Abstract
To observe the efficacy and safety of retrograde intrarenal surgery (RIRS) combined with a flexible vacuum-assisted ureteral access sheath (FV–UAS) in patients with large renal stones (LRS). A total of 149 patients with LRS were prospectively randomized into two groups: 75 in the FV–UAS group and 74 in the minimally invasive percutaneous nephrolithotomy (MPCNL) group. The primary outcome was the stone-free rates (SFRs) on the first postoperative day. Secondary endpoints included the total SFRs 1 month postoperatively, lithotripsy time, hemoglobin reduction, length of postoperative hospital stay, quality of life (QoL) score improvement, incidence of ureteral stricture at 3 months postoperatively, and any surgery-related complications. Patient demographics and preoperative clinical characteristics showed no apparent difference between the two groups (all P > 0.05). Postoperative data revealed a significantly longer lithotripsy time in the FV–UAS group than the MPCNL group (113.1 vs. 82.5 min, P < 0.001). The mean decrease in hemoglobin was significantly lower in the FV–UAS group than in the MPCNL group (8.2 vs. 17.7 g/L, P < 0.001). Similarly, the average hospital stay was shorter in the FV–UAS group than the MPCNL group (1.7 vs. 5.1 d, P < 0.001). Meanwhile, SFRs on the first postoperative day and 1 month postoperatively were statistically similar between the two groups (P > 0.05). QoL improvement was significantly higher in the FV–UAS group than in the MPCNL group (33.4 vs. 26.9, P < 0.001). The difference in ureteral stricture at 3 months postoperatively was not statistically significant (P > 0.05). Notably, the overall rate of postoperative complications was markedly lower in the FV–UAS group than in the MPCNL group (P < 0.05). Our study showed the safety and feasibility of applying RIRS combined with FV–UAS for LRS treatment, providing advantages such as high SFRs, minimal trauma, fast recovery, and low incidence of postoperative complications. It can be used as a clinical treatment alternative for LRS. The protocol for this study has been accepted by the Chinese Clinical Trial Registry (Ethics approval number: ChiCTR2200056402; Date of registration: 02-05-2022).
Similar content being viewed by others
Introduction
Urolithiasis is a major global health issue affecting millions of people. The incidence of renal stones varies significantly worldwide. Reports indicate that changes in diet and lifestyle associated with modernization have led to an incidence of approximately 10%–15% in developed countries, which continues to increase, particularly in industrialized nations1. Potential risk factors indicate that renal stones may recur in a significant proportion of cases, leading to upper urinary tract obstruction and infection-related complications. If these conditions are not treated promptly, irreversible damage may occur. Symptomatic and obstructive stones require proper management to achieve complete stone removal, minimize complications, and limit impact on quality of life.
Over the past 30–40 years, renal stone management has evolved from invasive surgical to minimally invasive methods. Currently, treatment methods for renal stones primarily include extracorporeal shock wave lithotripsy (ESWL), retrograde intrarenal surgery (RIRS), and percutaneous nephrolithotomy (PCNL)2. PCNL is the preferred surgical method for treating large renal stones (LRS) (> 2 cm)3. However, the establishment of artificial puncture access can damage the renal parenchyma and increase the risk of intraoperative and postoperative complications such as bleeding, infection, and peripheral organ damage4. While less invasive than PCNL, RIRS is limited by the power of flexible ureteroscope (fURS) fibers and the lower efficiency of traditional ureteral access sheaths (UAS) in stone removal5. The risk of postoperative renal colic, infection, and secondary surgery significantly increases with stone burden. Therefore, it remains still mostly limited to treating renal stones smaller than 2 cm6.
