonia digitata L.): The need for better conservation strategies. Afr. J. Ecol. 49, 234–245. https://doi.org/10.1111/j.1365-2028.2011.01257.x (2011).
Idohou, R., Assogbadjo, A. E., Kakaï, R. G. & Peterson, A. T. Spatio-temporal dynamic of suitable areas for species conservation in West Africa: Eight economically important wild palms under present and future climates. Agrofor. Syst. 91, 527–540. https://doi.org/10.1007/s10457-016-9955-6 (2017).
Gaisberger, H. et al. Spatially explicit multi-threat assessment of food tree species in Burkina Faso: A fine-scale approach. PLoS ONE 12, e0184457. https://doi.org/10.1371/journal.pone.0184457 (2017).
Hamilton, C. W. et al. Predicting the suitable habitat distribution of berry plants under climate change. Landsc. Ecol. 39, 18. https://doi.org/10.1007/s10980-024-01839-7 (2024).
Benítez, G. et al. Potential distribution of wild edible fruit trees under climate change scenarios: promoting food security in a Neotropical region. Reg. Environ. Change 24, 75. https://doi.org/10.1007/s10113-024-02231-6 (2024).
Ethiopian Statistical Service. Population Size by Sex, Region, Zone and Wereda: July 2023, https://www.statsethiopia.gov.et/wp-content/uploads/2023/08/Population-of-Zones-and-Weredas-Projected-as-of-July-2023.pdf (2023).
Teklehaymanot, T. An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state Ethiopia. J. Ethnobiol. Ethnomed. 13, 40. https://doi.org/10.1186/s13002-017-0166-7 (2017).
Ashagre, M., Asfaw, Z. & Kelbessa, E. Ethnobotanical study of wild edible plants in Burji District, Segan Area Zone of Southern Nations, Nationalities and Peoples Region (SNNPR) Ethiopia. J. Ethnobiol. Ethnomed. 12, 32. https://doi.org/10.1186/s13002-016-0103-1 (2016).
Feyssa, D. H., Njoka, J. T., Asfaw, Z. & Nyangito, M. Wild edible fruits of importance for human nutrition in semi-arid parts of East Shewa Zone, Ethiopia: Associated indigenous knowledge and implications to food security. Pak. J. Nutr. 10, 40–50 (2011).
Beche, D., Gebeyehu, G. & Feyisa, K. Indigenous utilization and management of useful plants in and around Awash National Park, Ethiopia. J. Plant Biol. Soil Health 3, 12 (2016).
Teketay, D. & Eshete, A. Status of indigenous fruits in Ethiopia. In Review and Appraisal on the Status of Indigenous Fruits in Eastern Africa: A Report Prepared for IPGRI-SAFORGEN in the Framework of AFRENA/FORENESSA 3–35 (Kenya Forestry Research Institute, 2004).
Teketay, D., Senbeta, F., Maclachlan, M., Bekele, M. & Barklund, P. Edible Wild Plants in Ethiopia. 575 (Addis Ababa University Press, 2010).
GBIF. Species occurrence download, https://www.gbif.org/occurrence/download/0003272-210914110416597 (2024).
QGIS Development Team. QGIS Geographic Information System 3.34.4-Prizren. (Open Source Geospatial Foundation Project, 2024).
Khokhar, J. S. et al. Variation in grain Zn concentration, and the grain ionome, in field-grown Indian wheat. PLoS ONE 13, e0192026. https://doi.org/10.1371/journal.pone.0192026 (2018).
WHO & FAO. Vitamin and mineral requirements in human nutrition. 2nd edn, (World Health Organization and Food and Agriculture Organization of the United Nations, 2004).
Delacre, M., Leys, C., Mora, Y. L. & Lakens, D. Taking parametric assumptions seriously: Arguments for the use of Welch’s F-test instead of the classical F-test in one-way ANOVA. Int. Rev. Soc. Psychol. 32, 13. https://doi.org/10.5334/irsp.198 (2019).
Gorelick, N. et al. Google earth engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 202, 18–27. https://doi.org/10.1016/j.rse.2017.06.031 (2017).
