Abstract
Cerebral collateral status is considered an independent prognostic factor in stroke patients. We aimed to assess whether collateral extent also modifies first-pass recanalization (FPR) and distal emboli generation across different mechanical thrombectomy (MT) strategies. Two in-vitro neurovascular models were created: good (GCM) and poor collaterals model (PCM). Both were identical up to the M2 segment of the middle cerebral artery (MCA), but only the GCM included primary and secondary anastomoses. Soft (stiffness = 53.72 ± 5.32 kPa) and stiff (stiffness = 110.02 ± 10.59 kPa) clot analogs embolized the M1-MCA. The study was randomized for thrombectomy technique: direct aspiration thrombectomy, partial stent retriever retraction and complete stent retriever retraction. Outcome measures were: complete (TICI2c-3), substantial (TICI2b-3) recanalization, and distal emboli parameters. A total of 240 MTs were performed (20 experiments per technique/model/clot type). Overall rates of complete and substantial recanalization were 23.8% and 68.8% respectively. Complete recanalization was higher in GCM (33.3%) than in PCM (14.2%; p < 0.01) regardless of clot type or technique. Across all clot types and collateral status, complete stent-retriever technique achieved highest rate of TICI 2b-3 and direct aspiration the highest rates of TICI 2c-3, particularly in PCM. Partial stent-retriever retraction technique was the least effective. In our experimental setup, there were no significant differences in distal embolization according to collaterals or clot types, however partial stent-retriever retraction technique generated the highest embolic load and direct aspiration the lowest. The degree of collateral circulation may modify MT angiographic outcomes with different impacts according to techniques or clot composition and could be used to guide therapeutic decisions.
Similar content being viewed by others
Introduction
Mechanical thrombectomy (MT) is now the most effective proven treatment of acute ischemic stroke caused by large vessel occlusion1. Despite significant advancements in endovascular techniques, perfect angiographic outcomes are still not systematically achieved, highlighting the importance of identifying factors that influence recanalization success. Among these, the collateral status (CS) of cerebral vasculature may be a significant modulator of success2.
CS plays a key role in maintaining cerebral perfusion during arterial occlusion by providing alternative routes for blood flow. Primary collaterals include the arterial segments of the circle of Willis, whereas the ophthalmic artery and leptomeningeal vessels constitute the secondary collateral network3. Their degree of development and robustness have an important interindividual variability and have been strongly linked to angiographic and clinical outcomes in ischemic stroke patients undergoing MT 4.
CS not only determines the speed and extension of infarct growth within the ischemic penumbra during the time the vessel remains occluded, but the resulting hemodynamic conditions may also influence the efficacy of MT by favouring or hampering clot retrieval with different devices or techniques3.
A deeper understanding of the interactions between CS and recanalization outcomes achieved with MT could significantly impact device selection and treatment strategies in clinical practice. Investigating these relationships in a controlled and replicable experimental setting may help optimize endovascular stroke therapies and ultimately enhance patient outcomes.
This study seeks to address unresolved questions regarding the role of CS in MT by evaluating whether specific thrombectomy techniques—such as stent retriever (SR) based methods or aspiration-based approach — yield superior outcomes according to the quality of the CS or the different clot characteristics. By integrating these factors into the experimental setup, we aim to provide insights into optimal treatment strategies across diverse clinical scenarios, bridging the gap between preclinical research and clinical application.
Methods
Neurovascular model
Two 3D-printed neurovascular models were created: the good collaterals model (GCM) and the poor collaterals model (PCM). The GCM includes a complete circle of Willis and anastomoses of the M2-middle cerebral artery (MCA) branches with anterior cerebral arteries (ACA) and vertebrobasilar circulation. In the PCM both primary and secondary collaterals were missing.
The models drained in an outlet tank that was feeding the pump creating a closed-loop system. The model included a simulated descending aorta that was also connected to the outlet mimicking the systemic circulation. Resistance in the descending aorta was adjusted with a Hoffmann clamp, so the cerebral/systemic circulating volume ratio was 20/80. The model was equipped with a compliance chamber to act as a reservoir, ensuring physiological systolic and diastolic pressure levels. The setup functions in a closed circulatory system connected to the pulsatile pump (Harvard Apparatus, Holliston, MA) to simulate physiological blood flow dynamics, with water temperature maintained at 37 [SD ± 1] °C. Before embolization, the internal pressure at the level of the right common carotid artery was measured before each experiment using a multiparameter hemodynamic monitor [Mindray iMEC8 Vet, China] to maintain consistent conditions. The systolic and diastolic cycles, pump’s strokes per minute, and volume per stroke were adjusted to achieve mean pressures of 130 (SD ± 2.94) / 70 (± 3.30) mmHg.