The novel flexible vacuum-assisted UAS (FV–UAS) can follow the fURS into the renal pelvises and calyces to suction and clear stones, substantially improving surgical efficiency7. In recent years, it has been increasingly applied in clinical practice and has become an effective alternative for treating LRS. Tang QL et al. study demonstrated that RIRS combined with FV–UAS achieved a high stone-free rate (SFR) and a low complication rate for treating 2–3 cm upper urinary tract stones7. In another study, for treating 2–3 cm renal stones, FV-UAS with disposable ureteroscope and 16 F tubeless PCNL both obtained high stone-free rates. The former reduced the rates of bleeding and hospital stay, and the latter shortened surgery duration8. However, its effectiveness for treating LRS (≥ 3 cm) remains uncertain, with few studies evaluating FV–UAS with RIRS in LRS over 3 cm. Thus, we conducted this prospective study to assess and compare the efficacy and safety of FV–UAS in RIRS versus minimally invasive PCNL (MPCNL) for patients with LRS (≥ 3 cm).
Materials and methods
Study design and patients
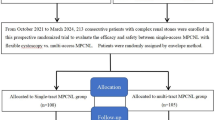
Patients with LRS referred to our institute between November 2022 and December 2024 were included in this prospective, randomized controlled study. After applying strict inclusion and exclusion criteria (Table 1), patients were randomly assigned to treatment groups by using the envelope method. The study ultimately included 149 patients (75 in the FV–UAS group and 74 in the MPCNL group), a number determined by a power analysis performed to estimate the sample size (Fig. 1). The pretreatment evaluation of participants included medical history, physical examination, laboratory investigations (i.e., urine analysis, urine culture and/or sensitivity, complete blood count, blood urea nitrogen, serum levels of creatinine, C-reactive protein, and procalcitonin), and radiological investigations. Patients with a known urinary tract infection (UTI) received antibiotic treatment until the infection was controlled. Meanwhile, The study was approved by the clinical research ethics committee of the Liaocheng People’s Hospital (ethics approval number: 2022–11313). Written informed consent was obtained from all participants. The study adhered to the principles of the Helsinki Declaration. The original source of method descriptions was referred to the article by Tang et al.7.
Flowchart for cases selection of the trial outlining enrollment, randomisation, allocation, follow-up, and analysis according to intention-to-treat standards. RIRS, Retrograde intrarenal surgery; FV-UAS, Flexible vacuum-assisted ureteral access sheath; MPCNL, minimally invasive percutaneous nephrolithotomy.
Randomization and masking
Parallel randomization was conducted using a stratified approach according to different surgical methods. Our center enrolled 149 participants, who were randomized in a 1:1 ratio to either the FV–UAS group or the MPCNL group. The randomization sequence was electronically generated before patient inclusion. Consecutively numbered and sealed envelopes were used for random sequence allocation and concealment. These sealed envelopes were opened by a designated nurse after the patients were subjected to general anesthesia. Subsequently, the ureteroscope was inserted into the urethra to visualize the specific surgical methods. After the procedure, the same individual automatically recorded the operative data.
Perioperative and surgical procedures
All patients underwent preoperative imaging, including CT and plain abdominal radiography of the kidneys, ureters, and bladder (KUB). Imaging was conducted to evaluate hydronephrosis and to assess the size, location, number, and specific details of the renal stones. Preprocedural urine cultures were performed, and appropriate antibiotic therapy was administered based on culture–antibiogram test results. Patients with negative urine cultures received broad-spectrum antibiotics before surgery. Otherwise, procedures were scheduled once infection indicators exhibited a downward trend and a negative urine culture was confirmed. Stone size was defined as the largest diameter of a single stone on preoperative non-contrast CT. For multiple stones, refer to the presence of two or more independent stones in the same renal simultaneously, it was defined as the sum of the largest diameters. Stone score include stone size (S), tract length (T), obstruction (O), number of involved calices (N), and essence or stone density (E). It was found to predict treatment success and the risk of perioperative complications9. For patients with recurrent urinary tract infections and severe renal colic, we often needed to place ureteral stents before surgery. All procedures were performed by two urologists, each with experience in more than 200 RIRS or MPCNL procedures annually. The surgical method for enrolled patients was randomly selected, excluding any subjective bias.
