Abstract
Cluster of differentiation 44 (CD44) is a transmembrane glycoprotein implicated in tumor progression and metastasis, while phosphorylated ERK1/2 (p-ERK1/2) plays central role in MAPK pathway-driven oncogenic signaling. We investigated the expression and clinical relevance of CD44 in colorectal cancer (CRC) and explored its synergistic interaction with p-ERK1/2 in predicting metastatic risk. Immunohistochemistry (IHC) for CD44 and p-ERK1/2 was performed in tissue microarray from 1,137 primary CRC cases. Associations with clinicopathological parameters and survival outcomes were analyzed using Chi-square tests, Kaplan-Meier curves and logistic regression models. CD44 was over expressed in 47.7% (542/1,137) cases and was significantly associated with lymph node metastasis (p = 0.0042), stage III disease (p = 0.0045), high-grade tumors (p = 0.0111), deficient mismatch repair status (p = 0.0007), high Ki-67 (p = 0.0051) and p-ERK1/2 expressions (p = 0.0012). However, CD44 alone did not predict survival outcomes (overall survival and disease-free survival). Co-expression of CD44 and p-ERK1/2 was observed in 284 cases (25.3%) and was significantly associated with stage III/IV disease (p = 0.0022), lymph node involvement (p = 0.0117) and metachronous distant metastasis (p = 0.0404). Co-expression emerged as an independent predictor of distant metastasis in multivariate analysis (Odds ratio = 1.73; 95% confidence interval = 1.11–2.69; p = 0.0149). CD44 and p-ERK1/2 co-expression defines a high-risk subset of CRC patients with increased metastatic potential. These findings highlight a clinically relevant biomarker axis that may aid in prognostic stratification and future therapeutic targeting.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is a major public health concern globally, ranking among the top causes of cancer-related morbidity and mortality worldwide1,2. In Saudi Arabia, CRC is the most commonly diagnosed cancer in men and ranks third among women, with an increasing incidence observed over recent decades3,4. Notably, a substantial proportion of CRC cases in Saudi Arabia are diagnosed at advanced stages and frequently present with regional or distant metastases5, highlighting the need for effective biomarkers to predict tumor aggressiveness, metastatic potential, and guide management.
A potential promising candidate is cluster of differentiation 44 (CD44), a transmembrane glycoprotein and principal receptor for hyaluronic acid6. CD44 plays a crucial role in cell-cell and cell-matrix interactions and is widely recognized for its involvement in various cancer related processes, including tumor initiation, epithelial-mesenchymal transition (EMT), invasion and metastasis7,8. Moreover, CD44 is considered a major marker of cancer stem cells (CSCs) in multiple solid tumors7,9,10. Its ability to maintain stemness, promote drug resistance and modulate the tumor microenvironment has made it an attractive target in cancer diagnostics and therapeutics8,11,12. In CRC specifically, CD44 has been associated with tumor progression and poor clinicopathological features, including high tumor grade, lymphovascular invasion and nodal involvement13,14. However, the prognostic significance of CD44 expression in CRC remains controversial, with some studies suggesting it correlates with poor outcomes, while others find no significant survival impact14,15,16. These discrepancies may reflect population heterogeneity, differences in detection methodology, or the dynamic role of CD44 isoforms and co-activated signaling pathways.
Consequently, there is growing interest in exploring CD44, not as an isolated marker, but rather as part of a large signaling network that drives tumor aggressiveness. One such network is the MAP/ERK signaling pathway, a key regulator of cell proliferation, differentiation and survival17. ERK1 and ERK2 when phosphorylated (p-ERK1/2), are activated downstream of receptor tyrosine kinases and RAS-RAF signaling and are known to promote tumor progression and metastasis in CRC18. Previous studies suggest that CD44 may facilitate ERK activation by acting as a co-receptor or scaffold, enhancing MAPK signaling in tumor cells19. Although CD44-ERK interaction has been characterized in cell lines20,21, its clinical significance in CRC tissues remains poorly defined.