The models were equipped with an inflow filter to prevent introduction of undesired particles into the flow-loop system. Outflow filters were placed to collect potential embolized clot particles in the primary and new vascular territories. In the GCM, the first outflow filter was positioned at the end of the occluded right MCA (embolization into primary territory) and second filter was placed after merging all draining collaterals (embolization into new territories). In the PCM, since ACA and MCA independently drained into the outlet tank, the first filter was collecting drainage from the occluded right MCA, and the second was collecting drainage from the right ACA, again collecting potential emboli to any vascular territory (Fig. 1).
Clot analogs
Soft and stiff elastic clot analogs (Life Model Design LLC, Marengo, OH), were embolized to create a 10 mm long occlusion within the middle M1 segment of the MCA. A length of 10 mm was chosen as it reflects clinically reported thrombus sizes in MCA occlusions and provided a balance between clinical relevance and experimental reproducibility5. Clot analogs used in the experiments were within the comparable stiffness range of thrombus retrieved from patients with stroke6,7. The mean secant modulus of elasticity at 0%–45% strain (E0%–45%) was 53.72 [SD ± 5.32] kPa and 110.02 [SD ± 10.59] kPa for soft and stiff clots, respectively.
In vitro MT techniques
To simulate femoral access, the 8 F sheath was inserted into a silicone tube that extended the descending aorta. The uniform synthetic clots, produced under standardized conditions to ensure consistent size, length, and mechanical properties and categorized into two groups (soft vs. stiff) based on reproducible elasticity values, were embolized from the external carotid artery port to the M1-MCA, and the type of embolized clot was known to the operator. After embolization, the clot was allowed to remain lodged in the M1 segment for approximately 5 min before thrombectomy was initiated, to ensure stable occlusion under physiological flow conditions. Once embolized, the thrombectomy approach was allocated (1:1:1) by randomization (studyrandomizer.com, NL) to one of the following arms: direct aspiration thrombectomy versus partial SR retraction technique versus complete SR retraction technique. A long sheath (Cerebase DA 8 F; Cerenovus) was then advanced to the distal C3 internal carotid artery segment. The total number and distribution of the experiments was predefined in the study protocol.
To replicate the clinical setting, continuous saline flush lines were connected to the long sheath, intermediate catheter, and microcatheter during the whole procedure.
For SR assisted techniques, as previously reported8 a triaxial system consisting of a intermediate catheter (React 71; Medtronic), a microcatheter (Phenom 21; Medtronic), and a microguidewire (Synchro 14; Stryker) was advanced to the proximal face of the clot. After crossing the clot with the microguidewire and microcatheter, the microguidewire was exchanged for a SR (Solitaire 4 × 40; Medtronic). SR deployment was performed using the push-and-fluff technique9 from the M2 to the M1 MCA segment, aligning the proximal ends of the clot and the stent. Following SR deployment, the microcatheter was removed, the intermediate catheter was advanced to contact the clot, and continuous pump aspiration (Penumbra, CA, USA) was initiated through the intermediate catheter for 1 min, after which SR was retrieved 2–3 mm into the intermediate catheter. Additional manual aspiration via 60-mL syringe (VacLok; Merit Medical) was applied through the long sheath. Prior to clot retrieval, slack in the system was actively removed to ensure optimal device tension. At this stage, in cases allocated to the complete SR retraction technique, the SR was fully retracted and extracted through the intermediate catheter before finally pulling it out under continuous aspiration. For cases allocated to the partial SR retraction technique, only the proximal 1/3 of the SR was retracted into the intermediate catheter before simultaneously pulling out both under continuous aspiration.
