Abstract
Long-term outcomes of postoperative tinnitus in vestibular schwannoma (VS) patients remain poorly characterized. This retrospective study aimed to evaluate predictors of long-term tinnitus outcomes following VS surgery. A questionnaire-based follow-up was conducted for patients who underwent retrosigmoid approach surgery between December 2018 and May 2024, with assessments performed at least 6 months postoperatively. Clinical parameters including tumor size, surgical outcomes, and facial and hearing function were analyzed. Univariate and multivariate analyses were used to identify predictors of tinnitus outcomes. Among 450 patients, 297 (66.0%) had preoperative tinnitus, with higher prevalence in males (39.1 vs. 25.5%, p = 0.004) and smaller tumors (26.00 vs. 29.00 mm, p = 0.020). Postoperative tinnitus incidence increased significantly. In preoperative tinnitus patients, outcomes correlated with age (p = 0.017), hearing level (p < 0.001), facial nerve function (p = 0.003 at discharge, p = 0.002 at follow-up), and tinnitus frequency (p < 0.001). Preoperative serviceable hearing was an independent risk factor for worsened tinnitus (OR 2.045, 95% CI 1.202–3.480, p = 0.008), while older age (OR 1.044, 95% CI 1.008–1.082, p = 0.017) and intermittent tinnitus (OR 7.145, 95% CI 2.897–17.621, p < 0.001) predicted recovery. In patients without preoperative tinnitus, both age (p = 0.001) and serviceable hearing (p < 0.001) correlated with outcomes, with serviceable hearing being an independent risk factor for new-onset tinnitus (OR 4.430, 95% CI 2.114–9.283, p < 0.001). Hearing preservation was not associated with tinnitus outcomes. Older age and intermittent tinnitus were predictive of recovery, while preoperative serviceable hearing increased the risk of worsened or new-onset tinnitus. Hearing preservation was not associated with a higher incidence of postoperative tinnitus. These findings provide important insights for counseling and long-term management of VS patients.
Similar content being viewed by others
Introduction
Vestibular schwannoma (VS), the most common type of central nervous system schwannoma, originates from the Schwann cells, primarily those of the vestibular nerve. It accounts for approximately 8% of all central nervous system tumors1. According to a 2023 epidemiological study, about 60% of VS patients report tinnitus, making it the second most common symptom after hearing loss2. Current treatment strategies for VS include microsurgery, radiotherapy, and a wait-and-scan approach3. Historically, the primary therapeutic goals have focused on complete tumor resection and the preservation of facial nerve (FN) function and hearing4,5,6. In contrast, tinnitus has received relatively little attention, despite its substantial impact on patient quality of life. As current treatment approaches often fail to adequately address tinnitus, further investigation into postoperative tinnitus is essential.
Tinnitus is defined as the perception of sound in the absence of an external auditory stimulus7. While its pathophysiology remains unclear and inconsistently explained, it is widely believed to arise from aberrant neural activity associated with hearing loss8. The severity of tinnitus and its impact on patients’ lives vary significantly. With recent advancements in microsurgical techniques, attention has shifted toward improving postoperative quality of life9. Among VS patients, tinnitus is frequently reported as the most distressing postoperative complaint, surpassing dizziness and fatigue10. However, the subjective nature of tinnitus and the lack of objective assessment tools continue to hinder research in this field.
Although several studies have examined the impact of microsurgery on tinnitus9,11,12,13,14,15,16, findings remain inconsistent, and data on long-term postoperative tinnitus outcomes are limited. In this study, we report the long-term tinnitus outcomes in VS patients following microsurgical resection and identify potential predictive factors.
Methods
Patient population
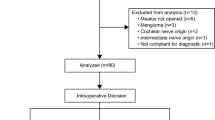
This study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University. Written informed consent was obtained from all participants (n = 450) who completed the questionnaire. All methods were carried out in accordance with relevant guidelines and regulations. We retrospectively analyzed patients who underwent VS resection via the retrosigmoid approach at our institution between December 2018 and May 2024. Inclusion criteria comprised patients with sporadic VS and a minimum postoperative follow-up of six months. Patients with predisposing genetic syndromes (e.g., neurofibromatosis type 2) or recurrent tumors were excluded.