FV–UAS group
Under general anesthesia, patients were positioned lithotomically for retrograde endoscopic access. Ureteroscopy was performed to assess ureteral condition and estimate the appropriate length of the indwelling FV–UAS for insertion into the affected renal pelvis. A loach guidewire (0.032 in) (Huamei Medical, Jiangsu, China) was then introduced to access the upper urinary tract, followed by an 12/14 Fr FV–UAS (length: 35 cm for females; 45 cm for males) (Huamei Medical, Jiangsu, China) placed into the affected upper ureter. The head of the FV–UAS had a 10 cm long, steel wire-reinforced structure. It could enter the renal pelvis and calyces via the ureteropelvic junction (UPJ) and passively bend with the fURS. The FV–UAS surface had a hydrophilic lubricating coating, facilitating entry into the urethra and ureter (Fig. 2). A 7.5-Fr disposable electronic fURS (Pusen Medical, Guangdong, China) was then inserted through the FV–UAS. Under direct fURS visualization, the sheath in the upper segment of the ureter was advanced across the UPJ and into the renal pelvis to position the sheath close to the target stone (Fig. 3). The negative pressure suction device set to 85–100 mmHg was subsequently connected. The fluid irrigation was set to flow at 60–80 mL/min to maintain a clear surgical field. The electronic fURS fragmented the renal stones by using a 200 μm holmium laser fiber (0.8–1.2 J, 25–35 Hz). Larger stone fragments were aspirated by retracting the fURS, whereas smaller stone fragments were irrigated with lavage fluid through the gap between the sheath and the fURS. After the procedure, the fURS was reinserted into the collection system to check for residual stones. The FV–UAS and fURS were removed under direct visualization to document and evaluate any ureteral injury10. A 6 Fr double-J stent (Bard, USA) was placed in all patients postoperatively. In cases where severe ureteral stenosis or distortion impeded FV–UAS insertion, balloon dilation was attempted as the first approach. If dilation proved infeasible, only a double-J stent was inserted for ureteral expansion.
MPCNL group
Under general anesthesia, the patient was placed in the lithotomy position. A 6 Fr ureteral catheter (Boston Scientific, USA) was inserted into the ureteropelvic junction of the target ureter. Subsequently, the patient was turned prone, and the percutaneous tract was punctured under ultrasonographic guidance with an 18 gauge coaxial needle (Hakko Medical, Japan). Considered an avascular area of the kidney, the posterior middle calyx was the preferred puncture site, reducing the risk of bleeding. The percutaneous tract was then incrementally dilated using fascial dilators (Copper Medical, China) up to 18 Fr until fluid efflux confirmed proper placement. Once the tract was established, a nephroscope (12 Fr; Wolf Medica, German) was introduced to inspect the collection system and proximal ureter. Stones were fragmented using a pneumatic ballistic (EMS, Swiss). Smaller stone fragments were washed out through the sheath via retrograde irrigation. Ultrasound was employed ultimately to verify stone clearance. The second access tracts (18 Fr) were established to achieve the maximum SFR according to the situation of residual stones during surgery. A 6 Fr double-J stent (Bard, USA) was inserted into the ureter using a loach guidewire, and nephrostomy tubes (14 Fr) were placed.
Postoperative follow-up
Postoperative monitoring included determining white blood cell count, C-reactive protein, and procalcitonin levels at 2 h postoperatively to screen for UTI. Ultra-low-dose CT scans with a section thickness of 1 mm were performed on all patients on the first postoperative day and 1 month after surgery to evaluate for residual stones. Patients without significant postoperative discomfort, such as lower back pain or fever, were typically discharged within 48 h. In addition, all patients underwent stone composition analysis for tailored follow-up and preventive treatment. All double-J stents were removed at 4 weeks postoperatively.
Stone-free status was defined as no radiological evidence of stone or the presence of asymptomatic fragments ≤ 2 mm in the urinary system11,12. The primary study outcome was the SFR on the first postoperative day. Secondary outcomes included the following: total SFRs 1 month postoperatively (evaluated via ultralow-dose CT); lithotripsy time; reduction in hemoglobin levels; length of postoperative hospital stay; improvement in quality of life, as measured by the QoL score; incidence of ureteral stricture at 3 months postoperatively; and any surgery-related complications.