For the cases allocated to the direct aspiration technique, the intermediate catheter was advanced over the microcatheter (that did not cross the clot) until achieving contact with the clot. The microcatheter and wire were then removed, and continuous aspiration with the pump was initiated for 1 min. After this time, the intermediate catheter was slowly retrieved into the long sheath, in which manual aspiration with the 60 ml syringe was previously initiated.
The same MT setup was used for conducting every experiment. Each experiment was video recorded. Only 1 MT attempt was performed per clot. After each experiment, the entire model and equipment were flushed to ensure no residual clot fragments were left in the circuit.
The primary endpoint was complete recanalization (TICI2c-3) previously defined as absence of remnant clot fragments in the model with no particles > 1 mm in the outflow filter. Secondary outcomes were: substantial recanalization (TICI 2b-3), defined as absence of remnant clot fragments in the model but presence of particles > 1 mm in the filter, and periprocedural distal embolization parameters.
Filter analysis
The analysis of the filter, as previously described10, involved several steps. Clean 100-micron filters were placed in the inflow and outflow before each experiment as described above. After each experiment, outflow filters were removed and stained with red dye to enhance particle visibility. Subsequently, high-resolution images were captured using a VZ-R camera (IPEVO, Pleasanton, CA.) for analysis. Using a previously validated10 Matlab R2020a algorithm, an independent researcher blinded to experimental data pre-processed the images and used the algorithm to automatically quantify several characteristics of distal emboli captured in the outflow filters Fig. 2. Emboli parameters included: major axis length of the largest embolus (Max Size), the area of the largest embolus (Max Area), the total embolic area (Total Area), and the total number of collected particles (Total Count).
Statistical analysis
Emboli characteristics were expressed as mean (± SD). The Shapiro-Wilk test was used to assess the normality of the data. The χ2 test was used to compare three MT techniques regarding the complete and substantial recanalization rates, and the Kruskal-Wallis test was used to analyze the distal emboli parameters. The statistical significance was defined as p < 0.05. Statistical analyses were performed using SPSS, Version 27.0 software (IBM, Chicago, IL).
Results
Following the predefined protocol, a total of 240 experiments were conducted, with 120 allocated to each model (GCM and PCM). Within each model, 20 experiments were performed per technique and clot type (Supplemental material 1 and 2).
Overall recanalization rates
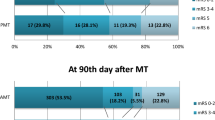
The overall rate of complete recanalization was 23.8%, where the direct aspiration technique demonstrated the highest success (31.3%), complete SR retraction technique achieved 25% and partial SR retraction technique 15% (Supplemental material 3).
The rate of substantial recanalization was 68.8%, with the highest success observed with complete SR retraction technique (90%), followed by direct aspiration (63.7%), and the lowest with partial SR retraction technique (52.5%).
Performance according to collaterals
The rate of complete recanalization was higher in the GCM compared to PCM (33.3%, vs. 14.2%; p < 0.001). This difference was consistent across techniques and clot types. GCM outperformed PCM both for stiff clots (33.3% vs. 15%; p < 0.05) and soft clots (33.3% vs. 13.3%; p < 0.05). Under poor collaterals conditions, the rates of complete recanalization, were consistently higher with the direct aspiration technique and particularly low with the partial SR retraction technique independently of clot type (Figs. 3 and 4).
The overall rate of substantial recanalization tended to be higher in the GCM (74.2%) than in the PCM (63.3%; p = 0.07). The difference was statistically significant for soft clots (80% vs. 63.3%; p = 0.043) but not for stiff clots (68.3% vs. 63.3%; p = 0.564). The complete SR retraction technique was the most effective approach in both models, particularly for soft clots, where the rate of substantial recanalization achieved 100% and 90% in the GCM and PCM, respectively (Fig. 4A and B) shows all recanalization results according to model, clot type, and technique.
Distal embolization measures
The analysis of distal embolization revealed significant differences between thrombectomy techniques, but no differences were observed according to collaterals or clot types in terms of distal emboli generation.
Embolization to the primary territory (Filter 1)
The complete SR retraction technique resulted in the highest level of embolization within the same territory, with the largest embolus area (Max Area = 6.63 ± 0.57 mm²) compared to partial SR retraction technique (3.28 ± 0.66 mm²) and direct aspiration (1.76 ± 0.43 mm²); all measured parameters followed a similar trend (Fig. 5).