Fick, S. E. & Hijmans, R. J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315. https://doi.org/10.1002/joc.5086 (2017).
Hengl, T. et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 12, e0169748. https://doi.org/10.1371/journal.pone.0169748 (2017).
Phillips, S. J., Anderson, R. P., Dudík, M., Schapire, R. E. & Blair, M. E. Opening the black box: An open-source release of Maxent. Ecography 40, 887–893. https://doi.org/10.1111/ecog.03049 (2017).
Phillips, S. J. & Dudík, M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31, 161–175. https://doi.org/10.1111/j.0906-7590.2008.5203.x (2008).
Phillips, S. J., Anderson, R. P. & Schapire, R. E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026 (2006).
U.S. Department of Agriculture, A. R. S. USDA Food and Nutrient Database for Dietary Studies 2019–2020, http://www.ars.usda.gov/nea/bhnrc/fsrg (2022).
Kumssa, D. B. et al. Variation in the mineral element concentration of Moringa oleifera Lam. and M. stenopetala (Bak. F.) Cuf.: Role in human nutrition. PLoS ONE 12, e0175503. https://doi.org/10.1371/journal.pone.0175503 (2017).
Reddy, M., Moodley, R. & Jonnalagadda, S. B. Elemental uptake and distribution of nutrients in avocado mesocarp and the impact of soil quality. Environ. Monit. Assess. 186, 4519–4529. https://doi.org/10.1007/s10661-014-3716-7 (2014).
Davies, J. N. & Hobson, G. E. The constituents of tomato fruit–the influence of environment, nutrition, and genotype. Crit. Rev. Food Sci. Nutr. 15, 205–280. https://doi.org/10.1080/10408398109527317 (1981).
Motyleva, S. M. et al. Mineral composition of repair raspberry (Rubus idaeus L.) fruits. Vavilovskii Zhurnal Genet Selektsii 26, 622–629. https://doi.org/10.18699/VJGB-22-76 (2022).
Joy, E. J. M. et al. Dietary mineral supplies in Africa. Plant Physiol. 151, 208–229. https://doi.org/10.1111/ppl.12144 (2014).
USGS EROS. USGS EROS Archive - Digital Elevation Shuttle Radar Topography Mission (SRTM) 1 Arc-Second Global. https://doi.org/10.5066/F7PR7TFT (2018).
GADM. Global Administrative Districts Maps, https://gadm.org/data.html (2022).
The Catalogue of Life Partnership. APG IV: Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants, https://www.gbif.org/dataset/fa8ab13c-52ed-4754-b838-aeff74c79718 (2017).
Acknowledgements
The researchers express gratitude to the local Ethiopian research participants, national/regional/district agricultural and environmental development and research workers, and field sampling teams who enabled this work. The Ethiopian Institute of Agriculture’s Agricultural Quality Research Laboratory and the National Agricultural Biotechnology Research Centre provided facilities for initial sample preparation. We acknowledge the contribution of the employees of these centres for facilitating a smooth operation of the equipment and access to the facilities.
Funding
Leverhulme Trust, ECF-2021-190, University of Nottingham, Future Food Beacon, Bill & Melinda Gates Foundation, INV-009129.
Author information
Authors and Affiliations
Contributions
D.B.K. developed the initial idea and overarching plan for the study. D.B.K., M.R.B., and T.D. obtained funding to support the research project and contributed to streamlining the research. D.B.K., Z.K., D.G., O.D.H., F.S., T.K.A., M.A.H., and K.T. participated in field surveys, species identification, and collecting samples and preparing them for analysis. Laboratory analyses, data management, data analysis, and visualizations were conducted by D.B.K., who also drafted the initial manuscript. All authors contributed to editing and reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumssa, D.B., Dew, T., Kebebew, Z. et al. Elemental concentration and spatial distribution of wild edible fruits and implications for dietary mineral intake in Ethiopia. Sci Rep 15, 42307 (2025). https://doi.org/10.1038/s41598-025-26400-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26400-7