Abstract
To evaluate the effect of ellagic acid (EA, extracted from pomegranate) on the quality and durability of the adhesive interface after tooth whitening. Tooth fragments were divided into (n = 10/group): G1 - control (no tooth whitening and no restoration), G2 – immediate whitening and restoration and G3 – whitening followed by EA application and restoration. The samples were subjected to an in-office bleaching technique and, the amount of residual oxygen released was measured. Resin composite blocks and sticks were fabricated and bonded to the dental surfaces. After 24 h and 12 months, microtensile bond strength was evaluated by microtensile shear test. The samples were coated with carbon, and the adhesive interface examined by scanning electron microscope (SEM). Data were analyzed with two-way ANOVA followed by Tukey’s test (α = 0.05). According to the bond strength test, higher values were observed in G1 > G2 < G3 at 24 h and 12 months, without statistically significant difference (p > 0.05). G1 showed the lowest level of nanoleakage, while G2 exhibited the highest. The prior application of EA in G3 resulted in intermediate nanoleakage. SEM analysis revealed a more stable adhesive interface in G3, with a lower amount of silver nitrate deposition compared to G2. EA appears to have a synergistic effect, qualitatively improving the in vitro quality of the adhesive interface by reducing oxygen in the dental structure. However, further studies are required to elucidate the mechanism of action of EA.
Similar content being viewed by others
Introduction
Tooth whitening is a dental procedure that enhances the aesthetics of the smile without requiring invasive interventions such as crowns or veneers1,2,3. However, immediately after bleaching, residual oxygen and other by-products from the bleaching agents remain within the dentinal tubules, interfering with the adhesion process. This condition can persist for up to 2 weeks after the end of bleaching treatment4,5. Consequently, the presence of residual oxygen affects the formation of the hybrid layer between the adhesive system and the dental substrate6,7. Oxygen retained on the enamel or dentin surface interacts with resin monomers, preventing the formation of a homogeneous hybrid layer by inhibiting or reducing the polymerization reaction8,9, which ultimately compromises the bond strength at the tooth-restoration interface.
To address this issue, pretreating dentin with antioxidants has been shown to reduce residual oxygen and improve bond strength values following bleaching. Therefore, the use of agents with antioxidant properties may contribute to the increased clinical longevity of adhesive restorations10,11. A 14-day waiting period between bleaching and restorative procedures has been recommended to allow sufficient time for the elimination of residual oxygen and its by-products12,13. However, some studies have shown no significant differences in bond strength or resin tag formation when restoration is performed 7, 14 or 21 days after the completion of tooth whitening14,15.
Thus, to enhance the elimination of residual oxygen and better define the appropriate timing for adhesive restoration, some researchers have investigated the use of antioxidant enzymes16,17. Among these, ellagic acid (EA) is a polyphenol synthesized by various plant species, known for its protective role against ultraviolet light, viruses, bacteria, and parasites. Additionally, ellagic acid is a biphenol classified as a hydrolysable tannins, and it has demonstrated satisfactory in vitro antioxidant potential18.
EA is a dimeric derivative of gallic acid, which is widely found in nuts and fruits such as blueberries, strawberries, and pomegranates19. It has been reported to possess several biological properties, including antioxidant and anti-inflammatory activities20,21. Its antioxidant effect is attributed to its ability to bind metal ions and neutralize hydroxyl, nitrogen, and peroxyl radicals18. Furthermore, studies have shown that ellagic acid can inhibit the growth of cancer cells, reduce inflammation, and protect brain function22,23. It also exhibits antioxidant potential through metal-chelating and radical-scavenging activities, as well as by modulating the activity of antioxidant enzymes24.
In this context, studies evaluating the antioxidant activity of EA on the adhesive interface are still scarce. Therefore, the aim of this study was to in vitro assess the effect of EA on the quality and durability of the adhesive interface following tooth whitening. The null hypothesis tested was that EA does not protect the adhesive interface after tooth whitening.