Embolization to a new territory (Filter 2)
The partial SR retraction technique was most prone to embolization into a new vascular territory, with the largest embolus area (Max Area = 3.68 ± 0.49 mm²), significantly exceeding values for complete SR retraction technique and direct aspiration; similar trends were observed for all parameters (Fig. 5).
Overall distal embolization (Filter 1 + 2)
When considering total embolization from both filters, partial SR retraction technique generated the highest embolic load, followed by complete SR retraction technique, while direct aspiration consistently produced the lowest values. For example, Max Area was 6.09 ± 0.68 mm² for partial SR retraction, 4.08 ± 0.55 mm² for complete SR retraction, and 1.90 ± 0.42 mm² for direct aspiration technique. This trend was consistent for all remaining embolization parameters detailed in Fig. 5.
Discussion
Our study investigated the impact of collateral flow on MT outcomes, focusing on the efficacy of different techniques and clot types in achieving successful recanalization. Using a previously validated in-vitro neurovascular model, we sought to standardize multiple replications of the experiments combining different scenarios to precisely isolate and characterize the influence of good versus poor collaterals on recanalization and distal embolization parameters.
The findings confirm that the collateral status significantly influences MT outcomes. The overall rates of complete and substantial recanalization as well as distal emboli measures were systematically more favourable in GCM scenarios. These results indicates that the pressures gradient and flow conditions associated with robust collaterals facilitate reperfusion by enhancing the likelihood of procedural success regardless of clot type and thrombectomy technique.
While the rates of complete recanalization were higher when using direct aspiration, the complete SR retraction technique was the most successful in achieving substantial recanalization. The differences in recanalization success between SR-based and aspiration-only techniques can be attributed to their distinct mechanisms of clot retrieval. It should also be acknowledged that the thrombectomy in our model was performed along a relatively straight vascular segment. Since direct aspiration is often more effective in straight anatomies, whereas combined approaches (Solumbra/SAVE) may perform better in angulated segments. Future studies should assess outcomes in models with different anatomical features.
Complete SR retraction technique, which combines SR deployment, with aspiration through a distal access catheter, provides a strong mechanical framework that grips the whole length of the clot, making it effective at achieving substantial recanalization and a moderate impact of conflicting pressure gradients. However, since it requires initial clot crossing with the microcatheter to deploy the SR, it may alter clot structure, contributing to the embolization into the same distal arterial territory of small but potentially meaningful fragments11 that preclude complete recanalization (Video 1). Additionally, the shaving effect during SR retrieval into the intermediate catheter, especially when clot composition is not soft and malleable, may further contribute to distal emboli formation by clot stripping and shearing off fragments, increasing the risk of incomplete reperfusion. As a result, complete SR retraction technique achieves high rates of substantial but not complete reperfusion.
In contrast, direct aspiration operates under an ‘all-or-nothing’ principle—if successful engagement and ingestion into the intermediate catheter occurs, the clot is successfully retrieved in one pass without fragmentation, achieving complete recanalization at higher rates than when SR is used (Video 2). For this reason, direct aspiration particularly favoured complete recanalization when retrieving softer clots that are easily ingested. However, an aspiration-only-based technique often fails following a specific sequence: aspiration first leads to intermediate catheter occlusion by the proximal part of the clot, which may not be progressively ingested into the intermediate catheter due to reduced catheter to clot diameter ratio and clot strain (i.e. hard clots). In these circumstances, the clot will remain corked at the tip of the intermediate catheter during retrieval, and procedural success (i.e. complete recanalization) will be determined by other factors such as clot friability (proneness of the distal non-ingested clot to be fragmented) or increased pressure gradient between proximal and distal ends of the clot (i.e. poor collaterals). As a result, significant clot fragments, if not the whole clot, may be lost during retrieval leading to a failed or incomplete recanalization (Video 3).