Lithotripsy time was characterized as the duration from the insertion of stone-broken equipment into the endoscope to the completion of stent placement. QoL improvement was assessed using the Wisconsin Stone QoL questionnaire. The improvement score was calculated as the difference between the preoperative and 1-month postoperative QoL scores13,14. Monitoring for ureteral strictures after surgery was also prioritized. Patients were scheduled for an ultrasound 3 months postoperatively. If hydronephrosis worsened relative to preoperative imaging, additional evaluation via IVU or enhanced CT was performed. Postoperative complications were classified using the modified Clavien grading system, including fever (≥ 38.5 ℃), hemorrhage, pain, and urosepsis15. Patients with residual stones underwent additional auxiliary procedures, such as external physical vibration lithotripsy, ESWL, or positional therapy as appropriate, at least 4 weeks postoperatively16,17.
Statistical analysis
Data were statistically analyzed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables are reported as means ± standard deviations. The independent-sample t-test was conducted to compare patient demographics, follow-up data, and surgical outcomes between groups, and the Shapiro–Wilk test assessed data normality. Categorical variables, including other pre- and postoperative clinical characteristics, were compared using the chi-squared test. A P value of < 0.05 was considered statistically significant.
Results
Demographics and preoperative clinical characteristics
In this study, 149 patients were randomly assigned to either the FV–UAS group (n = 75) or the MPCNL group (n = 74). The groups were similar in patient demographics and preoperative clinical characteristics (Table 2). The mean stone size was 3.5 cm in the FV–UAS group and 3.6 cm in the MPCNL group, with no significant difference (P = 0.233). Moreover, the two groups showed no substantial difference in mean age at diagnosis, body mass index, sex ratio, history of hypertension and diabetes, American Society of Anesthesiologists (ASA) classification, laterality, STONE score, stone distribution, stone essence, hydronephrosis grade, urine culture results, and previous upper urinary stone surgeries (all P > 0.05).
Postoperative clinical characteristics
In terms of effectiveness
Differences in postoperative clinical outcomes between the two groups are presented in Table 3. The lithotripsy time was longer in the FV–UAS group than in the MPCNL group (113.1 vs. 82.5 min, P < 0.001). The mean decrease in hemoglobin was significantly lower in the FV–UAS group than in the MPCNL group (8.2 vs. 17.7 g/L, P < 0.001), indicating reduced blood loss. Similarly, the average hospital stay was shorter in the FV–UAS group than in the MPCNL group (1.7 vs. 5.1 d, P < 0.001). However, SFRs on the first postoperative day and 1 month postoperatively were equivalent between the two groups (P > 0.05). QoL improvement, determined by the QoL score, was significantly higher in the FV–UAS group than in the MPCNL group (33.4 vs. 26.9, P < 0.001). At 3 months postoperatively, ureteral stricture was observed in three patients from the MPCNL group and in one patient from the FV–UAS group; no significant difference was found (P > 0.05).
In terms of safety
With regard to postoperative safety, the overall rate of complications was markedly lower in the FV–UAS group than in the MPCNL group (P < 0.05). Incidences of lower back pain and perirenal hematoma revealed notable significant differences (5.2% vs. 17.6%, P = 0.019; 2.6% vs. 14.9%, P = 0.008, respectively). In the MPCNL group, nine patients developed fever, two required blood transfusions, and three developed urosepsis necessitating antibiotic treatment. These complications showed higher rates in the MPCNL group than in the FV–UAS group; however, no statistically significant differences were found (all P > 0.05). Stone composition analysis, performed on all patients after surgery, exhibited no significant differences between the groups (P > 0.05).
Discussion
PCNL remains the gold standard for treating LRS (> 2 cm)3. Its major advantage is providing suitable artificial access for inserting large-diameter surgical instruments, allowing the removal of larger stone fragments. However, the creation of access can damage the renal parenchyma and blood vessels, leading to complications such as bleeding, organ damage, and infection during the perioperative period. Moreover, not all patients are suitable for PCNL, particularly those with an increased risk of bleeding or limited physical activity18. Consequently, many patients and urologists opt for RIRS with less damage instead of PCNL for treating renal stones19.