In this study, we aimed to investigate the expression profile of CD44 in a large, well characterized Saudi cohort of > 1100 CRC patients using immunohistochemistry. We then explored the relationship between CD44 and p-ERK1/2 expression as a potential predictor of patient prognosis and metastatic risk. Our findings offer a novel tissue based biomarker signature with potential clinical relevance in CRC.
Results
Patient characteristics
The clinicopathological characteristics of the 1137 CRC patients are summarized in Table 1. The median age of the study cohort was 56.0 years (inter quartile range [IQR], 47.0–68.0 years) with a male: female ratio of 1.1. Most of the tumors were located in the left colon (81.3%; 924/1137). 79.2% (900/1137) of patients had a moderately differentiated tumor and 72.0% (819/1137) were either stage II or stage III. Lymph node metastasis was noted in 50.3% (573/1137) of cases. Synchronous distant metastasis occurred in 13.0% (148/1137), whereas metachronous distant metastasis was seen in 15.9% (181/1137) of CRC cases. 9.1% (104/1137) of tumors were MMR deficient by immunohistochemistry (Table 1).
CD44 immuno-expression and its association with clinicopathological characteristics
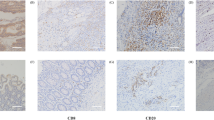
CD44 protein expression was assessed immunohistochemically, with membrane staining considered for scoring (Fig. 1). CD44 overexpression was noted in 47.7% (542/1137) of CRC cases and was significantly associated with lymph node metastasis (p = 0.0042), stage III tumors (p = 0.0045), poorly differentiated (grade 3) tumors (p = 0.0111), dMMR status (p = 0.0007) and high Ki-67 proliferation index (p = 0.0051). Interestingly, a significant association was also noted between CD44 overexpression and p-ERK1/2 overexpression (p = 0.0012) (Table 2).
Tissue microarray (TMA) based immunohistochemistry analysis of CD44 and p-ERK1/2 in colorectal cancer (CRC) patients. CRC TMA spots showing overexpression of CD44 (A) and p-ERK1/2 (C). In contrast, another set of TMA spots showing reduced expression of CD44 (B) and p-ERK1/2 (D). 20 X/0.70 objective on an Olympus BX 51 microscope. (Olympus America Inc, Center Valley, PA, USA).
We next analyzed the survival outcomes for CD44 in CRC. However, CD44 expression was not associated with either overall survival (p = 0.7836) or disease-free survival (p = 0.2150) (Fig. 2).
p-ERK1/2 expression analysis
p-ERK1/2 overexpression was observed in 47.1% of cases (545/1157) and was significantly associated with lymph node involvement (p = 0.0001) and stage IV disease (p = 0.0001) (Table 3). High p-ERK1/2 expression was significantly associated with shorter overall survival in univariate analysis (p = 0.0156; Fig. 3A). However, it did not retain independent significance in the multivariate Cox regression model (HR = 1.32, 95% CI: 0.99–1.77, p = 0.0584; Table 4), likely due to collinearity with advanced tumor stage and nodal involvement, which are established predictors of outcome. No significant association was found with disease-free survival (Fig. 3B).
CD44 and p-ERK1/2 co-expression predicts metachronous distant metastasis
To further delineate the clinical relevance of co-expression, we stratified cases into four groups based on CD44 and p-ERK1/2 status. Among the 1,120 cases with available dual-marker data, 284 (25.3%) were double-positive (CD44high and p-ERK1/2 high), 256 (22.9%) were CD44high and p-ERK1/2 low, 254 (22.7%) were CD44 low and p-ERK1/2 high, and 326 (29.1%) were double-negative. The double-positive group showed significant association with advanced disease, including increased lymph node metastasis (p = 0.0117), metachronous distant metastasis (p = 0.0404), as well as higher tumor stage (III and IV, p = 0.0022), compared to other subgroups (Table 5). However, CD44 and p-ERK1/2 co-expression was not associated with either overall survival or disease-free survival (Fig. 4).
Considering the association of CD44 and p-ERK1/2 co-expression with metachronous distant metastasis, we sought to determine if co-expression could independently predict development of metachronous distant metastasis, using logistic regression analysis. Univariate analysis revealed T stage, N stage, MMR status and CD44/p-ERK1/2 co-expression as predictors of distant metastasis. On multivariate analysis, we found CD44 and p-ERK1/2 co-expression to be an independent predictor of metachronous distant metastasis (Odds ratio = 1.73; 95% confidence interval = 1.11–2.69; p = 0.0149) (Table 6).
Discussion
In this large-scale IHC study of 1,137 CRC cases from Saudi Arabia, we evaluated the clinicopathological and prognostic significance of CD44 and its potential synergy with p-ERK1/2. CD44 expression was observed in 47.7% of tumors and was significantly associated with lymph node metastasis, advanced tumor stage (Stage III), poor differentiation (grade III), MSI high status and elevated Ki-67 index, all features consistent with tumor aggressiveness. However, CD44 expression alone showed no significant impact on patient survival, which prompted further exploration of its co-expression with p-ERK1/2. Interestingly, we found that 26% of CRC cases co-expressed CD44 and p-ERK1/2, with this subset exhibiting a stronger association with advanced stage (III and IV), lymph node metastasis and most notably, metachronous distant metastasis. Co-expression emerged as an independent predictor of metachronous distant metastasis in multivariate analysis, highlighting its clinical and biological relevance. Our findings build and extend a growing body of literature implicating CD44 in tumor progression. CD44 is widely regarded as a key marker of cancer stem cells (CSCs), contributing to tumor initiation, invasion, EMT and resistance to chemotherapy22,23.
In CRC, CD44 overexpression has been linked to advanced disease stage and poor differentiation14,24. However, its role as a prognostic marker has remained unclear. Some studies have reported a survival disadvantage associated with CD44 positivity14,25,26, while others, in concordance with our own study, have found no significant association with overall or disease-free survival16,27. Although CD44 overexpression was significantly associated with adverse clinicopathological features—including lymph node metastasis, high tumor grade, and MSI-high status—it did not independently predict survival in our cohort. This apparent disconnect aligns with prior literature suggesting that CD44 primarily facilitates local invasion and early metastatic steps, whereas its influence on long-term outcomes is modulated by other oncogenic pathways and tumor-intrinsic factors19,28,29,30. In our analysis, only co-expression of CD44 with p-ERK1/2—an activated effector of the MAPK signaling cascade—identified a biologically aggressive subgroup with increased risk of distant metastasis. These findings support the view that CD44 functions as a context-dependent facilitator of tumor progression, whose prognostic relevance is enhanced in the presence of concurrent MAPK pathway activation. Additionally, population-specific genetic backgrounds and treatment patterns may have attenuated the observable survival impact of CD44 alone in our Saudi cohort, underscoring the need for broader validation in diverse populations. Notably, despite the association between CD44/p-ERK1/2 co-expression and an elevated risk of distant metastasis, this did not translate into a significant survival difference in our cohort. This discrepancy may reflect variability in post-metastatic treatment regimens, or suggest that the CD44–ERK axis predominantly contributes to the initiation phase of metastatic spread. Its influence on long-term outcomes may be mitigated by effective systemic therapies following metastasis. Also, the inconsistencies in prognostic outcomes may reflect methodological differences, population heterogeneity, or context-dependent expression of CD44, which exists in multiple isoforms and engages in dynamic signaling interactions14.
Notably our study showed a synergistic interaction between CD44 and ERK signaling. The MAP/ERK pathway plays a critical role in CRC biology, driving cell proliferation, differentiation and metastasis in response to upstream growth factor and oncogenic RAS/RAF signaling18,31. Our findings that p-ERK1/2 expression correlated with CD44 overexpression is supported by mechanistic studies in other tumor types19,32,33,34. CD44 can facilitate ERK activation by clustering with receptor kinases such as EGFR or c-MET and promoting downstream signal transduction8. Moreover, CD44 may function as signaling scaffold, enhancing MAPK pathway activation and ERK nuclear translocation19,35.
In the context of cancer stemness, ERK activation has been shown to maintain the self-renewal capacity of CD44 + CSCs and promote metastatic dissemination19,36. This layered molecular interaction may explain why CD44 alone is insufficient to predict outcomes, but its co-activation with p-ERK1/2 defines a biologically aggressive subset of CRC. Overall, our study demonstrates a strong clinical synergy between CD44 and p-ERK1/2 using large, well-characterized cohort. Importantly, while CD44 or ERK may variably impact prognosis, their co-expression defines a high-risk subset of CRC with enhanced metastatic potential, providing a more refined biomarker axis than either proteins alone. This co-expression profile may help identify patients who are more likely to develop distant metastasis, and who may benefit from more intensive follow-up or targeted interventions. While CD44/p‑ERK1/2 co‑expression correlated with an increased risk of metachronous distant metastasis, it was not associated with a shorter disease‑free survival interval. This distinction reflects the differing endpoints analyzed: metachronous metastasis represents the specific occurrence of distant spread, whereas disease‑free survival encompasses all recurrence types and is influenced by adjuvant therapy and post‑recurrence management. Hence, CD44/p‑ERK1/2 co‑expression may mark tumors with enhanced metastatic potential without necessarily predicting overall recurrence timing or survival outcome. Furthermore, the CD44–ERK axis represents a promising therapeutic target. Preclinical studies have suggested that MEK inhibitors may be more effective in CD44 expressing tumors, and co-targeting CD44 and ERK may help overcome resistance in CRC models21,37.
While our findings suggest that CD44 and p-ERK1/2 co-expression marks a biologically aggressive subset of colorectal cancer, the therapeutic implications remain speculative. Although prior studies in CRC models have shown that CD44 can activate ERK signaling and promote invasion, EMT, and chemoresistance38,39, these mechanistic insights require broader validation across CRC systems. At this stage, our data support the co-expression pattern as a prognostic biomarker rather than an immediately targetable pathway.
The strength of this study is the population it represents, focusing on an under-represented Middle Eastern population. Most prior studies on CD44 in CRC have focused on Western or Asian populations, while this study is composed entirely of the Saudi population, which may help in forming region-specific risk stratification strategies. Nevertheless, some limitations should be acknowledged, including the retrospective design of the study, lack of isoform-specific CD44 staining (e.g. CD44v6) and absence of functional assays to directly test pathway interaction. Another limitation of this study is the incomplete documentation of treatment history in approximately 20% of cases, largely due to archival constraints from earlier years (1990–2011). Given the potential impact of therapy on biomarker expression, treatment variables were intentionally excluded from multivariate models to minimize the risk of bias. This warrants validation in future cohorts with comprehensive treatment annotation. However, the clinical associations observed here are consistent with known mechanisms from in vitro studies, and our findings provide a potential foundation for future translational or therapeutic research.
In conclusion, our study provides novel evidence that co-expression of CD44 and p-ERK1/2 is a clinically meaningful biomarker for aggressive disease in CRC. While CD44 alone lacks prognostic value, its synergy with ERK activation identifies a high risk subgroup with propensity for distant metastasis. These findings have potential implications for biomarker guided prognostic as well as therapeutic stratification, and future drug development targeting the CD44-ERK axis.
Materials and methods
Sample selection and clinicopathological data
Archival samples from 1137 CRC patients diagnosed between 1990 and 2015 at King Faisal Specialist Hospital and Research Center (Riyadh, Saudi Arabia) were included in the study. Clinicopathological data were collected from patient medical records, which are summarized in Table 1. Distant metastasis was divided into synchronous (detected within 6 months of diagnosis) and metachronous (detected more than 6 months after diagnosis) metastasis. Overall survival was defined as the length of time from the date of diagnosis that patients are still alive. Of the 1137 patients, 218 (19.2%) died and the remaining 919 (80.8%) patients were censored at the time of last follow-up. Disease-free survival was defined as the length of time after patient’s initial surgery that the patient survives without any signs or symptoms of that cancer (such as local, regional and distant recurrence or disease-related deaths). Of the 1137 patients, 352 (31.0%) progressed and the remaining 785 (69%) patients were censored at the time of last follow-up. The median follow-up duration for the entire cohort was 41.2 months.
Institutional Review Board of King Faisal Specialist Hospital and Research Centre provided ethical approval for the current study. Research Advisory Council (RAC) granted waiver of informed consent for use of retrospective patient case data and archival tissue samples under project RAC# 2190 016. All the methods were carried out in accordance with the Declaration of Helsinki.
Tissue microarray construction & immunohistochemistry
Tissue microarray (TMA) format was utilized for immunohistochemical analysis of samples. For the construction of TMA, representative tumor regions from each donor tissue block were chosen and tissue cylinders with a diameter of 0.6 mm were punched and brought into recipient paraffin block with the help of a modified semiautomatic robotic precision instrument (Beecher Instruments, Wood-land, WI, USA). Two spatially distinct tumor cores (0.6 mm each) were selected per case by two independent pathologists, with one core taken from the invasive front when identifiable. This approach has demonstrated reproducibility for CD44 immunohistochemical assessment in prior studies, including consistent inter-core and whole-section concordance40.
Tissue microarray slides were processed and stained manually as described previously41. Primary antibody against CD44 and p-ERK1/2 were used, details of which are provided in Table 7. A normal colon tissue microarray was also stained to validate the antibody. Normal tissues of different organ system were also included in the TMA to serve as positive controls. Negative control was performed by omission of the primary antibody. For CD44, membranous staining was considered for scoring. Positive staining in more than 10% of tumor cells was considered as overexpression of CD44 42. Nuclear staining was considered for scoring p-ERK1/2. H score was used to analyze p-ERK1/2 staining43. Briefly, each TMA spot was assigned an intensity score from 0 to 3 (I0, I1–I3) and the proportion of tumor staining for that intensity was recorded as 5% increments from a range of 0–100 (P0, P1–P3). A final H score (range 0–300) was obtained by adding the sum of scores obtained for each intensity and proportion of area stained (H score = I1 × P1 + I2 × P2 + I3 × P3). p-ERK1/2 expression was dichotomized based on the median H score, with H score ≤ 60 classified as low expression and H score > 60 classified as overexpression.
Evaluation of mismatch repair protein staining was performed as described previously44. Briefly, MMR protein expression was evaluated using MSH2, MSH6, MLH1 and PMS2 proteins. Details of the primary antibodies used are provided in Table 7. Tumor was classified as deficient MMR (dMMR) if any of the four proteins showed complete loss of staining in tumor cells with concurrent positive staining in the nuclei of normal epithelial cells. Otherwise, they were classified as proficient MMR (pMMR).
IHC scoring was done by two pathologists, blinded to the clinicopathological characteristics. Discordant scores were reviewed together to achieve agreement.
Statistical analysis
Associations between clinicopathological variables and protein expression was analyzed using contingency table analysis and Chi square test. Kaplan-Meier method was used to generate survival curves and Mantel Cox log rank test was used to evaluate significance. Univariate and multivariate analysis was performed using Cox proportional hazards model to determine factors predicting overall survival and logistic regression model to determine factors predicting metachronous distant metastasis. Two-sided tests were used for the calculations and limit of significance was defined as p value of < 0.05 for all analyses. Data analyses was performed using JMP14.0 (SAS Institute, Inc.,Cary, NC) software package.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 74, 229–263 (2024).
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. Cancer J. Clin. 74, 12–49 (2024).
Al-Eid, H. & Arteh, S. Cancer incidence report Saudi Arabia. Riyadh Kingd. Saudi Arabia: Ministry Health Saudi Cancer Registry, 1–99 (2005).
Alessa, A. M. & Khan, A. S. Epidemiology of colorectal cancer in Saudi arabia: A review. Cureus 16 (2024).
Elwali, N. E. et al. Colorectal cancer in Saudi arabia: the way forward. Asian Pac. J. Cancer Prevention: APJCP. 24, 13 (2023).
Naor, D., Sionov, R. V. & Ish-Shalom, D. CD44: structure, function and association with the malignant process. Adv. Cancer Res. 71, 241–319 (1997).
Hassn Mesrati, M., Syafruddin, S. E., Mohtar, M. A. & Syahir, A. CD44: a multifunctional mediator of cancer progression. Biomolecules 11, 1850 (2021).
Chen, C., Zhao, S., Karnad, A. & Freeman, J. W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 11, 1–23 (2018).
Ziranu, P. et al. CD44: A new prognostic marker in colorectal cancer? Cancers 16, 1569 (2024).
Skandalis, S. S., Karalis, T. T., Chatzopoulos, A. & Karamanos, N. K. Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell. Signal. 63, 109377 (2019).
Guo, Q., Yang, C. & Gao, F. The state of CD44 activation in cancer progression and therapeutic targeting. FEBS J. 289, 7970–7986 (2022).
Yaghobi, Z. et al. The role of CD44 in cancer chemoresistance: A concise review. Eur. J. Pharmacol. 903, 174147 (2021).
Zhao, L. et al. CD44v6 expression in patients with stage II or stage III sporadic colorectal cancer is superior to CD44 expression for predicting progression. Int. J. Clin. Exp. Pathol. 8, 692 (2015).
Wang, Z. et al. The prognostic and clinical value of CD44 in colorectal cancer: a meta-analysis. Front. Oncol. 9, 309 (2019).
Lugli, A. et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br. J. Cancer. 103, 382–390 (2010).
Iseki, Y. et al. Significance of E-cadherin and CD44 expression in patients with unresectable metastatic colorectal cancer. Oncol. Lett. 14, 1025–1034 (2017).
Lavoie, H., Gagnon, J. & Therrien, M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 21, 607–632 (2020).
Bahar, M. E., Kim, H. J. & Kim, D. R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal. Transduct. Target. Therapy. 8, 455 (2023).
Wang, Y. Y. et al. Cd44 promotes lung cancer cell metastasis through erk–zeb1 signaling. Cancers 13, 4057 (2021).
Bourcier, S. et al. CD44-ligation induces, through ERK1/2 pathway, synthesis of cytokines TNF-α and IL-6 required for differentiation of THP-1 monoblastic leukemia cells. Leukemia 24, 1372–1375 (2010).
Herishanu, Y. et al. Activation of CD44, a receptor for extracellular matrix components, protects chronic lymphocytic leukemia cells from spontaneous and drug induced apoptosis through MCL-1. Leuk. Lymphoma. 52, 1758–1769 (2011).
Jaggupilli, A. & Elkord, E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. J. Immunol. Res. 708036. (2012).
Yan, Y., Zuo, X. & Wei, D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl. Med. 4, 1033–1043 (2015).
Yan, B., Mu, Y., Cui, M. & Liu, L. Clinicopathological significance and prognostic implication of CD44 and its splice variants (v3 and v6) in colorectal cancer. Transl. Cancer Res. 9, 1215 (2020).
Ziranu, P. et al. New horizons in metastatic colorectal cancer: prognostic role of CD44 expression. Cancers 15, 1212 (2023).
Wu, Q. et al. Evaluation of the correlation of KAI1/CD82, CD44, MMP7 and β-catenin in the prediction of prognosis and metastasis in colorectal carcinoma. Diagn. Pathol. 10, 1–9 (2015).
Ribeiro, K. B. et al. KRAS mutation associated with CD44/CD166 Immunoexpression as predictors of worse outcome in metastatic colon cancer. Cancer Biomarkers. 16, 513–521 (2016).
Wielenga, V. J. et al. Expression of CD44 in apc and Tcfmutant mice implies regulation by the WNT pathway. Am. J. Pathol. 154, 515–523 (1999).
Zeilstra, J. et al. CD44 expression in intestinal epithelium and colorectal cancer is independent of p53 status. PLoS One. 8, e72849 (2013).
Gao, Y. et al. Knockdown of CD44 inhibits the invasion and metastasis of hepatocellular carcinoma both in vitro and in vivo by reversing epithelial-mesenchymal transition. Oncotarget 6, 7828 (2015).
Koveitypour, Z. et al. Signaling pathways involved in colorectal cancer progression. Cell. Biosci.. 9, 97 (2019).
Yu, S. et al. Adhesion glycoprotein CD44 functions as an upstream regulator of a network connecting ERK, AKT and Hippo-YAP pathways in cancer progression. Oncotarget 6, 2951 (2014).
Xie, P. et al. CD44 potentiates hepatocellular carcinoma migration and extrahepatic metastases via the AKT/ERK signaling CXCR4 axis. Ann. Transl. Med 10, 689 (2022).
Judd, N. P. et al. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 72, 365–374 (2012).
Bourguignon, L. Y., Gilad, E., Rothman, K. & Peyrollier, K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J. Biol. Chem. 280, 11961–11972 (2005).
Han, X. et al. Post-translational modifications: the potential ways for killing cancer stem cells. Heliyon 10 (2024).
Cash, H. et al. mTOR and MEK1/2 Inhibition differentially modulate tumor growth and the immune microenvironment in syngeneic models of oral cavity cancer. Oncotarget 6, 36400 (2015).
Lv, L. et al. Upregulation of CD44v6 contributes to acquired chemoresistance via the modulation of autophagy in colon cancer SW480 cells. Tumor Biol.. 37, 8811–8824 (2016).
Liu, B. et al. TSG-6 promotes cancer cell aggressiveness in a CD44-dependent manner and reprograms normal fibroblasts to create a pro-metastatic microenvironment in colorectal cancer. Int. J. Biol. Sci. 18, 1677 (2022).
Serkad, C. P. V. et al. Validation of CanAssist breast immunohistochemistry biomarkers on an automated platform and its applicability in tissue microarray. Int. J. Clin. Exp. Pathol. 14, 1013 (2021).
Bavi, P. et al. Prevalence of fragile histidine triad expression in tumors from Saudi arabia: a tissue microarray analysis. Cancer Epidemiol. Biomarkers Prev. 15, 1708–1718 (2006).
Qu, J. et al. Prognostic value of E-cadherin-, CD44-, and MSH2-associated nomograms in patients with stage II and III colorectal cancer. Transl. Oncol. 10, 121–131 (2017).
Pinhel, I. F. et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res. 12, 1–7 (2010).
Siraj, A. K. et al. Prevalence of Lynch syndrome in a middle Eastern population with colorectal cancer. Cancer 121, 1762–1771 (2015).
Acknowledgements
The authors would like to thank Kaleem Iqbal for his assistance.
Author information
Authors and Affiliations
Contributions
S.K.P.: Study design, Data analysis, Interpretation of data, Revised the article critically for important intellectual content. A.K.S.: Study conception, Study design, Interpretation of data, Revised the article critically for important intellectual content. P.A.: Performed experiments. F.A.D.: Acquisition of data, Interpretation of dataK. S.A.: Study conception, Study design, Interpretation of data, Revised the article critically for important intellectual content. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Parvathareddy, S.K., Siraj, A.K., Annaiyappanaidu, P. et al. CD44 and phosphorylated ERK1/2 coexpression predicts distant metastasis in colorectal cancer based on a study of 1137 Saudi patients. Sci Rep 15, 44084 (2025). https://doi.org/10.1038/s41598-025-27690-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-27690-7