The primary aim of our experimental setup was to define the impact of CS on the efficacy of the different MT techniques. A good collateral circulation ensures higher pressures in the arterial segments beyond the occluding clot and therefore a lower trans-clot pressure gradient. The theoretical benefit of a lower trans-clot pressure gradient was confirmed in our experiments since recanalization rates were consistently higher in the GCM. In the PCM, the clot retrieval had to overcome higher resistance due to a higher-pressure gradient. The unfavourable influence of PCM was observed in all studied techniques, but the negative impact was greater when the procedural strategy included unsecured dragging of the clot outside of the intermediate catheter without the support of SR. This is the case of hard clots retrieved with the direct aspiration technique that are not immediately ingested before retrieval of the intermediate catheter, in which we could observe a marked difference in the rate of complete recanalization between the GCM and the PCM scenarios (Figs. 3 and 4). In techniques using SR the negative impact of a poor collateral status is dampened by the fact that the deployed SR may create a channel through the occlusion, reducing the trans-clot pressure gradient. Also, the SR itself confers an active support and interaction over the whole length of the clot improving the efficiency when dragging the clot against resistances as compared to unsupported pull.
The relatively poor performance of partial SR retraction could be attributed to its hybrid nature, which involves partial retraction of the SR into the intermediate catheter. Unlike complete SR retraction and direct aspiration technique, where aspiration occurs at a fixed location and ingestion can occur at the occlusion site, partial SR retraction always requires pulling the clot outside of the intermediate catheter through a longer distance and curves until the long sheath is reached at the level of the ICA. A common mechanism of failure when performing partial SR retraction technique was the loss of intermediate catheter contact with the clot’s proximal edge, which resulted in a reduction in aspiration force and subsequent clot fragmentation (Video 4). Additionally, this technique led to a significantly higher rate of embolization in filter #2, which in clinical practice could translate into a higher risk of embolization of new vascular territories (i.e. ACA), further worsening outcomes. The partial SR retraction technique however may have different advantages: compared to the other techniques, partial SR retraction technique relies on the ingestion of the clot into the largest bore catheter (i.e. long sheath at the level of the ICA) with the assistance of the SR 8 which potentially diminish the risk of clot shaving, especially when the clot is stiff and not malleable.
Our findings align with prior studies investigating the impact of CS on MT outcomes and our previous research comparing SR-assisted MT techniques 8,12. This randomized in vitro study demonstrated that complete SR retraction technique achieved higher first pass recanalization (FPR) rates and resulted in fewer and smaller distal emboli compared to the partial SR retraction technique. These results further support the advantage of complete SR retraction technique over partial SR retraction technique in terms of procedural efficiency and reduced embolic complications, reinforcing the importance of selecting an optimal thrombectomy strategy based on patient-specific factors. Clinical research has demonstrated that patients with robust collateral circulation exhibit better functional outcomes and higher rates of successful recanalization13. Additionally, in vitro and computational models have emphasized the protective role of collaterals in maintaining cerebral perfusion during ischemic events14.
In summary, poor collateral status and stiffer clot composition negatively influenced, to different extend, the evaluated MT techniques. Complete SR retraction technique was the most effective in achieving substantial recanalization irrespective of collateral status at the price of generating smaller embolic fragments due to its mechanical interaction with the clot, which reduced the probability of complete recanalization. Direct aspiration, when successful, removed the clot entirely but was dependent on clot characteristics and collateral status. Partial SR retraction technique exhibited the highest embolization risk, especially in new territories, due to clot fragmentation during retrieval.
Our study adds to this body of knowledge by providing quantitative evidence from a controlled experimental setting, reinforcing the importance of collateral assessment in treatment planning and propose a specific approach to thrombectomy strategy based on CS and clot composition. At present, clot composition and physical characteristics are difficult to predict before retrieval and, therefore cannot be used to plan the therapeutic approach. However, an evaluation of the collateral circulation status on baseline angiographic run, could guide neurointerventionalists selecting the frontline technique: while under a good collateral scenario any technique may suffice to achieve the optimal rates of complete recanalization, in situations where collaterals are poor, the complete SR retraction approach ensures higher rates of substantial recanalization, but first line direct aspiration increases the chances of complete recanalization.
Limitations
While our in vitro study provides valuable insights, it is essential to acknowledge its limitations. Despite the highly realistic reproduction of the neurovasculature of our model, the materials may not entirely capture the dynamic nature of human vasculature or accurately replicate interactions between vessel wall, device, and clot. Furthermore, the use of clot analogs, while facilitating experimental control, may not fully emulate the heterogeneous structure and diverse characteristics of human thrombi. Moreover, our study exclusively utilized one type of SR and intermediate catheter, suggesting caution in generalizing our findings to other device combinations or sizes.
Another constraint of our study pertains to the adoption of a uniform clot size and occlusion location. This deliberate choice aimed to minimize the introduction of confounding variables and maintain consistency across experimental conditions. Nonetheless, we recognize that variations in clot sizes could significantly influence the efficacy of different MT techniques.
Therefore, we advocate for further investigation in this regard to enhance our comprehension and optimize treatment outcomes.
Conclusion
Our study demonstrates that CS significantly influences the success of MT. A robust collateral network facilitates improved reperfusion, leading to higher rates of FPR regardless of clot type and thrombectomy technique.
Among the evaluated techniques, complete SR retraction technique achieved the highest substantial recanalization rates, while direct aspiration was the most effective in achieving complete recanalization, likely due to differences in clot fragmentation during retrieval maneuvers. Our findings suggest a patient-specific approach to MT based on CS.
Despite the limitations inherent to an in vitro study, our research provides valuable insights that could optimize and individualize thrombectomy strategies. Future studies should focus on validating these findings in clinical settings, exploring the impact of different thrombectomy devices under different collateral circulation scenarios, and refining patient selection criteria to enhance stroke treatment outcomes.
Data availability
Data relevant to the study are included in the article. All data are available upon reasonable request to the corresponding author.
References
Goyal, M. et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 387, 1723–1731 (2016).
Bang, O. Y. et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 42, 693–699 (2011).
Liebeskind, D. S. Collateral circulation. Stroke 34, 2279–2284 (2003).
Wang, Y. J. et al. Association between collaterals, cerebral circulation time and outcome after thrombectomy of stroke. Ann. Clin. Transl Neurol. 10, 266–275 (2023).
Ganeshan, R. et al. Assessment of thrombus length in acute ischemic stroke by post-contrast magnetic resonance angiography. J. Neurointerv Surg. 10, 756–760 (2018).
Juega, J. et al. Granulocytes-rich thrombi in cerebral large vessel occlusion are associated with increased stiffness and poorer revascularization outcomes. Neurotherapeutics 20, 1167–1176 (2023).
Boodt, N. et al. Mechanical characterization of thrombi retrieved with endovascular thrombectomy in patients with acute ischemic stroke. Stroke 2510-2517 https://doi.org/10.1161/STROKEAHA.120.033527 (2021).
Jablonska, M. et al. Partial (SAVE) versus complete (Solumbra) stent retriever retraction technique for mechanical thrombectomy: A randomized in vitro study. Am. J. Neuroradiol. 44, 1165–1170 (2023).
Haussen, D. C., Rebello, L. C. & Nogueira, R. G. Optimizating clot retrieval in acute stroke: The push and fluff technique for closed-cell stentrievers. Stroke 46, 2838–2842 (2015).
Li, J. et al. Impact of stent-retriever tip design on distal embolization during mechanical thrombectomy: A randomized in vitro evaluation. 1–5 (2023). https://doi.org/10.1136/jnis-2023-020382
Chueh, J. Y., Puri, A. S., Wakhloo, A. K. & Gounis, M. J. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J. Neurointerv Surg. 8, 197–202 (2016).
Lee, J. S. & Bang, O. Y. Collateral status and outcomes after thrombectomy. Transl Stroke Res. 14, 22–37 (2023).
Wufuer, A. et al. Impact of collateral circulation status on favorable outcomes in thrombolysis treatment: A systematic review and meta-analysis. Exp. Ther. Med. 15, 707–718 (2018).
Otani, T. et al. Computational modeling of multiscale collateral blood supply in a whole-brain-scale arterial network. PLoS Comput. Biol. 19, 1–17 (2023).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conception and design: MR, MJ. Data acquisition: MJ, JL, EP, MR. Data analysis and interpretation: MJ, JL, MR. Drafting the manuscript: MJ, MR. Technical/material support: MR. Critically revising the article: MR, JL, RT, MR. Reviewed submitted version of the manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent for publication
Not required.
Ethics approval
Not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jablonska, M., Li, J., Tiberi, R. et al. Impact of collateral flow on recanalization in different thrombectomy techniques. Sci Rep 15, 43531 (2025). https://doi.org/10.1038/s41598-025-27592-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-27592-8