Materials and methods
Ethical aspects
This research was approved by the Human Research Ethics Committee of the University of Campinas (UNICAMP), and the teeth were obtained throught donation, in accordance with resolution No. 466/2012 of the National Health Council and the Declaration of Helsinki. Informed consent was obtained from all participants.
Tooth sample preparation and experimental groups
Freshly extracted human molars were collected. The teeth were cleaned using Gracey-type periodontal curettes (types 5, 6, 7 and 8; Hu-Friedy, IL, USA), pumice paste (SSWhite, Rio de Janeiro, RJ, Brazil), and water. Then, stored for one week at 4 °C in a 0.5% thymol solution until use25.
A cut was made 3 mm below the amelocementary junction using a precision metallographic cutter (IsoMet 1000, Buehler Inc., Lake Bluff, IL, USA) to remove the roots. Tooth fragments were obtained from the buccal and lingual surfaces of each crown, with an area of 25 mm2 (5 mm x 5 mm). A stereoscopic loupe (Leica Microsystems, Wetzlar, Germany) was used to examine the specimens for cracks, fissures or discolorations that could interfere with the whitening procedure. Thirty fragments were selected and randomly assigned to 3 experimental groups (n = 10/group): G1 - control (no tooth whitening), G2 – immediate whitening and restoration and G3 – whitening followed by ellagic acid application and restoration. An overview of the experimental design is presented in Fig. 1.
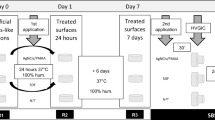
Experimental design of the study. (A,B) Separation of crown and root, followed by sectioning of tooth fragments to obtain buccal and lingual surfaces. (C) Embedding in acrylic resin discs. (D) Sample polishing. (E) Experimental groups. (F) Application of 35% phosphoric acid gel. (G) Application of ellagic acid. (H) Application of the adhesive system. (I) Application of the resin composite. (J,K) The resin composite block bonded to each fragment was sectioned into multiple sticks for the bond strength test.
Whitening procedure
Opalescence Boost 38% hydrogen peroxide (Ultradents Products, INC, South Jordan, UT) was used according to the manufacturer’s instructions. The gel was applied to the tooth fragment for 15 min, then removed with a suction cannula and rinsed off with water. This procedure was repeated 3 consecutive times per clinical session. A total of 3 clinical whitening sessions were performed, with a one-week interval between each session.
Ellagic acid
Ellagic acid (EA) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). A 10 µmol/L of EA (in 0.1% DMSO) was prepared based on previous study16. The solution was applied to the surface of the fragment using a microbrush in a circular motion for 60 s. Following this, the samples were rinsed with a water spray for 30 s and then stored in jars containing deionized water.
Adhesive restorative procedure
The procedure was performed according to the manufacturer’s instructions. Conditioning was carried out using 35% phosphoric acid gel (ScotchbondTM, 3 M ESPE, St. Paul, MN, USA) for 15 s, followed by rinsing with water for 30 s. The dental fragment was then gently dried with absorbent paper to preserve dentin moisture. The conditioned dental fragment was dried using absorbent paper to maintain dentin moisture5. After the application of ellagic acid, the adhesive system (Adper Single Bond 2, 3 M ESPE) was applied and light-cured for 10 s using the RadiiCal high-power LED device (SDI, São Paulo, SP, Brazil).
Titrations
The first titrimetric analysis was performed immediately after the bleaching treatment, with the value from the control group considered the baseline. Following the application of ellagic acid, the fragment was stored for either 24 h or 12 months, after which a new analysis was conducted. Titrimetric analyses of dissolved oxygen, modified by azide iodide, were carried out using a graduated pipette with the tip immersed in the deionized water in which the specimen had been stored. To each sample, 1mL of 50% manganese solution and 1mL of alkaline azide iodide solution were added, followed by vigorous shaking. A brown precipitate of manganese hydroxide formed and was allowed to settle for 15 min. Subsequently, 2 ml of 85% phosphoric acid was added, and the contents were mixed thoroughly. The precipitate dissolved, releasing free iodine into the solution, initiating the iodine titration with a standard sodium thiosulfate solution. When the solution turned pale yellow, 2 ml of starch indicator was added, and titration continued until the solution became colorless. The dissolved oxygen concentration was calculated in mg/L-1; 1 ml of thiosulphate = 1 mg of dissolved oxygen26.
Specimens preparation for the bond strength test
After the adhesive procedure, a 5 mm x 5 mm composite resin block was fabricated using an addition silicone guide with FiltekTM Z250 composite (3 M ESPE). The resin was placed in three increments of approximately 2 mm each, totaling a height of 6 mm. Each increment was light-cured for 20 s using the RadiiCal high-power LED device (SDI, São Paulo, SP, Brazil). 24 h after the restorative procedure, the blocks were sectioned longitudinally along the long axis of the fragment using a precision metallographic cutter (IsoMet 1000, Buehler Inc., Lake Bluff, IL, USA), producing slices approximately 0.8 mm thick. The fragment was then rotated 90°, and additional cuts were made to obtain sticks with an adhesive area of approximately 0.64 mm2. The number of slices that fractured prematurely during handling was recorded. Half of the sticks obtained for the microtensile test were analyzed after 24 h, while the remaining half were stored in distilled water—replaced weekly—for testing after 12 months. All samples were stored at a temperature of − 20 °C25.
Bond strength test
For this assay, the composite resin block bonded to each fragment was sectioned into multiple sticks for the bond strength test used. The thickness of the cross-section at the adhesive interface was measured with a digital caliper (Mitutoyo Sul Americana, Suzano, SP, Brazil), and the adhesive area (in mm2) was recorded to calculate the bond strength values in Megapascals (MPa). The sticks were mounted on a EZ-Test L universal testing machine (Shimadzu co, Kyoto, Japan) and secured at both ends using a cyanoacrylate-based adhesive (Super Bonder Gel, Loctite, Itapevi, SP, Brazil). The adhesive interface was positioned perpendicular to the direction of the applied force, and tensile testing was performed at a crosshead speed of 0.5 mm/min using a 20 kg force (kgf) load cell, until specimen failure occurred. At the point of rupture, the machine automatically stopped, and the microtensile bond strength values were tabulated (kgf). The bond strength (MPa) was calculated using the formula: Bond strength (MPa) = (Fracture load in kgf × 9.8) / Adhesive area (mm2). All values were coverted to MPa, tabulated and submitted to statistical analysis. The sticks were stored in distilled water to avoid direct exposure to the storage medium and kept at a temperature of -20 °C25.
Nanoinfiltration and scanning electron microscope (SEM)
Three slices from each group (24 h and 12 months) were immersed in a 50% ammoniacal silver nitrate solution. The solution was prepared by dissolving 10 g of silver nitrate crystals in 10 mL of deionized water, by the addition of drops of 28% ammonium hydroxide until the solution cleared. The samples were submerged in this solution for 24 h at 37 °C, then rinsed in distilled water for 2 min until completely clean. Subsequently, they were immersed in a developer solution for 8 h and exposed to direct fluorescent lighting (via a lamp) and indirect fluorescent lighting (via ambient lighting)26.
The samples were processed for scanning electron microscope (SEM) to visualize nanometric spaces within the hybrid layer. They were embedded in polystyrene resin and sequentially ground using 600, 1200, and 2000 grit sandpaper on a metallographic polishing machine. After grinding, polishing was performed using felt disks and diamond pastes of decreasing grits (3 μm, 1 μm and 0.25 μm). Between each grit change, the samples were immersed in distilled water in an ultrasonic tank for 10 min to remove surface debris. Next, the samples were dried with absorbent paper and etched in 85% phosphoric acid for 30 s, rinsed in distilled water, and immersed in a 10% sodium hypochlorite solution for 10 min. After being washed (2x) and dried at room temperature, the samples were dehydrated in a graded ethanol series (25%, 50%, 75%, 90%, and 100%), 10 min per step26. Finally, the samples were coated with carbon for observation under SEM (JSM 5600 LV, Jeol, Tokyo, Japan) at 50x magnification, operating under high vacuum at 20 kV. Backscattered electron images were obtained, and the silver nitrate-infiltrated areas were calculated using Image Processing and Analysis in Java (ImageJ, version 1.53t, National Institutes of Health, USA. https://imagej.net/ij/) software for qualitative analysis26.
Statistical analysis
Statistical analysis was performed to determine which technique was most effective. The data were analyzed using two-way ANOVA with the SAS statistical software (version 9.1.3; SAS Institute Inc., Cary, NC, USA), followed by Tukey’s post hoc test. A significance level of α = 0.05 was adopted.
Results
Bond strength test
For group 1, the bond strength values after 12 months of storage showed no statistically significant difference compared to those observed after 24 h (p > 0.05). Group 2 exhibited the lowest bond strength values at both time points when compared to the other groups. In group 3, the bond strength values were not statistically different from the control group at 24 h, however, a reduction was observed after 12 months of aging (Fig. 2).
Nanoinfiltration and scanning electron microscope
Samples aged for 12 months in deionized water showed a slight increase in nanoleakage values, regardless of the group evaluated. As expected, G1 showed the lowest values, particularly in the 24-hour specimens. On the other hand, G2 presented the highest nanoleakage values, especially after 12 months of storage. G3, which received a prior application of ellagic acid, showed intermediate nanoleakage values when compared to the other groups (Table 1).
Figures 3, 4 and 5 are representative SEM images of each group. According to the qualitative evaluation of the samples, a slight deposition of silver nitrate was observed (Fig. 3A), along with similar nanoleakage patterns after 12 months of storage (Fig. 3B). Extensive nanoleakage was noted in G2 at both 24 h and 12 months (Fig. 4A,B). In G3, which contained ellagic acid, a lower level of nanoleakage was observed in comparison to the other groups (Fig. 4), although a slight increase was seen after 12 months compared to the 24-hour specimens. Notably, in G3, improved sealing was evident, with longer resin tags and a thicker, more homogenous hybrid layer. As expected, extensive silver precipitates were observed along the adhesive interface during the immediate evaluation of G2 (Fig. 3; Table 1), whereas significantly fewer precipitates were observed in the ellagic acid group (Fig. 5; Table 1).
Representative image of interfacial silver nanoleakage from SEM (50×, scale bar = 500 μm) in the control group after 24 h (A) or 12 months (B). Minimal deposition of silver nitrate is observed in A, while a similar pattern of nanoleakage is observed after 12 months of storage in B. The white arrow indicates ammoniacal silver nitrate; red arrows indicate nanoleakage; T resin tag; the area between the two white arrows represents the hybrid layer; C composite; A adhesive.
Representative image of interfacial silver nanoleakage from SEM (50×, scale bar = 500 μm) in the group 2 after 24 h (A) or 12 months (B). Extensive nanoinfiltration was observed, both 24 h and 12 months after storage, as seen in A and B. The white arrow indicates ammoniacal silver nitrate; red arrows indicate nanoleakage; T resin tag; the area between the two white arrows represents the hybrid layer; C composite; A adhesive.
Representative image of interfacial silver nanoleakage from SEM (50×, scale bar = 500 μm) in the group 3 after 24 h (A) or 12 months (B). A smaller amount of nanoinfiltration was observed compared to the other groups, with a slight increase in nanoinfiltration after 12 months compared to 24 h. The white arrow indicates ammoniacal silver nitrate; red arrows indicate nanoleakage; the area between the two white arrows represents the hybrid layer; C composite; A adhesive.
Discussion
The present study compared three adhesive interfaces: control (Group 1), dental bleaching with restoration (Group 2), and dental bleaching with ellagic acid (EA) application prior to restoration (Group 3) for nanoleakage and nanoinfiltration. The null hypothesis is partially rejected, as a lower level of nanoleakage was qualitatively observed after ellagic acid application. However, no specimens were free of microleakage or microgap formation. This finding is consistent with previous studies that demonstrate gingival margins located below the dentin-enamel junction (DEJ) present a higher risk of marginal leakage in class I resin composite restorations compared to the margins placed above the DEJ27,28,29,30. The complex nature of the dentin substrate - characterized by a 45% inorganic composition, randomly arranged dentinal hydroxyapatite within an organic matrix primarily composed of type I collagen31,32,33,34,35 - and its intimate connection with the pulp through numerous fluid-filled tubules from the pulp to the DEJ36,37 complicate bonding. The presence of dentinal fluid can interfere with adhesion, as hydrophobic resins do not effectively bond to hydrophilic substrates, even when resin tags form within the dentin tubules38.
Aesthetic anterior restorations often require replacement following bleaching, as they do not respond to whitening agents in the same manner as tooth structure. A 14-day waiting period before restorative procedures is typically recommended to allow the dissipation of residual oxygen and by-products, ensuring optimal bond strength39,40,41,42,43. To accelerate this process and better define the necessary interval before adhesive restoration, this study is the first to investigate the use of ellagic acid - due to its antioxidant properties - for reducing residual oxygen post-bleaching and potentially shortening the delay between bleaching and restoration.
Ellagic acid is a natural antioxidant found in various fruits and vegetables, including chestnuts, raspberries, strawberries, blueberries, walnuts, and pomegranates. It has demonstrated properties against breast, pancreas, esophagus, colon, skin and prostate cancers, as well as protective effects against cardiovascular diseases and skin pigmentation disorders. In Dentistry, the application of ellagic acid prior to adhesive procedures may reduce residual oxygen after bleaching, thereby minimizing its interference with the resin polymerization and potentially enhancing bond strength. However, the efficacy of this approach depends on several factors, including the concentration of ellagic acid, application time, and the specific clinical protocol used. While preliminary findings are promising, further research is needed to quantify its effects and to optimize application protocols for clinical use.
In relation to our previous study44, a different storage period was adopted, as no statistically significant differences were observed after 6 months. In the present study, the improved performance of the DMSO and ellagic acid combination may be attributed to enhanced wettability of the dentin organic matrix exposed by acid etching45, as well as reduced dentinal fluid flow into the hybrid layer during in vitro bonding. These factors may explain the observed differences. It is also possible that DMSO acted as a solvent for the adhesive components, contributing to the protection of the adhesive interface after dental bleaching (G3).
One limitation of this study is the sample size; however, as the first to investigate the interaction between ellagic acid, the dental substrate, and the adhesive interface following tooth bleaching, it serves as a foundation for future research. Further in vitro studies are warranted to validate these findings, given the known systemic and oral health benefits of ellagic acid. In addition, future studies should explore various post-bleaching time intervals (48 h, 72 h, 1 week, 2 weeks, and 1 month) to determine the optimal timing for resin restoration following ellagic acid treatment. Moreover, incorporating ellagic acid into mouthwashes or toothpastes should be explored in future in situ and in vivo studies.
According to our findings, silver uptake was lower in the group treated with ellagic acid compared to Group 2, which may be attributed to enhanced protection of the adhesive interface. Additionally, SEM analysis enabled a qualitative assessment of hybrid layer degradation. Greater nanoleakage and hybrid layer failure were observed in Group 2 than in group Group 3; however, there was no statistically significant difference were found between the groups regarding bond strength values. The complex and sometimes contradictory relationship between bond strength and nanoleakage warrants further investigation. One possible explanation for this discrepancy is the polymerization shrinkage of the restorative material used. Future studies should consider evaluating different resin composites associated with ellagic acid. In the present study, SingleBond 2 - an etch-and-rinse adhesive - was used to assess the effect of ellagic acid on interface bonding durability. In this system, collapsed collagen fibrils can be rehydrated and re-expanded due to the presence of the hydrophilic monomer HEMA, which reduces water evaporation. It is also worth noting that the adhesive used may have interacted negatively with certain molecular chains of ellagic acid, potentially affecting the bonding performance. Therefore, comparing different adhesive systems in future experimental designs is recommended. Moreover, the samples were stored in deionized water after EA application to prevent potential chemical interactions between the teste solution and the various components found in artificial saliva, such as ions, enzymes, or organic molecules. Because EA has known chelating and antioxidant properties, its interaction with calcium, phosphate, or protein components in artificial saliva could have influenced its activity or interfered with the adhesive interface. Deionized water, being chemically inert and standardized, was chosen to minimize confounding variables and ensure better control of the experimental conditions. However, future studies involving in situ or in vivo conditions should consider artificial saliva or more biologically relevant media to simulate the oral environment.
To date, the only polyphenol studied at the adhesive-dentin interface has been epigallocatechin-3-gallate (EGCG), which has demonstrated the ability to preserve dentin bond strength, reduce interfacial nanoleakage after collagenase ageing, suppress MMP activity, and inhibit biofilm formation46. Therefore, it is important to highlight that no studies have yet investigated ellagic acid in this context, making data comparison difficult. Nevertheless, the findings presented open new avenues for incorporating this compound into Restorative Dentistry.
Conclusions
Under the protocol of this in vitro study, qualitative analysis revealed protection of the adhesive interface following the application of ellagic acid. However, further studies are needed to investigate the mechanical, chemical, and biological effects of ellagic acid in association with dental substrates.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Fioresta, R., Melo, M., Forner, L. & Sanz, J. L. Prognosis in home dental bleaching: a systematic review. Clin. Oral Investig. 27 (7), 3347–3361 (2023).
Barbosa, I. F. et al. The in vitro effect of solutions with or without sugar in dental bleaching. J. Mech. Behav. Biomed. Mater. 142, 105821 (2023).
Mukka, P. K. et al. An In-vitro comparative study of shear bond strength of composite resin to bleached enamel using three herbal antioxidants. J. Clin. Diagn. Res. 10 (10), 89–92 (2016).
Young, J. C., Clesceri, L. S. & Kamhawy, S. M. Changes in the biochemical oxygen demand procedure in the 21st edition of standard methods for the examination of water and wastewater. Water Environ. Res. 77 (4), 404–410 (2005).
Basílio, M. et al. Influence of different photoinitiators on the resistance of union in bovine dentin: experimental and microscopic study. J. Clin. Experimental Dentistry. 13 (2), e132–e139 (2021).
Hardan, L. et al. Immediate dentin sealing for adhesive cementation of indirect restorations: A systematic review and Meta-Analysis. Gels 8 (3), 175 (2022).
Zou, Y., Armstrong, S. R. & Jessop, J. L. Quantitative analysis of adhesive resin in the hybrid layer using Raman spectroscopy. J. Biomed. Mater. Res. A. 94 (1), 288–297 (2010).
Betancourt, D. E., Baldion, P. A. & Castellanos, J. E. Resin-Dentin bonding interface: mechanisms of degradation and strategies for stabilization of the hybrid layer. Int. J. Biomater. 3, 5268342 (2019).
Zaniboni, J. F. et al. Hybrid layer formation and bond strength to dentin impregnated with endodontic sealer after cleaning protocols. J. Conserv. Dent. 24 (2), 179–183 (2021).
Mazzoni, A. et al. MMP activity in the hybrid layer detected with in situ zymography. J. Dent. Res. 91 (5), 467–472 (2012).
Breschi, L. et al. Chlorhexidine stabilizes the adhesive inteface: a 2-year in vitro study. Dent. Mat. 26, 20–25 (2010).
Franco, L. M. et al. Surface effects after a combination of dental bleaching and enamel microabrasion: an in vitro and in situ study. Dent. Mater. J. 35 (1), 13–20 (2016).
Farawati, F. A. L. et al. Effect of carbamide peroxide bleaching on enamel characteristics and susceptibility to further discoloration. J. Prosthet. Dent. 121 (2), 340–346 (2019).
Henn, S. et al. Addition of zinc methacrylate in dental polymers: MMP-2 Inhibition and ultimate tensile strength evaluation. Clin. Oral Invest. 16 (2), 531–536 (2012).
Sanabe, M. E., Costa, C. A. & Hebling, J. Exposed collagen in aged resin-dentin bonds produced on sound and caries-affected dentin in the presence of chlorhexidine. J. Adhes. Dent. 13 (2), 117–124 (2011).
Flemming, J. et al. Olive oil as a transport medium for bioactive molecules of Plants?-An in situ study. Molecules 28 (9), 3803 (2023).
Kharouf, N. et al. Antibacterial and bonding properties of universal adhesive dental polymers doped with pyrogallol. Polym. (Basel). 13 (10), 1538 (2021).
Han, D. H., Lee, M. J. & Kim, J. H. Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 26 (5A), 3601–3606 (2006).
Kikuchi, H., Harata, K., Madhyastha, H. & Kuribayashi, F. Ellagic acid and its fermentative derivative urolithin A show reverse effects on the gp91-phox gene expression, resulting in opposite alterations in all-trans retinoic acid-induced superoxide generating activity of U937 cells. Biochem. Biophys. Rep. 25, 100891 (2021).
Gupta, A. et al. Antioxidant, anti-inflammatory and hepatoprotective activities of terminalia Bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol. Rep. 24, 8:44–52 (2020).
Ren, L., Zhang, J. & Zhang, T. Immunomodulatory activities of polysaccharides from ganoderma on immune effector cells. Food Chem. 340, 127933 (2021).
Djedjibegovic, J., Marjanovic, A., Panieri, E. & Saso, L. Ellagic Acid-Derived urolithins as modulators of oxidative stress. Oxid. Med. Cell. Longev. 28, 5194508 (2020).
Yoganathan, S., Alagaratnam, A., Acharekar, N. & Kong, J. Ellagic acid and schisandrins: natural biaryl polyphenols with therapeutic potential to overcome multidrug resistance in cancer. Cells 10 (2), 458 (2021).
Vekiari, S. A., Gordon, M. H., García-Macías, P. & Labrinea, H. Extraction and determination of ellagic acid contentin chestnut bark and fruit. Food Chem. 110 (4), 1007–1011 (2008).
Araújo, C. T. P. et al. Active application of primer acid on acid-treated enamel: influence on the bond effectiveness of self-etch adhesives systems. Microsc. Res. Tech. 80 (8), 943–949 (2017).
Prieto, L. T. et al. Nanoleakage evaluation of resin Luting systems to dental enamel and leucite-reinforced ceramic. Microsc. Res. Tech. 75 (5), 671–676 (2012).
Ferreira-Barbosa, I. et al. Effect of alternative solvent evaporation techniques on mechanical properties of primer-adhesive mixtures. Acta Odontol. Latinoam. 33 (2), 135–142 (2020).
Brkanović, S. et al. Comparison of different universal adhesive systems on dentin bond strength. Mater. (Basel). 16 (4), 1530 (2023).
Mosharrafian, S., Farahmand, N., Poorzandpoush, K., Hosseinipour, Z. S. & Kahforushan, M. In vitro microleakage at the enamel and dentin margins of class II cavities of primary molars restored with a bulk-fill and a conventional composite. Clin. Exp. Dent. Res. 9 (3), 512–517 (2023).
Takamizawa, T. et al. Bond durability of a two-step adhesive with a universal-adhesive-derived primer in different etching modes under different degradation conditions. Dent. Mater. J. 42 (1), 121–132 (2023).
Yamauchi, K. et al. Etch-and-rinse vs self-etch mode for dentin bonding effectiveness of universal adhesives. J. Oral Sci. 61 (4), 549–553 (2019).
Reis, F. N. et al. Solutions containing a Statherin-Derived peptide reduce enamel erosion in vitro. Caries Res. 57, 52–58 (2023).
Frazão Câmara, J. V. et al. Effect of a sugarcane Cystatin on the profile and viability of microcosm biofilm and on dentin demineralization. Arch. Microbiol. 203 (7), 4133–4139 (2021).
Lima, F. S. et al. Scaffold based on castor oil as an osteoconductive matrix in bone repair: biocompatibility analysis. Polímeros: Ciência E Tecnologia. 32 (1), e2022003 (2022).
Lima, F. et al. Chitosan-based hydrogel for treatment of temporomandibular joint arthritis. Polímeros: Ciência e Tecnologia 31(2), e2021019 (2021).
Reis, F. N. et al. Gels containing statherin-derived peptide protect against enamel and dentin erosive tooth wear in vitro. J. Mech. Behav. Biomed. Mater. 137, 105549 (2023).
Santos, L. A. et al. Solutions and gels containing a Sugarcane-Derived Cystatin (CaneCPI-5) reduce enamel and dentin erosion in vitro. Caries Res. 55, 594–602 (2021).
Costa, D. et al. Microabrasion effect on enamel susceptibility to penetration of hydrogen peroxide: an experimental and computational study. Odontology 109 (4), 770–778 (2021).
Amaral, W. S. et al. Surface and micromechanical analysis of polyurethane plates with hydroxyapatite for bone structure. Polímeros: Ciência e Tecnologia 32(4), e2022040 (2022).
Kamiya Coppini, E. et al. Surface roughness evaluation and whitening efficiency on tooth enamel after using whitening toothpaste: a randomized double-blinded study. Biosci. J. 38, e38056 (2022).
Passos, I. A. G. et al. Effect of non-thermal argon plasma on the shear strength of adhesive systems. Polímeros: Ciência E Tecnologia. 32 (1), e2022012 (2022).
Baldion, P. A. & Betancourt, D. E. Dataset on the effect of flavonoids on the stabilization of the resin-dentin interface. Data Brief. 35, 106984 (2021).
Guo, R. et al. Evaluation of resveratrol-doped adhesive with advanced dentin bond durability. J. Dent. 114, 103817 (2021).
Araújo Pierote, J. J. et al. Ellagic acid on the quality of the adhesive interface of class I composite resin restorations after aging. Biosci. J. 38, e38055 (2022).
Tjäderhane, L. et al. The effect of dimethyl sulfoxide (DMSO) on dentin bonding and Nanoleakage of etch-and-rinse adhesives. Dent. Mater. 29 (10), 1055–1062 (2013).
Yu, J. et al. Enhancing adhesive-dentin interface stability of primary teeth: from ethanol wet-bonding to plant-derived polyphenol application. J. Dent. 126, 104285 (2022).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Prieto LT, Paulillo LAMS: Conceptualization, Methodology, Validation, Visualization, Formal analysis, Data curation, Investigation, Writing - Original Draft. Câmara JVF, Pierote JJA, Santos LA, Ferrari CR, Lima DANL: Methodology, Validation, Visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This research was approved by the Human Research Ethics Committee and the teeth by donation, following resolution No. 466/2012 of the National Health Council. Informed consent was obtained from all subjects. in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prieto, L.T., Câmara, J.V.F., Pierote, J.J.A. et al. Protective role of ellagic acid in the adhesive interface after dental bleaching. Sci Rep 15, 38989 (2025). https://doi.org/10.1038/s41598-025-26885-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26885-2