All preoperative MRIs were reviewed by a multidisciplinary team, including a neuroradiologist, neurologist, and skull base neurosurgeon. Cystic VS (cVS) was defined as a tumor with one or more cystic components comprising more than one-third of the maximal average tumor diameter on MRI17.
Surgical and evaluation criteria
Tumor size was determined as the maximum diameter measured on preoperative MRI, including both the cerebellopontine angle and internal auditory canal components. FN function was assessed using the House-Brackmann (HB) grading system (Grades I-VI); grades I and II were considered good function. Hearing was classified based on the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) classification system (Classes A-D), with serviceable hearing defined as Class A or B. Near-total resection (NTR) was defined as intraoperative identification of a thin residual tumor layer with no radiographically visible remnant on postoperative imaging. Subtotal resection (STR) was defined as gross residual tumor evident on postoperative MRI.
Questionnaire content
A simplified, self-designed questionnaire was used for postoperative follow-up (Fig. 1a). This tool was not derived from or validated against standardized instruments such as the Tinnitus Handicap Inventory (THI) or the Tinnitus Functional Index (TFI). We chose this approach due to the subjective nature of tinnitus and the large sample size, which would have rendered standardized assessments impractical. Additionally, many patients had difficulty recalling their preoperative tinnitus in detail, which could compromise the accuracy of structured scales. Our questionnaire focused on binary reporting of tinnitus status and frequency at a minimum of 6 months postoperatively. Worsened tinnitus was defined as a subjective increase in tinnitus severity postoperatively, including louder sound, more frequent episodes, or greater impact on daily life, based on patient self-report during structured follow-up.
Statistical analysis
Figure 1b illustrates the process by which patients were categorized, facilitating subsequent analysis. Univariate analyses were performed using the chi-square test to assess associations between categorical variables. Variables with statistical significance (p < 0.05) in univariate analysis were subsequently entered into multivariate logistic regression models to identify independent predictors. To assess multicollinearity among independent variables in the logistic regression models, we performed a separate linear regression analysis using the same independent variables. Variance inflation factors (VIFs) and tolerance values were examined, with VIF > 10 or tolerance < 0.1 indicating significant multicollinearity. No multicollinearity was detected among the independent variables included in our multivariable analysis. All statistical analyses were performed using IBM SPSS Statistics (Version 26.0; IBM Corp., Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
The cohort included 155 male and 295 female patients, with a median age of 45 years (interquartile range [IQR]: 19 years). A total of 297 patients presented with preoperative tinnitus, while 153 did not. The median follow-up duration was 32.0 months (IQR: 31.0) in the tinnitus group and 31.0 months (IQR: 32.0) in the non-tinnitus group (p = 0.602). The prevalence of preoperative tinnitus was significantly higher in male patients (39.1% vs. 25.5%, p = 0.004) and in those with smaller tumors (median diameter: 26.0 mm vs. 29.0 mm, p = 0.020). The incidence of tinnitus increased significantly after surgery (p < 0.001).
There were no significant differences between groups regarding age (p = 0.980), tumor side (p = 0.708), preoperative hearing level (p = 0.060), hearing outcomes at discharge (p = 0.833), FN outcomes at discharge (p = 0.894), FN outcomes at follow-up (p = 0.358), tumor classification (p = 0.842), extent of resection (p = 0.248), or surgical position (p = 0.906) (Table 1).
Predictors of postoperative long-term tinnitus status in patients with preoperative tinnitus
Age was significantly associated with postoperative tinnitus status (p = 0.017): patients who worsened had a median age of 44.5 years (IQR: 18.0), those who remained unchanged were 45.0 years (IQR: 20.0), and those who recovered were 54.0 years (IQR: 22.0) (Table 2).
Preoperative hearing level also showed a significant association (p < 0.001). Among patients with worsened tinnitus, 46.6% had serviceable hearing preoperatively; this proportion was 31.9% in the unchanged group and only 11.4% in the recovered group (Table 2).
FN function at discharge was significantly associated with outcome (p = 0.003), with good function observed in 83.9% of patients in the worsened group, 88.2% in the unchanged group, and 62.9% in the recovered group. Similar associations were seen at follow-up (p = 0.002), with good FN function observed in 95.8%, 95.1%, and 77.1% of patients in the respective groups (Table 2).
Preoperative tinnitus frequency varied significantly across outcome groups (p < 0.001). In the worsened group, 34 patients (28.8%) had intermittent tinnitus, while 84 (71.2%) reported continuous tinnitus. Similarly, in the unchanged group, 41 patients (28.5%) experienced intermittent tinnitus and 103 (71.5%) had continuous tinnitus. In contrast, the recovered group displayed a markedly different distribution: 27 patients (77.1%) reported intermittent tinnitus, whereas only 8 (22.9%) experienced continuous tinnitus preoperatively (Table 2).
A multivariate logistic regression model was constructed using age, preoperative hearing level, FN function at discharge and follow-up, and tinnitus frequency. The analysis revealed that having serviceable hearing preoperatively was an independent risk factor for worsened tinnitus (odds ratio [OR] = 2.045; 95% confidence interval [CI]: 1.202–3.480; p = 0.008), compared to patients with unchanged symptoms. In contrast, older age (OR = 1.044; 95% CI: 1.008–1.082; p = 0.017) and preoperative intermittent tinnitus (OR = 7.145; 95% CI: 2.897–17.621; p < 0.001) were independently associated with a higher likelihood of tinnitus recovery (Fig. 2).
Independent predictors of tinnitus outcomes in patients with preoperative tinnitus. (a) Preoperative serviceable hearing was an independent risk factor for worsened tinnitus (p = 0.008). (b) Older age (p = 0.017) and preoperative intermittent tinnitus (p < 0.001) independently predicted tinnitus recovery.
Predictors of postoperative long-term tinnitus status in patients without preoperative tinnitus
Among patients who developed new-onset postoperative tinnitus, the median age was 42.0 years (IQR: 16.0), compared to 50.0 years (IQR: 23.0) in those who remained tinnitus-free (p = 0.001). Preoperative serviceable hearing was significantly more common in the tinnitus group (64.9%) than in those who remained tinnitus-free (25.3%) (p < 0.001) (Table 3).
Binary logistic regression incorporating age and hearing level identified preoperative serviceable hearing as an independent predictor of new-onset tinnitus (OR = 4.430; 95% CI: 2.114–9.283; p < 0.001) (Fig. 3).
Association between preoperative AAO-HNS hearing classification and tinnitus outcomes
Figure 4a and b illustrate the relationship between preoperative tinnitus outcomes and AAO-HNS hearing classification in patients who had tinnitus before surgery. As shown in Fig. 4a, the distribution of hearing grades significantly differed among the three tinnitus outcome groups. Figure 4b further reveals that tinnitus outcomes significantly varied between hearing grades A and D, B and C, and B and D. Overall, better preoperative hearing was associated with a higher likelihood of postoperative tinnitus worsening.
Association between preoperative AAO-HNS classification and tinnitus outcomes. (a,b) Among patients with preoperative tinnitus, worse hearing was associated with better tinnitus outcomes. c and d. Among patients without preoperative tinnitus, better hearing was associated with increased risk of new-onset tinnitus. AAO-HNS: American Academy of Otolaryngology-Head and Neck Surgery. *p < 0.05; **p < 0.01; ***p < 0.001; ns = not significant.
Figure 4c and d show the relationship between tinnitus outcomes and preoperative AAO-HNS hearing classification in patients without tinnitus before surgery. Figure 4d indicates that hearing grades significantly differed between the two tinnitus outcome groups. Figure 4d demonstrates significant differences in tinnitus outcomes between hearing grades A and C, A and D, B and C, B and D, and C and D. In general, better preoperative hearing was associated with a higher risk of new-onset postoperative tinnitus.
Relationship between hearing preservation and tinnitus outcomes
Further subgroup analysis was performed in patients with preoperative serviceable hearing. Regardless of preoperative tinnitus status, no significant association was found between hearing preservation and tinnitus outcomes (Fig. 5). That is, hearing preservation did not increase the risk of tinnitus.
Relationship between postoperative hearing preservation and tinnitus outcomes in patients with preoperative serviceable hearing. (a) No significant association was found between hearing preservation and tinnitus outcomes in patients with preoperative tinnitus. (b) Similarly, no significant association was observed in patients without preoperative tinnitus. ns = not significant.
Discussion
Over recent decades, the prognosis for VS patients has markedly improved, with mortality rates reduced to negligible levels. Advances in microsurgical techniques and intraoperative electrophysiological monitoring have significantly increased preservation rates of both facial and cochlear nerve functions6. As a result, clinical focus has shifted toward quality-of-life outcomes, particularly tinnitus, which is now recognized as the second most distressing symptom after hearing loss10,12.
Tinnitus is highly individualized. While some patients adapt even to continuous tinnitus, others experience considerable distress, contributing to reduced quality of life18.
In our cohort, the prevalence of preoperative tinnitus was 66%, which is consistent with previous studies11,12. Postoperatively, the overall tinnitus rate increased by 8.7% following the retrosigmoid approach. Among patients with preoperative tinnitus, 11.8% experienced symptom resolution, whereas 48.4% of those without preoperative tinnitus developed new-onset symptoms after surgery.
Our results suggest possible associations between preoperative tinnitus and both sex and tumor size. Male patients were more likely to report tinnitus, and those with tinnitus tended to have smaller tumors (Table 1). This aligns with findings by Kameda et al.19, who noted similar trends. Harun et al.20 also reported a non-significant but higher prevalence of tinnitus in male patients. We speculate that biological, psychological, and social factors may contribute to sex-based differences in tinnitus perception. Additionally, tinnitus may prompt earlier medical consultation, leading to detection of smaller tumors.
Age was a significant predictor of long-term tinnitus outcomes. Patients who recovered were older than those whose tinnitus worsened or remained unchanged. Similarly, in patients without preoperative tinnitus, older individuals were less likely to develop new-onset symptoms. These findings are supported by Bell et al.21, who reported that patients aged ≥ 50 were more likely to experience tinnitus recovery. While other studies have failed to confirm age as a predictor22,23,24,25. Although the pathophysiology is both heterogeneous and incompletely understood, tinnitus is thought to result from abnormal neural activity in response to hearing loss8. What’ more, neural plasticity has been identified as a driver of tinnitus26, and refers to the ability of the nervous system to alter with respect to organization and functionality. Our results suggest that age-related reductions in neuronal plasticity or excitability may reduce aberrant neural activity, possibly explaining the protective effect. Further pathophysiological investigation is warranted.
Preoperative hearing level was strongly associated with tinnitus outcomes. Worse preoperative hearing was associated with greater likelihood of recovery among patients with tinnitus, and better hearing increased the risk of new-onset symptoms. Multivariate analysis confirmed that preoperative serviceable hearing was an independent risk factor for both worsened and new-onset tinnitus. These findings are consistent with previous reports15,21,22. Hearing loss may reduce afferent input, triggering maladaptive neural plasticity27, and animal models have shown that auditory deprivation alters protein expression in cochlear nucleus neurons, potentially increasing excitability. In patients with serviceable hearing, a larger postoperative reduction in auditory input may exacerbate this plasticity and worsen tinnitus.
For patients with preoperative serviceable hearing, especially those with small tumors (< 25 mm), individualized treatment strategies are recommended. A “wait-and-scan” approach may help preserve hearing and reduce the risk of new or worsened tinnitus, with final decisions made jointly by physicians and patients. The impact of radiotherapy on tinnitus remains controversial: one recent study28 found no significant change after Gamma Knife, while a 2025 meta-analysis29 reported better tinnitus outcomes after microsurgery than radiosurgery. These findings highlight the need for personalized treatment planning that balances tumor control, hearing preservation, and tinnitus outcomes.
We also found that intermittent tinnitus was a strong predictor of postoperative recovery. In fact, 77.1% of patients in the recovered group had intermittent symptoms preoperatively. This observation has not been reported in previous literature. We hypothesize that patients with intermittent tinnitus may have incomplete neural plasticity, and the removal of tumor-related stimuli could potentially lead to tinnitus recovery.
Although univariate analysis suggested an association between FN function and tinnitus outcomes, this was not supported by multivariate analysis. This discrepancy may be explained by confounding factors: preoperative serviceable hearing was identified as an independent risk factor for worsened tinnitus, and both preoperative serviceable hearing and FN function are closely associated with tumor size. Therefore, it is likely that the observed association between FN function and tinnitus outcomes in the univariate analysis reflects confounding by tumor size.
The potential link between hearing preservation and tinnitus remains controversial. In our study, hearing preservation was not associated with increased risk of worsened or new-onset tinnitus. These findings align with reports by Kameda et al.19 and Catalano et al.30, who observed no correlation between tinnitus outcomes and hearing preservation surgery. However, other studies, such as Kanzaki et al.31, suggested that attempts at hearing preservation may exacerbate tinnitus. Variability in surgical technique, tumor characteristics, and patient selection may contribute to these discrepancies.
This study adds valuable evidence to a limited and inconsistent body of literature on postoperative tinnitus in VS patients. With a median follow-up of 32 months, we provide one of the largest datasets on long-term outcomes to date. Most importantly, this is the first study to systematically investigate tinnitus frequency—intermittent versus continuous—as a predictor of recovery. This novel factor may provide insight into the underlying neural mechanisms and inform clinical decision-making.
However, several limitations should be acknowledged. First, we used a non-validated, simplified tinnitus questionnaire, which limits comparability with studies employing standardized scales such as the TFI or THI. Second, the lack of standardized longitudinal follow-up prevented us from capturing the dynamic course of tinnitus over time, including possible fluctuations or delayed recovery. Third, potential recall bias exists, particularly in assessing preoperative symptoms. Fourth, psychological and psychosocial factors such as anxiety, depression, pain, and sleep quality, which may influence tinnitus perception, were not routinely assessed in the original database and could not be included retrospectively. Future prospective studies should incorporate comprehensive assessments of these factors, along with validated tinnitus instruments, to better clarify their associations with long-term outcomes.
Conclusions
In summary, this study identified several key predictors of long-term tinnitus outcomes following vestibular schwannoma surgery. Among patients with preoperative tinnitus, older age and intermittent symptoms were associated with recovery, whereas serviceable preoperative hearing predicted worsened outcomes. In patients without preoperative tinnitus, serviceable hearing significantly increased the risk of new-onset tinnitus. Notably, hearing preservation was not associated with increased tinnitus risk. These findings highlight the complex relationship between auditory function and tinnitus perception and emphasize the importance of individualized patient counseling. To our knowledge, this is the first study to incorporate tinnitus frequency as a predictive factor, offering a novel contribution to the understanding of postoperative tinnitus in VS patients.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Carlson, M. L. & Link, M. J. Vestibular schwannomas. N Engl. J. Med. 384 (14), 1335–1348 (2021).
Fernandez-Mendez, R., Wan, Y., Axon, P. & Joannides, A. Incidence and presentation of vestibular schwannoma: a 3-year cohort registry study. Acta Neurochir. (Wien). 165 (10), 2903–2911 (2023).
Huo, X. et al. Treatment options for unilateral vestibular schwannoma: a network meta-analysis. BMC Cancer. 24 (1), 1490 (2024).
Song, G. et al. Outcomes after semisitting and lateral positioning in large vestibular Schwannoma surgery: A single-center comparison. Clin. Neurol. Neurosurg. 207, 106768 (2021).
Song, G. et al. Facial nerve length influence on vestibular Schwannoma microsurgery outcomes. World Neurosurg. 150, e400–e407 (2021).
Samii, M., Gerganov, V. & Samii, A. Improved preservation of hearing and facial nerve function in vestibular Schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J. Neurosurg. 105 (4), 527–535 (2006).
Walker, D. D., Cifu, A. S., Gluth, M. B. & Tinnitus JAMA 315 (20), 2221–2222 (2016).
Knipper, M., Van Dijk, P., Nunes, I., Ruttiger, L. & Zimmermann, U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 111, 17–33 (2013).
Park, S. H. et al. Change in tinnitus after treatment of vestibular schwannoma: microsurgery vs. gamma knife radiosurgery. Yonsei Med. J. 55 (1), 19–24 (2014).
Leong, S. C. & Lesser, T. H. A united Kingdom survey of concerns, needs, and priorities reported by patients diagnosed with acoustic neuroma. Otol Neurotol. 36 (3), 486–490 (2015).
Zhang, C. et al. Identification of factors associated with tinnitus outcomes following the microsurgical treatment of vestibular Schwannoma patients. Acta Otolaryngol. 141 (4), 334–339 (2021).
Sardhara, J. et al. Postoperative tinnitus after vestibular Schwannoma surgery: A neglected entity. Neurol. India. 68 (2), 333–339 (2020).
Cao, W. et al. Larger tumor size and female gender suggest better tinnitus prognosis after surgical treatment in vestibular Schwannoma patients with tinnitus. Acta Otolaryngol. 140 (5), 373–377 (2020).
Wang, J. J. et al. Changes in tinnitus after vestibular Schwannoma surgery. Sci. Rep. 9 (1), 1743 (2019).
Kohno, M. et al. Prognosis of tinnitus after acoustic neuroma surgery–surgical management of postoperative tinnitus. World Neurosurg. 81 (2), 357–367 (2014).
Van Gompel, J. J. et al. Acoustic neuroma observation associated with an increase in symptomatic tinnitus: results of the 2007–2008 acoustic neuroma association survey. J. Neurosurg. 119 (4), 864–868 (2013).
Jian, B. J. et al. Implications of cystic features in vestibular schwannomas of patients undergoing microsurgical resection. Neurosurgery 68 (4), 874–880 (2011). discussion 879 – 80.
Grauvogel, J., Kaminsky, J. & Rosahl, S. K. The impact of tinnitus and vertigo on patient-perceived quality of life after cerebellopontine angle surgery. Neurosurgery 67 (3), 601–609 (2010). discussion 609 – 10.
Kameda, K., Shono, T., Hashiguchi, K., Yoshida, F. & Sasaki, T. Effect of tumor removal on tinnitus in patients with vestibular Schwannoma. J. Neurosurg. 112 (1), 152–157 (2010).
Harun, A., Agrawal, Y., Tan, M., Niparko, J. K. & Francis, H. W. Sex and age associations with vestibular Schwannoma size and presenting symptoms. Otol Neurotol. 33 (9), 1604–1610 (2012).
Bell, J. R., Anderson-Kim, S. J., Low, C. & Leonetti, J. P. The persistence of tinnitus after acoustic neuroma surgery. Otolaryngol. Head Neck Surg. 155 (2), 317–323 (2016).
Chovanec, M. et al. Does attempt at hearing preservation microsurgery of vestibular Schwannoma affect postoperative tinnitus? Biomed. Res. Int. 2015, 783169 (2015).
Jufas, N., Flanagan, S., Biggs, N., