Abstract
Functional constipation (FC), a prevalent gastrointestinal disorder in early childhood associated with gut microbial dysbiosis, shows significant connections with specific constitutional types in Traditional Chinese Medicine (TCM). While TCM’s nine constitutional classifications identify damp-heat constitution as a potential risk factor for FC, pediatric-specific evidence remains scarce regarding this correlation. Using the Constitutional Medicine Questionnaire, 49 children under 2 years were stratified into two groups: 18 children with damp-heat constitution who suffered from constipation (DHC) and 31 children with balanced constitution who did not suffer from constipation (BC). Clinical assessments (Bristol Stool Scale, symptom profiles), gut microbial profiling (16 S rRNA sequencing), and urinary metabolomics (UPLC-Q-TOF/MS) revealed marked intergroup differences. DHC subjects demonstrated reduced stool consistency scores, heightened symptom severity, distinct microbial communities, and altered sphingolipid-related metabolic pathways. A diagnostic panel combining six microbial markers and six metabolites achieved effective discrimination. This multi-omics investigation delineates the pathophysiological characteristics of FC in DHC children, proposing novel therapeutic targets through TCM constitution-based microbial-metabolite interactions.
Similar content being viewed by others
Introduction
Functional constipation (FC) is a functional bowel disorder clinically defined by persistent defecatory dysfunction (infrequent and/or incomplete evacuation) in the absence of organic pathology, posing significant morbidity and substantial healthcare burden due to its chronicity and treatment complexity1. In pediatric populations, organic causes of constipation are exceedingly rare, with studies indicating that over 90% of cases are of functional origin2,3. Globally, the prevalence of FC in children was reported to be 9.5% in 20184, while in Asia, the prevalence reached up to 12% in 20245. FC is the most common gastrointestinal disorder in children, with the majority of cases developing during infancy and toddlerhood6,7,8. If childhood constipation remains undiagnosed or inadequately treated, approximately 25% of affected individuals may experience persistent symptoms into adulthood, significantly impacting their quality of life9.
According to Traditional Chinese Medicine Constitution (TCMC) theory, individuals are classified into nine constitutional types, each with unique causes and distinct manifestations. A balanced constitution represents a state of general health with minimal susceptibility to disease. Among the unbalanced constitutions, damp-heat constitution is one of the most common types, characterized by an excess of dampness and heat in the body. This condition is typically manifested by symptoms such as dry or sticky stools, deep yellowish urine, and a red tongue with a yellowish greasy coating10. In preliminary research, we observed that the gut microbiota and metabolomics of children with damp-heat constitution differ from those of healthy children11. Our team conducted a statistical analysis of the constitutional distribution among 1069 children under the age of 2 in China. The results indicated that a balanced constitution accounted for 56.22%, followed by damp-heat constitution at 16.00%11. These findings demonstrate that damp-heat constitution is highly prevalent among children. Individuals with a damp-heat constitution are predisposed to various diseases12,13. For instance, this constitution is characterized by excessive dampness and heat in the body, which can impair intestinal peristalsis and delay fecal elimination, ultimately leading to constipation. Song14 utilized the chi-square test to analyze the relationship between functional constipation (FC) and TCMC, concluding that there is a statistically significant correlation between FC and damp-heat constitution. Furthermore, Wang15 investigated the distribution of constitutional types among children with FC and found that damp-heat constitution accounted for as high as 13.18%. Based on these insights, it is essential to conduct further research into the underlying mechanisms of damp-heat constitution and its association with FC in children, as this may provide valuable information for developing targeted interventions and improving clinical outcomes.
With the rapid advancement of modern research techniques, an increasing number of studies have explored the correlation between different constitutional types and intestinal flora16,17,18,19. However, the majority of these investigations have focused on adult populations, with limited attention devoted to children under 2 years of age. The gut microbiota of infants and toddlers plays a critical role in shaping their health at various developmental stages and exerts long-term effects on the maturation of the immune and endocrine systems throughout life20,21. In this study, we used a cross-sectional study with children aged 0–2 years and screened children with damp-heat constitution and functional constipation (DHC) as well as those with balanced constitution but without functional constipation (BC). Fecal and urinary samples were collected from these participants and subjected to 16 S rRNA gene sequencing and UPLC-Q-TOF/MS analysis, respectively. This approach was employed to investigate the characteristics of the intestinal flora in DHC children and elucidate the underlying metabolic mechanisms associated with this condition. Such insights may provide a foundation for developing targeted interventions and improving clinical outcomes in pediatric populations.
Materials and methods
Study design and participants
Children aged 0–2 years from Guangdong and Beijing, China, were recruited between November 2022 and April 2023. Participants were screened using Wang Qi’s nine body types in Constitutional Medicine Questionnaire (Supplementary file 1). This questionnaire has been integrated into national public health management systems and is widely utilized in countries such as Japan, Korea, and the USA22,23,24. The questionnaire comprises 43 items corresponding to nine constitutional types, and individuals are classified as a specific constitution based on their transformation scores meeting predefined criteria11.
Participants were required to meet the following criteria: (1) infants aged 0–2 years old; (2) parents who had cared for the infants for a long time and had a detailed knowledge of the infant’s basic condition; (3) infants with damp-heat constitution or balanced constitution diagnosed using the Constitutional Medicine Questionnaires. Participants meeting one or more of the following criteria were excluded: (1) infants who received antibiotics and probiotics within 3 months prior to sample collection; (2) infants with a mental illness or severe disease that were unable to participate in and complete clinical studies in a standardized manner; (3) infants living far from the hospital where stool and urine samples were not readily available; (4) infants with incomplete clinical data.
FC was diagnosed according to the Rome IV criteria8. Children classified as DHC (damp-heat constitution with functional constipation) and BC (balanced constitution without functional constipation) were included in the fecal flora and urinary metabolomics study. The study was approved by the Ethics Committee of Beijing University of Chinese Medicine (Approval No. 2020BZYLL122). All guardians of the subjects were provided with detailed information regarding the trial procedures and provided written informed consent. The study flow chart was presented in Fig. 1.
Fecal sample collection, DNA extraction and 16 S rRNA sequencing
Fecal samples were collected and processed following standardized procedures, as previously described25. Shortly, freshly produced stool samples that did not contain urine were collected every morning; > 3 g (two scoops) from the middle of the stool was scooped into a sterile stool cup. Initially, the child’s guardian collected freshly produced, urine-free fecal samples at home and transported them to the hospital within one hour, maintaining low temperatures using an ice pack. Upon arrival, the samples were immediately flash-frozen in liquid nitrogen and subsequently stored at − 80 °C for long-term preservation. Genomic DNA was extracted from the fecal samples using the CTAB/SDS method. The quality and concentration of the extracted DNA were assessed via agarose gel electrophoresis. The hypervariable V3–V4 region of the 16 S rRNA gene was amplified by PCR using barcode indexed primers (341 F-806 R), and the resulting amplicons were purified to construct sequencing libraries. Finally, the libraries were sequenced on the Illumina MiSeq platform to generate high-throughput sequencing data for analysis.
Gut microbiota analysis
Sequencing analysis was performed using the DATA2 workflow following the merging of paired-end reads and assignment to respective samples. Sequences with similarity ≥ 97% were grouped into the same ASV. The α and β diversity of the microbial community were evaluated using QIIME2 2019.4 software26. To identify differentially abundant microbial taxa, LEfSe analysis was conducted, with a threshold of LDA score > 2.027. Additionally, PICRUSt software (v 1.1.1) was employed to predict functional differences by analyzing gene composition based on the KEGG database28,29,30,31.
Urine samples collection and UPLC-Q-TOF/MS processing
The middle portion of morning urine was collected at home by the child’s guardian and transported to the hospital within 1 h, maintained at a low temperature using an ice pack. Upon arrival, the samples were centrifuged, and the supernatant was removed for rapid freezing and stored at − 80 °C. For metabolite extraction, the supernatant samples were shipped on dry ice to Shanghai Applied Protein Technology Co. Ltd. for further processing32. Briefly, the samples were thawed, mixed with methanol/acetonitrile solvent, sonicated, and centrifuged. The resulting supernatant was collected and dried, then reconstituted in acetonitrile/water solvent, vortexed, and centrifuged again. Finally, the last supernatant was analyzed using ultra-high-performance liquid chromatography (UHPLC) coupled with quadrupole time-of-flight mass spectrometry (Q-TOF MS) employing a TOF 5600 + system. This comprehensive workflow ensured accurate and reliable metabolomic profiling of the urine samples.
Metabolomics data analysis
For data analysis, the mzXML format was utilized for data processing33. Metabolite characteristics were detected using the XCMS 3.10.1 software package (Scripps, La Jolla, CA, USA), with peak alignment performed via the obiwarp algorithm34. Subsequently, the data underwent metabolite structure identification and preprocessing, followed by quality evaluation of experimental data and final statistical analysis. Univariate analysis, exemplified by fold change analysis, was employed as one of the most commonly used statistical methods. Orthogonal partial least squares discriminant analysis (OPLS-DA) was conducted for multivariate analysis to identify global metabolic differences between the DHC and BC groups. The variable importance in projection (VIP) was used to determine characteristic metabolites in the two groups. MetaboAnalyst web software was utilized for metabolic pathway analysis, and Fisher’s exact test was applied to evaluate the significance level of enriched pathways.
Correlation analysis, biomarker identification and statistical analysis
Spearman analysis and matrix heat map were utilized to show the correlation between differential flora and metabolites in the two groups by R and Cytoscape software. The area under a receiver operating characteristic curve (AUC) was adopted to mine markers of flora and metabolites in DHC children. For microbiome studies, multiple comparison corrections were performed as follows: box plots were generated using R scripts to visually illustrate α diversity differences between DHC and BC groups. Kruskal–Wallis rank-sum tests and Dunn’s post-hoc tests were employed for multiple comparison correction to validate statistical significance. Inter-group differential analysis utilised PERMANOVA tests. For metabolomics, statistical analysis employed t-tests supplemented with FDR (False Discovery Rate) correction for multiple comparisons. Statistical analyses were performed using SPSS and GraphPad Prism software. P < 0.05 was taken as a significant difference.
Results
General information of participants
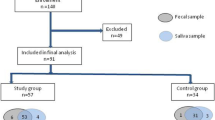
A total of 812 questionnaires were received, 31 BC children and 18 DHC children were enrolled in the study after data screening, constitution identification and FC diagnosis. It is widely believed that age35,36, gender37,38, delivery mode39,40,41, and feeding pattern42,43,44 can influence the gut bacterial composition; therefore, in this study, the above variables were statistically analyzed in the DHC and BC groups and the results showed no significant differences (Table 1).
DHC children exhibited changes in stool shape and frequency
Given the young age of the participants, who may not be able to articulate their perceptions, parents utilized the Bristol Stool Scale (BSS), a widely recognized and validated tool that combines fecal images with descriptive terms, to evaluate stool consistency and assess constipation. For BC children, the predominant stool shapes were soft blobs, followed by smooth snake-like forms and fluffy pieces. These characteristics may be attributed to the young age and the typically thinner diameter of feces in this population. In contrast, DHC children predominantly exhibited sausage-shaped stools with cracks, with a smaller proportion showing fluffy pieces. This pattern was influenced not only by age-related characteristics but also by the presence of FC. Compared to BC children, the BSS scores were significantly lower in the DHC group (P = 0.0006), indicating more pronounced constipation in DHC children (Table 2).
In addition to BSS assessments, the prevalence of various FC symptoms was also evaluated. The results demonstrated that nearly half of the DHC children had a history of excessive stool retention (41.46%), followed by painful or hard bowel movements (29.27%) and fewer than two defecations per week (19.51%). By comparison, only 12.9% of BC children experienced one or two constipation-related symptoms (Table 3). These findings underscore the distinct gastrointestinal manifestations associated with DHC and highlight the importance of considering constitutional differences in pediatric constipation management.
DHC children changed the diversity of gut flora
Fecal samples from the DHC and BC groups were processed using the DADA2 method, which included dereplication, size filtering, denoising, merging, and chimera removal. The DADA2 algorithm performs exact sequence matching without clustering, effectively corresponding to 100% similarity clustering. Each sequence generated after DADA2 quality control is referred to as an amplicon sequence variant (ASV), representing a precise microbial sequence. This approach is more accurate than the traditional 97% similarity-based operational taxonomic unit (ASV) clustering45. The DHC and BC groups contained 27,962 and 15,101 specific ASVs, respectively, with 2041 overlapping ASVs identified between the two groups (Fig. 2A). These results provide a high-resolution view of the microbial community structure in both groups.
To comprehensively evaluate bacterial diversity within the samples, α diversity analysis was conducted in this study. Metrics such as Chao 1 and Observed species were used to assess species richness, Shannon and Simpson indices for species diversity, Faith’s PD for phylogenetic diversity, Pielou’s evenness for species evenness, and Good’s coverage for sampling adequacy. No statistically significant differences were observed between the DHC and BC groups for any of these metrics (P > 0.05) (Fig. 2B).
To investigate the differences in microbial community composition between groups, β diversity analysis was conducted. PCoA, based on unweighted and weighted unifrac distance, was used to examine the clustering patterns of microbial communities in the DHC and BC groups. The results demonstrated distinct separations between the microbial profiles of two groups (PERMANOVA P = 0.003, P = 0.012), suggesting significant differences in bacterial composition between the DHC and BC groups (Fig. 2C,D).
DHC children changed the gut microbial diversity. (A) Venn diagram of ASVs in two groups. (B) α diversity of microbiota. (C) β diversity of microbiota based on unweighted unifrac distance. (D) β diversity of microbiota based on weighted unifrac distance. DHC, damp-heat constitution with functional constipation; BC, balanced constitution without functional constipation.
DHC children changed the composition of gut flora
The gut flora composition was analyzed at five taxonomic levels, namely, phylum, class, order, family, and genus, and the top 20 flora were listed at each level (Fig. 3A–E). Compared with that in the BC group, the relative abundance of phylum Actinobacteria and its genus Bifidobacteria increased in the DHC group, whereas the relative abundance of phylum Firmicutes decreased, which was consistent with the results on constipation by Wang et al.46 LEfSe analyses allowed simultaneous analysis of differences at all taxonomic levels to be presented via the cladogram (Fig. 3F), and placed considerable emphasis on finding robust biomarkers across groups (Fig. 3G). A total of 38 characteristic flora were found, of which 15 were detected in the DHC group and 23 in the BC group. In the DHC group, the characteristic flora at the phylum level were Actinobacteria and Synergistetes, and at the genus level were Bifidobacterium, Oscillospira, Eggerthella, Alistipes, Robinsoniella, and Desulfovibrio. In the BC group, the characteristic phyla were Firmicutes and Cyanobacteria, and the characteristic genera were Veillonella, Megamonas, Lachnospira, Campylobacter, Gemella, Selenomonas, Haemophilus, and Turicibacter. Using the PICRUSt2 software, KEGG and MetaCyc pathways were used to predict the functional potential of DHC. The results consistently indicated that the function of DHC is primarily concentrated in three areas: carbohydrate, amino acid, cofactor and vitamin metabolism/biosynthesis (Fig. 3H–I).
DHC children changed the gut microbial composition. (A–E) Histogram showing the relative abundance of the top 20 taxa in phyla, class, orders, family, genera, respectively. (F) Cladogram of the phylogenetic distribution at all taxonomic levels. (G) LEfSe analysis showing gut flora with an LDA (log10)>2. The length of the bar graph representing the size of the LDA. (H) KEGG pathway. (I) MetaCyc pathway. DHC, damp-heat constitution with functional constipation; BC, balanced constitution without functional constipation.
DHC children altered the metabolomics profile
Intestinal bacteria play a direct role in the development of gastrointestinal disorders, and their metabolites can significantly influence the body’s physiological and pathological states. Urine, as the end product of human metabolism, contains a rich array of metabolites that reflect the body’s biochemical metabolic status. It is also non-invasive, non-infectious, and highly suitable for analyzing metabolic changes in young children. In this study, urine samples were collected from 31 BC children and 16 DHC children for untargeted metabolomic analysis.
The total ion flow plots of the quality control (QC) samples showed substantial overlap, indicating that the response intensities and retention times of the various chromatographic peaks were essentially identical. This suggests that variation caused by instrument error was minimal throughout the entire experimental process (Supplementary file 2). Differential analysis of all metabolites was performed using univariate statistical analysis and visualized through volcano plots (Fig. 4A, B). OPLS-DA revealed an overall trend of separation between DHC and BC samples (Fig. 4C,D), suggesting that the OPLS-DA model effectively differentiated the two groups. These findings indicate that the metabolite composition in DHC children underwent significant alterations compared to BC children.
DHC children changed the metabolome. (A) Volcano plots of positive ion mode. (B) Volcano plots of negative ion mode. Rose represented differential metabolites with Fold change > 1.5, suggesting up-regulation of their expression in the DHC group. Blue represented differential metabolites with Fold change < 0.67, suggesting down-regulation of their expression in the BC group. Black represented metabolites with no statistically significant difference. (C) OPLS-DA score graph for positive ion mode. (D) OPLS-DA score graph for negative ion mode. DHC, damp-heat constitution with functional constipation; BC, balanced constitution without functional constipation.
Differential metabolite screening between two groups
The differences in metabolite expression profiles between groups can be quantified and analyzed using variable importance in projection (VIP) values derived from OPLS-DA, allowing for the identification of significantly altered metabolites. In this study, a stringent screening criterion of VIP > 1.0 and P < 0.05 was applied to select differential metabolites. As illustrated in Fig. 5A,B, a total of 59 metabolites were identified in positive ion mode and 9 in negative ion mode. The horizontal coordinates revealed distinct expression trends of metabolites across the two groups, indicating significant differences in their metabolic profiles. From the vertical coordinates, certain metabolites clustered together, suggesting potential functional similarities or involvement in shared biological processes. Detailed parameters of each differential metabolite are provided in Supplementary file 3.
Metabolic pathway annotation in DHC children
To further explore the metabolic mechanisms of DHC, the differential metabolites in urine were mapped to relevant physiological pathways. Analysis of the differential abundance scores indicated that the DHC group was associated with alterations in sphingolipid signaling pathways, sphingolipid metabolism, and apoptosis-related pathways (Fig. 5C). These findings provide insights into the potential metabolic alterations and biological processes involved in DHC.
Differential metabolite analysis. (A) Hierarchical clustering heatmap of differential metabolites in positive ion mode. (B) Hierarchical clustering heatmap of differential metabolites in negative ion mode. The horizontal axis represents groups, the vertical axis represents metabolites. (C) Differential abundance score plot of differential metabolites. DHC, damp-heat constitution with functional constipation; BC, balanced constitution without functional constipation.
Correlation analysis between the differential flora and metabolites
To investigate the potential relationship between metabolic changes and gut microbiota, Spearman correlation analysis was performed. A total of 119 significant correlations were identified between microbial taxa and metabolites, with 6 pairs exhibiting strong correlations (absolute correlation coefficients > 0.6). Specifically, Oscillospira showed strong positive correlations with indoxyl sulfate and coniferyl aldehyde. Selenomonas demonstrated negative correlations with fingolimod, palmitic acid, and octadecanoic acid. Additionally, Veillonella exhibited a negative association with indoxyl sulfate (Fig. 6). These findings suggest that specific gut microbes may play a role in regulating the levels of certain metabolites, potentially influencing the metabolic profile observed in DHC individuals.
Potential biomarker of DHC children identification
To identify potential biomarkers for DHC children, receiver operating characteristic (ROC) analysis was employed. This method is widely recognized for its ability to evaluate the predictive accuracy of models by plotting sensitivity against 1-specificity across various thresholds.
The top six metabolites identified as potential biomarkers were oleamide, palmitamide, sphingosine, linoleoylglycine, 2-4-6-tri-tert-butylaniline, and 4-5-epoxy-7Z-10Z-13Z-16Z-19Z-docosapentaenoic acid methyl ester. Additionally, the top six microbial taxa included Bifidobacterium, Eggerthella, Megamonas, Oscillospira, Selenomonas, and Turicibacter.
When combining these metabolites and microbial taxa, the resulting model demonstrated excellent discriminatory power, with an area under the curve (AUC) of 0.975. This indicates a highly accurate classification of DHC children. Furthermore, the sensitivity of this potential biomarker panel was 88.89%, while the specificity reached 100%, suggesting that the model not only effectively identifies DHC children but also minimizes false positives (Fig. 7). These results highlight the potential utility of this combined metabolite-microbiota panel as a robust biomarker for DHC children. Such findings underscore the importance of integrating both metabolic and microbial profiles in identifying constitution-specific biomarkers, which could have significant implications for personalized medicine and early intervention strategies.
Discussion
Academician Wang Qi of the Chinese Academy of Engineering and founder of TCMC theory proposed that human can be systematically categorized according to age stages, including childhood, youth, middle age, menopause, and old age. Each age group exhibits distinct constitutional characteristics, reflecting unique morphological structures, physiological functions, and psychological states. Interestingly, different constitutions demonstrate varying susceptibilities and predispositions to specific diseases47.
The first 1000 days from conception to 2 years of age is a critical period for early childhood growth and development, during which the gastrointestinal microbial community plays a critical role in immune, endocrine, metabolic and other host developmental pathways. And the emerging view of human developmental biology suggests that trillions of microorganisms and their genes are formed and stabilized for survival in the human body during the first 2 years of life48. Therefore, we focused on the gut microbiota of children with damp-heat constitution aged 0–2 years. China’s earliest surviving pediatric monograph, Lu Xin Jing, records that children under 3 years of age have vigorous Yang Qi, corresponding mainly to the damp-heat constitution of nine constitutions, which is consistent with the results of our cross-sectional survey of children’s constitution in China11. The individuals with damp-heat constitution are more susceptible to functional constipation due to excessive dampness and heat in the intestines, which impairs their conduction function.
A survey investigating the TCMC distribution in FC-affected children revealed that damp-heat constitution constituted 5.26% of all constipated children49. Dysbiosis of the intestinal flora is increasingly recognized as a critical etiological factor in pediatric FC. Emerging evidence from metagenomic studies highlights the significance of bacterial composition and metabolic capacity in the pathogenesis and progression of FC50,51. Based on these findings, we conducted the investigation into the intestinal flora and urinary metabolomics of children aged 0–2 years with damp-heat constitution who suffer from FC. This study aimed to elucidate the microbial community characteristics of this specific population in a targeted manner.
The study highlights significant differences in the gut microbiota composition between the DHC (damp-heat constitution with functional constipation) group and the BC (balanced constitution without functional constipation) group. Specifically, two phyla and six genera exhibited significantly higher relative abundances in the DHC group compared to the BC group. Notably, the phylum Actinobacteria and its associated genus Bifidobacteria showed increased relative abundance in the DHC group, while the phylum Firmicutes demonstrated a decrease. This finding aligns with previous studies on constipation, such as the work by Wang et al.47 which emphasized the role of gut microbiota in the pathogenesis of functional constipation.
Bifidobacterium, a well-known beneficial bacterium, contributes to alleviating constipation through mechanisms such as modulating gastrointestinal regulatory peptides and stimulating the production of short-chain fatty acids in the intestinal tract. Additionally, Bifidobacterium promotes lactic acid production, leading to a lower intestinal pH, which enhances intestinal peristalsis52,53. However, in this study, the level of Bifidobacterium was significantly higher in the constipation group. It is important to note that the therapeutic effects of Bifidobacterium on constipation are strain-dependent. For instance, the presence of the abfA cluster in Bifidobacterium longum has been shown to influence its efficacy in treating constipation54.
In contrast, the phylum Synergistetes, often considered an opportunistic pathogen, exhibited low relative abundance in individuals with damp-heat constitution in prior studies11, which was elevated in the DHC group in this investigation. This suggests a strong association between Synergistetes and constipation, corroborated by findings in studies examining antipsychotic drug-induced constipation, where an increase in Synergistetes abundance was observed55.
Another potentially pathogenic bacterium, Eggerthella, was found to be significantly more abundant in patients with Rett syndrome who experienced constipation. Although the altered intestinal flora in these patients was not entirely dependent on their constipation status, the dysbiotic flora produced a distinct SCFA profile, possibly linked to the abundance of Eggerthella56. Furthermore, Eggerthella has been implicated in inducing intestinal inflammation and leakage, which are closely associated with gastrointestinal symptoms such as bleeding and bloating57.
The relationship between fecal concentration and microbial composition is also noteworthy. For example, rapid colonic transit time selects for fast-growing microorganisms, whereas slow transit times associated with constipation allow slow-replicating microorganisms to persist in the intestinal lumen. In the context of constipation, the genus Oscillospira tends to exhibit high abundance58,59, a finding consistent with the results of this study. This observation underscores the importance of transit time in shaping the gut microbiota profile and its potential implications for constipation management.
Alistipes is one of the abundant members of the gut microbiome, mainly indole-positive, and is able to metabolize tryptophan. Tryptophan has two metabolic pathways: One is converted into 5-hydroxytryptophan, which is then converted into serotonin, stimulating intestinal motility. The other is the kynurenine pathway, a major pathway in which the synthesis of kynurenine reduces the amount of tryptophan available for the synthesis of 5-hydroxytryptophan. Reduced levels of 5-hydroxytryptophan have been demonstrated in a number of patients with constipation who are slow transporters. Sugitani et al. indicated that the mucosa-associated microbiome of FC was characterized by elevated levels of Alistipes. In addition, the consumption of Bifidobacteria showed inter-species differences in relieving constipation, and that several species of Bifidobacteria could improve constipation by increasing acetic acid concentration and the relative abundance of Lactobacillus and by decreasing the levels of Alistipes and others. These findings are consistent with the elevated abundance of Alistipes in the DHC group.
The gut microbiome plays a critical role in the pathophysiology of FC, and specific bacterial genera have been implicated in its development and alleviation. Among these, Alistipes is an abundant member of the gut microbiota, characterized by its ability to metabolize tryptophan through two primary pathways: the serotonin pathway and the kynurenine pathway. In the serotonin pathway, tryptophan is converted into 5-HTP, which is subsequently transformed into serotonin, a neurotransmitter that stimulates intestinal motility. Conversely, the kynurenine pathway reduces the availability of tryptophan for serotonin synthesis, potentially contributing to reduced intestinal motility60. Studies have demonstrated that patients with constipation who exhibit slow colonic transit times often have lower levels of 5-HTP61. Elevated levels of Alistipes have been observed in the mucosa-associated microbiome of individuals with FC62, suggesting a potential link between this genus and impaired intestinal motility. Furthermore, certain species of Bifidobacteria have been shown to alleviate constipation by increasing acetic acid concentrations and the relative abundance of Lactobacillus, while simultaneously reducing levels of Alistipes63. These findings align with the elevated abundance of Alistipes observed in the DHC group.
Another bacterial genus associated with gastrointestinal health is Desulfovibrio, a dominant sulfate-reducing bacterium in the human colonic flora. This genus has the ability to colonize the intestine via the mucus layer, and its metabolites may contribute to chronic gastrointestinal diseases. In studies involving constipated mice treated with varying concentrations of cellulose, Desulfovibrio levels were reduced in the intestinal tract, exhibiting a significant negative correlation with the total content of SCFAs64. SCFAs, particularly acetate, propionate, and butyrate, play crucial roles in maintaining colonic health and promoting regular bowel movements.
Megamonas, belonging to the phylum Mycobacteriaceae and family Weillonococcaceae, exhibits a strong degrading effect on cellulose. Its impact on the intestinal flora is marked by an increase in butyric acid levels, accompanied by a shift in the balance between acetic and lactic acids. Specifically, Megamonas leads to a decrease in acetic acid and an increase in lactic acid65. Elevated lactic acid levels can increase intestinal osmotic pressure, resulting in higher water content in feces, which may not be conducive to alleviating constipation66.
Lachnospira is another genus that differs significantly between constipated and non-constipated groups. Higher abundances of Lachnospira have been observed in non-constipated individuals, with levels being more than four times higher in healthy controls compared to constipated patients67. Supplementation with plantain has been shown to significantly increase Lachnospira levels, with fecal water content positively correlated with acetic acid levels. Members of Lachnospira are capable of producing lactic and acetic acids, with lactic acid further metabolized into butyric or propionic acid. While low concentrations of butyric acid inhibit mucin secretion, leading to constipation, high concentrations of butyrate and acetic acid may exacerbate constipation symptoms. These findings underscore the complex interplay between gut microbiota composition and functional constipation, highlighting the potential of targeted microbial interventions for managing this condition68.
The liquid-quantity coupledomics technology employed in this study identified a distinct metabolite profile in children with DHC. A total of 68 differential metabolites were screened, along with three associated metabolic pathways. Notably, oleamide, an amide derivative of oleic acid and a member of the fatty acid amide family, emerged as a key modulator of enterodynamics. Oleamide has been shown to slow intestinal motility in mice by activating the cannabinoid receptor CB169. The traditional Chinese herbal formula MaZiRenWan was found to attenuate oleamide-induced intestinal motility slowness in mice, likely by enhancing the degradation of oleamide mediated by colonic fatty acid amide hydrolase, thereby improving intestinal motility in functional constipation70.
Another significant finding pertains to the involvement of sphingolipid metabolism in DHC children. Sphingolipid metabolism generates several biologically active metabolites, including sphingosine-1-phosphate (S1P), phytosphingosine, and dihydrosphingosine. These metabolites play critical roles in regulating cardiovascular function, smooth muscle contraction, and acting as signaling molecules involved in cell survival, proliferation, migration, differentiation, and apoptosis71,72. Specifically, S1P induces intestinal smooth muscle contraction via interstitial cells of Cajal (ICC), which are essential for gastrointestinal motility regulation73,74. Low levels of S1P may disrupt normal ICC function, leading to decreased or inhibited colonic motility and contributing to constipation75. Altered levels of dihydrosphingosine in aged mice have also been associated with gastric contractile dysfunction76, while dietary supplementation with phytosphingosine and dihydrosphingosine has been shown to promote gastrointestinal motility77. Therefore, decreased levels of these metabolites may result in reduced gastric motility.
Furthermore, variations in the levels of S1P, dihydrosphingosine, and phytosphingosine are linked to disturbances in lipid and glucose metabolism78, potentially increasing susceptibility to metabolic disorders in individuals with damp-heat constitution. Previous studies have demonstrated that changes in sphingolipid distribution are associated with neurological disorders, diabetes, and cardiovascular diseases79,80,81,82. This study is the first to identify a potential role for sphingolipid metabolism in DHC children.
Additionally, the combination of characteristic metabolites and gut flora provided an excellent discriminatory marker for DHC children, with an AUC of 0.975. This highlights the potential utility of metabolomic and microbiomic profiles in diagnosing and understanding the underlying mechanisms of functional constipation in this population. These findings underscore the importance of integrating metabolomics and microbiomics in elucidating the pathophysiology of functional constipation and identifying potential therapeutic targets.
This study presents several limitations that warrant consideration for future research. First, the study population consisted of Chinese infants and toddlers, which introduces potential confounding factors although factors such as mode of birth, feeding style, and age showed no significant differences between groups. To enhance the precision of the study, future investigations could focus on a narrower age range. Second, the study was limited by the number of questionnaires distributed and the relatively small sample size of the cohort. Cross-sectional designs cannot establish causal relationships. These constraints could potentially lead to sampling errors and reduce the external validity of the study. A larger, globally representative study with a broader geographic scope would be beneficial in addressing these limitations and ensuring more robust conclusions. Third, the phylogenetic resolution of 16 S rRNA sequencing used to analyze the gut flora was insufficient for identifying microbial species accurately. While this method is effective for profiling bacterial communities at higher taxonomic levels, its ability to resolve differences at the species level is limited. This limitation affects the precision of microbial identification, particularly for certain genera where finer distinctions are critical. Future studies could employ alternative techniques, such as shotgun metagenomic sequencing, to achieve higher-resolution analysis of the gut microbiota. Additionally, mechanistic insights could be gained through experimental approaches such as fecal microbiota transplantation and targeted drug interventions. These methods would allow researchers to explore causal relationships and validate the observed associations between specific microbial profiles and functional constipation. By addressing these limitations, future research can provide more comprehensive and actionable insights into the role of gut microbiota in DHC children.
Conclusions
This study represents a pioneering effort in elucidating the gut flora and metabolomic profiles of children with DHC. The findings offer critical insights into the pathogenesis of this condition by identifying specific differential flora and metabolites that influence key biological pathways, including sphingolipid signaling, sphingolipid metabolism, and apoptosis. It is noteworthy that specific combinations of microbiota and metabolites may serve as potential biomarkers, offering prospects for advancing precision interventions tailored to individual constitutions in managing constipation. Subsequent validation of identified microbial and metabolite biomarkers through larger-scale or longitudinal studies will be undertaken to deepen existing findings. This approach not only enhances our understanding of the underlying mechanisms but also opens new avenues for targeted interventions and personalized treatments in pediatric populations. Overall, the study highlights the potential of microbiome-based diagnostics and therapeutics in managing DHC and related conditions.
Data availability
The datasets generated and/or analysed during the current study are available in the National Center for Biotechnology Information repository. The Sequence Read Archive (SRA) ID is PRJNA1262707.
References
Vriesman, M. H. et al. Quality of life in children with functional constipation: A systematic review and meta-analysis. J. Pediatr. 214, 141–150 (2019).
Loening-Baucke, V. Chronic constipation in children. Gastroenterology 105, 1557–1564 (1993).
Loening-Baucke, V. Prevalence, symptoms and outcome of constipation in infants and toddlers. J. Pediatr. 146, 359–363 (2005).
Koppen, I. J. N. et al. Prevalence of functional defecation disorders in children: A systematic review and meta-analysis. J. Pediatr. 198, 121–130 (2018).
Djurijanto, F. et al. Prevalence and determinants of constipation in children in asia: a systematic review and meta-analysis. EClinicalMedicine 71, 102578 (2024).
Chogle, A. et al. A population-based study on the epidemiology of functional gastrointestinal disorders in young children. J. Pediatr. 179 (.e1), 139–143 (2016).
Malowitz, S., Green, M., Karpinski, A., Rosenberg, A. & Hyman, P. E. Age of onset of functional constipation. J. Pediatr. Gastroenterol. Nutr. 62, 600–602 (2016).
Benninga, M. A. et al. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 150, 1443–1455 (2016).
Pijpers, M. A., Bongers, M. E., Benninga, M. A. & Berger, M. Y. Functional constipation in children: a systematic review on prognosis and predictive factors. J. Pediatr. Gastroenterol. Nutr. 50, 256–268 (2010).
Chen, J. et al. Gut microbiome and metabolome alterations in traditional Chinese medicine damp-heat constitution following treatment with a Chinese patent medicine and lifestyle intervention. Phytomed. Int. J. Phytother. Phytopharmacol. 131, 155787 (2024).
Zhao, H. et al. Damp-heat constitution influences gut microbiota and urine metabolism of Chinese infants. Heliyon 9, e12424 (2022).
Jiang, Z. et al. The correlation between traditional chinese medicine constitution and hyperuricemia and gout: A systematic review and meta-analysis. Evid.-Based Complement. Altern. Med. eCAM. 2023, 5097490 (2023).
Liang, X. et al. Clinical research linking traditional Chinese medicine constitution types with diseases: a literature review of 1639 observational studies. J. Tradit. Chin. Med. 40, 690–702 (2020).
Song, Y. The study of correlation among function constipation, constitution of TCM and dietary habits. Guangzhou Univ. Tradit. Chin. Med. (2015).
Wang, F. Study on the distribution of Chinese medicine patterns and constitution types in children with functional constipation and Professor Liu Fang’s experience in the use of medicine. Liaoning Univ. Tradit. Chin. Med. (2023).
Jing, Y. et al. Gut microbiota and urine metabonomics alterations in constitution after Chinese medicine and lifestyle intervention. Am. J. Chin. Med. 49, 1165–1193 (2021).
Ma, K. et al. Qi-deficiency related increases in disease susceptibility are potentially mediated by the intestinal microbiota. Evid.-Based Complement. Altern. Med. eCAM 2018, 1304397 (2018).
Jing, C. et al. Study on the composition and abundance of intestinal flora in Phlegm-Dampness constitution subjects based on 16S rDNA sequencing. J. Tradit. Chin. Med. 60, 2045–2049 (2019).
Liu, Q. et al. Analysis of blood biochemical indexes and intestinal microflora diversity in women with constitution of Yin deficiency and constitution of Yin-yang harmony. ACTA Chin. Med. 35, 1514–1519 (2020).
Sarkar, A., Yoo, J. Y., Ozorio Dutra, V., Morgan, S. & Groer, K. H. The association between early-life gut microbiota and long-term health and diseases. J. Clin. Med. 10, 459 (2021).
Clarke, G. et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 18, 666–673 (2013).
Wang, J., Li, Y. & Wang, Q. Identification of Chinese medicine constitution in public health services. Chin. J. Integr. Med. 25, 550–553 (2019).
Lu, T. et al. Valid and convenient questionnaire assessment of Chinese body constitution: item characteristics, reliability, and construct validation. Patient Prefer. Adher. 16, 1875–1884 (2022).
Li, L., Yao, H., Wang, J., Li, Y. & Wang, Q. The role of Chinese medicine in health maintenance and disease prevention: application of constitution theory. Am. J. Chin. Med. 47, 495–506 (2019).
Lv, J. et al. Alterations of gut microbiota are associated with blood pressure: a cross-sectional clinical trial in Northwestern China. J. Transl. Med. 21, 429 (2023).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 7, 335–336 (2010).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Langille, M. G. et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31, 814–821 (2013).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677 (2025).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. Publ. Protein Soc. 28, 1947–1951 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Wen, M. et al. Correlation analysis between gut microbiota and metabolites in children with systemic lupus erythematosus. J. Immunol. Res. 2021, 5579608 (2021).
Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012).
Jia, H. et al. Predicting the pathological response to neoadjuvant chemoradiation using untargeted metabolomics in locally advanced rectal cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 128, 548–556 (2018).
Palmer, C., Bik, E. M., DiGiulio, D. B., Relman, D. A. & Brown, P. O. Development of the human infant intestinal microbiota. PLoS Biol. 5, e177 (2007).
Fallani, M. et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 157, 1385–1392 (2011).
Kozyrskyj, A. L., Kalu, R., Koleva, P. T. & Bridgman, S. L. Fetal programming of overweight through the microbiome: boys are disproportionately affected. J. Dev. Origins Health Dis. 7, 25–34 (2016).
McClorry, S. et al. Anemia in infancy is associated with alterations in systemic metabolism and microbial structure and function in a sex-specific manner: an observational study. Am. J. Clin. Nutr. 108, 1238–1248 (2018).
Biasucci, G. et al. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 86 (Suppl 1), 13–15 (2010).
Dominguez-Bello et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U.S.A. 107, 11971–11975 (2010).
Munyaka, P. M., Khafipour, E. & Ghia, J. E. External influence of early childhood establishment of gut microbiota and subsequent health implications. Front. Pead. 2, 109 (2014).
Li, N. et al. Distinct gut microbiota and metabolite profiles induced by different feeding methods in healthy Chinese infants. Front. Microbiol. 11, 714 (2020).
Le Huërou-Luron, I., Blat, S. & Boudry, G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 23, 23–36 (2010).
Dimitrakopoulou, E. I. et al. The metagenomic and metabolomic profile of the infantile gut: can they be predicted by the feed type? Child. (Basel Switzerland). 9, 154 (2022).
Callahan, B. J. et al. DADA2: High-resolution sample inference from illumina amplicon data. Nat. Methods. 13, 581–583 (2016).
Wang, M. et al. Application of probiotics in patients with intestinal dysbiosis. Dairy. Ind. 51, 15–18 (2023).
Wang, Q. TCM Constitution (People’s Health Publishing House, 2008).
Robertson, R. C., Manges, A. R., Finlay, B. B. & Prendergast, A. J. The human Microbiome and child growth-first 1000 days and beyond. Trends Microbiol. 27, 131–147 (2019).
Feng, Y. Study on the correlation of paediatric functional constipation patterns with related factors and traditional Chinese medicine constitution. Nanjing Univ. Tradit. Chin. Med. (2019).
de Meij, T. G. et al. Characterization of microbiota in children with chronic functional constipation. PloS One. 11, e0164731 (2016).
Mancabelli et al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci. Rep. 7, 9879 (2017).
Wang, J. K. & Yao, S. K. Roles of gut microbiota and metabolites in pathogenesis of functional constipation. Evid.-Based Complement. Altern. Med. eCAM. 2021, 5560310 (2021).
Wang, T. & Xu, X. Research progress of pregnant women’s emotional impact on fetuses and infants. Chin. J. Women Child. Health. 6, 71–74 (2015).
Zhang, C. et al. A key genetic factor governing arabinan utilization in the gut microbiome alleviates constipation. Cell Host Microbe. 31, 1989–2006.e8 (2023).
Xu, Y. et al. Antipsychotic-induced Gastrointestinal hypomotility and the alteration in gut microbiota in patients with schizophrenia. Brain. Behav. Immun. 99, 119–129 (2022).
Strati, F. et al. Altered gut microbiota in Rett syndrome. Microbiome 4, 41 (2016).
Gardiner, B. J. et al. Clinical and microbiological characteristics of Eggerthella lenta bacteremia. J. Clin. Microbiol. 53, 626–635 (2015).
Vandeputte, D. et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65, 57–62 (2016).
Parthasarathy, G. et al. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology. 150, (2016). 367 – 79.e1.
Tyrrell, K. L., Warren, Y. A., Citron, D. M. & Goldstein, E. J. Re-assessment of phenotypic identifications of bacteroides putredinis to alistipes species using molecular methods. Anaerobe 17, 130–134 (2011).
Mearin, F. et al. Bowel disorders. Gastroenterology 17, 130–134 (2016).
Sugitani, Y. et al. Mucosa-associated gut microbiome in Japanese patients with functional constipation. J. Clin. Biochem. Nutr. 68, 187–192 (2021).
Wang, L. et al. Bifidobacteria exert species-specific effects on constipation in BALB/c mice. Food Funct. 8, 3587–3600 (2017).
Fu, X., Li, R., Zhang, T., Li, M. & Mou, H. Study on the ability of partially hydrolyzed Guar gum to modulate the gut microbiota and relieve constipation. J. Food Biochem. 43, e12715 (2019).
Sandri, M., Monego, D., Conte, S., Sgorlon, G. & Stefanon, S. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 13, 65 (2017).
Yu, X. et al. Characteristics of intestinal flora in infants aged 0–26 months in Beijing. China J. Child. Health Care. 30, 124–129 (2022). 139.
Jalanka, J. et al. The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int. J. Mol. Sci. 20, 433 (2019).
Canani, R. B. et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 17, 1519–1528 (2011).
Capasso, R. et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology 129, 941–951 (2005).
Huang, T. et al. Chinese herbal medicine (MaZiRenWan) improves bowel movement in functional constipation through down-regulating oleamide. Front. Pharmacol. 10, 1570 (2020).
Watterson, K. R., Ratz, P. H. & Spiegel, S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell. Signal. 17, 289–298 (2005).
Maceyka, M., Harikumar, K. B., Milstien, S. & Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 (2012).
Dragusin, M. et al. Effects of sphingosine-1-phosphate and ceramide-1-phosphate on rat intestinal smooth muscle cells: implications for postoperative ileus. FASEB J. 20, 1930–1932 (2006).
Kim, Y. D. et al. Effects of sphingosine-1-phosphate on pacemaker activity of interstitial cells of Cajal from mouse small intestine. Mol. Cells. 35, 79–86 (2013).
Mostafa, R. M., Moustafa, Y. M. & Hamdy, H. Interstitial cells of Cajal, the maestro in health and disease. World J. Gastroenterol. 16, 3239–3248 (2010).
Choi, S. et al. Altering sphingolipid composition with aging induces contractile dysfunction of gastric smooth muscle via K(Ca) 1.1 upregulation. Aging cell. 14, 982–994 (2015).
Wang, S. et al. Mechanism of fructus aurantii flavonoids promoting Gastrointestinal motility: from organic and inorganic endogenous substances combination point of view. Pharmacognosy Mag. 13, 372–377 (2017).
Sui, J. et al. Sphingolipid metabolism in type 2 diabetes and associated cardiovascular complications. Exp. Ther. Med. 18, 3603–3614 (2019).
Lam, S. M. et al. Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol. Aging. 35, 2369–2381 (2014).
Novgorodov, S. A. et al. Lactosylceramide contributes to mitochondrial dysfunction in diabetes. J. Lipid Res. 57, 546–562 (2016).
Trayssac, M., Hannun, Y. A. & Obeid, L. M. Role of sphingolipids in senescence: implication in aging and age-related diseases. J. Clin. Investig. 128, 2702–2712 (2018).
Hadas, Y. et al. Altering sphingolipid metabolism attenuates cell death and inflammatory response after myocardial infarction. Circulation 141, 916–930 (2020).
Acknowledgements
The authors would like to thank all the participants in the study, including children and their guardians.
Funding
This work was supported by the High level Key Discipline of National Administration of Traditional Chinese Medicine-Traditional Chinese constitutional medicine [No. zyyzdxk-2023251]; and Qi-Huang Scholar, Chief Scientist Program of National Administration of Traditional Chinese Medicine Leading Talents Support Program [National TCM Human Education Letter 2022 No. 6].
Author information
Authors and Affiliations
Contributions
H.Z.: designed the study and performed the microbiological analyses; X.L. and Y.Y.: some experiments, bioinformatic analysis; W.L. and H.Y.: analysis and interpretation of data; Z.L.: patients data collection; X.Z., J.W. and J.W. conceived and designed the study. all authors contributed to writing and revising the manuscript, have seen and approved the submission of the final version of the manuscript, and take full responsibility for the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study protocol was reviewed and approved by the Ethics Committee of Beijing University of Chinese Medicine (No. 2020BZYLL122) and was conducted in accordance with the 1964 Helsinki Declaration and its later amendments. Informed consent was obtained from children’s parents to participate in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, H., Lu, X., Ye, Y. et al. Dysbiosis of gut microbiota in children with functional constipation and damp-heat constitution: a cross-sectional multi-omics analysis. Sci Rep 15, 42256 (2025). https://doi.org/10.1038/s41598-025-26439-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26439-6