Despite the rising popularity of RIRS, traditional UAS often struggles to pass through UPJ and is typically placed 1–2 cm below the renal pelvis outlet. Stone fragments produced by fURS rely on repeated retrieval by a stone basket and subsequent removal via the sheath; this process limits the effectiveness of stone removal20. By contrast, the FV–UAS can enter the renal collection system via the UPJ under fURS guidance. This approach avoids the influence of UPJ on the sheath, allowing for a higher irrigation flow rate, a clearer surgical field, and lower intrarenal pressure (IRP). Concurrently, the negative pressure suction device can collapse the internal space of the renal pelvis and calyx, reducing stone displacement during lithotripsy. If necessary, FV–UAS can also be used to stabilize stones and improve the efficiency of lithotripsy21. In addition, the FV–UAS can enter the renal pelvis and calyx, approach target stones, and use a method similar to PCNL to flush stones at close range. The stones can then be attracted and removed by retracting the fURS without a stone basket, significantly improving the efficiency of stone removal22. The current study demonstrates that SFRs on the first postoperative day and 1 month postoperatively were equivalent between the two groups (P > 0.05). This finding aligns with the results obtained by Tang QL et al.23.
Stone removal primarily involved the repeated withdrawal of stone fragments via natural channels in the FV-USA group. The long and narrow diameter of the natural pathway, formed by the ureter and urethra, limited the passage of stone fragments. This restriction led to lithotripsy time being mainly consumed during stone removal. The artificial channel established via PCNL offers a shorter path and larger diameter, allowing the quick passage of larger stone fragments. Thus, the FV-USA group had a significantly longer lithotripsy time than the MPCNL group (113.1 vs. 82.5 min, P < 0.001). Research indicates that longer lithotripsy time may increase the probability of ureteral stricture due to the compression of the ureteral sheath in RIRS24. However, the present study demonstrated no significant difference in ureteral stricture rates at 3 months postoperatively between the two groups (P > 0.05). Moreover, the FV–UAS group exhibited significantly lower hemoglobin levels and shorter postoperative hospital stays than the MPCNL group (P < 0.05); this difference reflected the minimally invasive nature of FV–UAS. The FV–UAS group also markedly exceeded the MPCNL group in postoperative QoL improvements (P < 0.05).
High IRP during RIRS is a risk factor for infectious complications, such as fever, sepsis, and septic shock25. In traditional UAS, the lavage fluid must enter the renal collection system via the UPJ, which is relatively narrow and can affect intraoperative IRP control. Meanwhile, the mucosa and stone fragments below the UPJ may block the sheath, leading to high IRP, renal pelvis venous reflux, and the systemic spread of bacteria and endotoxins. This process can cause fever, sepsis, and even septic shock26. Corrales M et al. found a significantly higher risk of infection after RIRS than PCNL25. Reducing perfusion flow can prevent high IRP; however, it inevitably compromises the surgical field of view and diminishes stone fragmentation efficiency. The FV–UAS can pass through the UPJ with fURS, preventing UPJ obstruction and high IRP formation. Through its negative pressure suction device, bacteria and endotoxins exposed during lithotripsy can be promptly eliminated, reducing the risk of infection. The present study shows that nine patients developed fever and three had urosepsis in the MPCNL group; meanwhile, five patients developed fever and one had urosepsis in the FV–UAS group. Using sensitive antibiotics, all these patients achieved good outcomes. Notably, these differences were not statistically significant (Both P > 0.05), but the FV–UAS group reported lower incidences of lower back pain and perirenal hematoma than the MPCNL group (Both P < 0.05). The renal parenchyma injury associated with percutaneous tract establishment led to the significantly lower overall postoperative complications in the FV–UAS group than in the MPCNL group (P < 0.05).
However, this study has certain limitations. First, the follow-up period of 3 months may be inadequate to capture long-term complications such as ureteral strictures, likely affecting the observed outcomes. Second, the high level of experience in FV–UAS of the surgeons who participated in the trial may limit the generalizability of the findings to broader clinical settings. Nonetheless, this consistent skill level among surgeons was necessary to minimize surgeon bias when comparing RIRS and MPCNL outcomes. Finally, as a single-center study with a modest sample size, it exhibits a potential for sampling error. Optimal procedures will likely emerge from extended clinical applications and observations over time.
Conclusions
Combining RIRS with FV–UAS for LRS treatment is safe and feasible, presenting advantages such as high SFRs, minimal trauma, fast recovery, and a low incidence of postoperative complications. This approach can be used as a clinical treatment alternative for LRS. However, large-scale multicenter prospective studies are necessary to confirm these findings.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
American Urological Association. Medical Management of Kidney Stones: AUA Guideline. https://www.auanet.org/guidelines-and-quality/guidelines/kidney-stones-medical-mangement-guideline (2019).
European Association of Urology. EAU Guidelines on Urolithiasis. https://uroweb.org/guidelines/urolithiasis (2024).
Assimos, D. et al. Surgical management of stones: American urological Association/Endourological society Guideline, PART II. J. Urol. 196 (4), 1161–1169. https://doi.org/10.1016/j.juro.2016.05.091 (2016).
Soderberg, L. et al. Percutaneous nephrolithotomy versus retrograde intrarenal surgery for treatment of renal stones in adults. Cochrane Database Syst. Rev. 11 (11), CD013445. https://doi.org/10.1002/14651858.CD013445 (2023).
Lai, S. et al. Comparing different kidney stone scoring systems for predicting percutaneous nephrolithotomy outcomes: A multicenter retrospective cohort study. Int. J. Surg. 81, 55–60. https://doi.org/10.1016/j.ijsu.2020.07.025 (2020).
Emmott, A. S. et al. Re-Intervention Rates, and natural history of residual stone fragments after percutaneous nephrolithotomy. J. Endourol. 32 (1), 28–32. https://doi.org/10.1089/end.2017.0618 (2018).
Tang, Q. L. et al. RIRS with flexible vacuum-assisted UAS versus MPCNL for impacted upper ureteral stones: a prospective, randomized controlled study. Urolithiasis 53 (1),105. https://doi.org/10.1007/s00240-025-01781-6 (2025).
Deng, G. et al. Comparison of the efficacy of ureteroscopy through a flexible vacuum-assisted ureteral access sheath with tubeless-mini percutaneous nephrolithotomy for the treatment of 2–3 cm renal calculi. Urol. J. 1. https://doi.org/10.22037/uj.v22i.8368 (2025).
Okhunov, Z. et al. S.T.O.N.E. Nephrolithometry: novel surgical classification system for kidney calculi. Urology 81 (6), 1154–1159. https://doi.org/10.1016/j.urology.2012.10.083 (2013).
Karakan, T. et al. Evaluating ureteral wall injuries with endoscopic grading system and analysis of the predisposing factors. J. Endourol. 30 (4), 375–378. https://doi.org/10.1089/end.2015.0706 (2016).
Ghani, K. R. & Wolf, J. S. Jr What is the stone-free rate following flexible ureteroscopy for kidney stones? Nat. Rev. Urol. 12 (5), 281–288. https://doi.org/10.1038/nrurol.2015.74 (2015).
Dauw, C. A. et al. Contemporary practice patterns of flexible ureteroscopy for treating renal stones: results of a worldwide survey. J. Endourol. 29 (11), 1221–1230. https://doi.org/10.1089/end.2015.0260 (2015).
Penniston, K. L. et al. Validation and reliability of the Wisconsin stone quality of life questionnaire. J. Urol. 197 (5), 1280–1288. https://doi.org/10.1016/j.juro.2016.11.097 (2017).
Zhong, W. et al. Translation and validation of the Chinese version of Wisconsin stone quality of life questionnaire in patients with kidney stones. Minerva Urol. Nephrol. 75 (3), 353–358. https://doi.org/10.23736/S2724-6051.22.04905-9 (2023).
Mitropoulos, D. et al. Validation of the Clavien-Dindo grading system in urology by the European association of urology guidelines ad hoc panel. Eur. Urol. Focus. 4 (4), 608–613. https://doi.org/10.1016/j.euf.2017.02.014 (2018).
Tao, R. Z. et al. External physical vibration Lithecbole facilitating the expulsion of upper ureteric stones 1.0–2.0 cm after extracorporeal shock wave lithotripsy: a prospective randomized trial. Urolithiasis 48 (1), 71–77. https://doi.org/10.1007/s00240-018-1100-8 (2020).
Yang, J. et al. Efficacy analysis of self-help position therapy after holmium laser lithotripsy via flexible ureteroscopy. BMC Urol. 18 (1), 33. https://doi.org/10.1186/s12894-018-0348-1 (2018).
Setthawong, V., Srisubat, A., Potisat, S., Lojanapiwat, B. & Pattanittum, P. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database Syst. Rev. 8 (8), CD007044. https://doi.org/10.1002/14651858.CD007044.pub4 (2023).
Cosmin, C., Georgescu, D. A., Geavlete, P., Popescu, R. I. & Geavlete, B. Comparison between Retrograde Flexible Ureteroscopy and Percutaneous Nephrolithotomy for the Treatment of Renal Stones of 2–4 cm. Med. (Kaunas). 59 (1), 124. https://doi.org/10.3390/medicina59010124 (2023).
Sari, S. et al. Outcomes with ureteral access sheath in retrograde intrarenal surgery: a retrospective comparative analysis. Ann. Saudi Med. 40 (5), 382–388. https://doi.org/10.5144/0256-4947.2020.382 (2020).
Chen, Y. et al. A novel flexible vacuum-assisted ureteric access sheath in retrograde intrarenal surgery. BJU Int. 130 (5), 586–588. https://doi.org/10.1111/bju.15873 (2022).
Chen, Y. et al. Novel flexible Vacuum-Assisted ureteral access sheath can actively control intrarenal pressure and obtain a complete Stone-Free status. J. Endourol. 36 (9), 1143–1148. https://doi.org/10.1089/end.2022.0004 (2022).
Tang, Q. L. et al. RIRS with flexible vacuum-assisted UAS versus MPCNL for impacted upper ureteral stones: a prospective, randomized controlled study. Urolithiasis 53 (1), 105. https://doi.org/10.1007/s00240-025-01781-6 (2025).
Grosso, A. A. et al. Intraoperative and postoperative surgical complications after ureteroscopy, retrograde intrarenal surgery, and percutaneous nephrolithotomy: a systematic review. Minerva Urol. Nephrol. 73 (3), 309–332. https://doi.org/10.23736/S2724-6051.21.04294-4 (2021).
Corrales, M., Sierra, A., Doizi, S. & Traxer, O. Risk of sepsis in retrograde intrarenal surgery: A systematic review of the literature. Eur. Urol. Open. Sci. 44, 84–91. https://doi.org/10.1016/j.euros.2022.08.008 (2022).
Somani, B. K. et al. Complications associated with ureterorenoscopy (URS) related to treatment of urolithiasis: the clinical research office of endourological society URS global study. World J. Urol. 35 (4), 675–681. https://doi.org/10.1007/s00345-016-1909-0 (2017).
Author information
Authors and Affiliations
Contributions
YB Ma and YF Ding: Project development. T Lin and GL Huang: Data Collection. Y Cheng and YF Ding: Data analysis and Manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of Liaocheng People’s Hospital (ethics approval number: 2022–11313) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The clinical trial registration number for study is ChiCTR2200056402.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, Yf., Lin, T., Cheng, Y. et al. RIRS with flexible vacuum-assisted ureteral access sheath for large renal stones: a prospective randomized controlled study. Sci Rep 15, 42780 (2025). https://doi.org/10.1038/s41598-025-26987-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26987-x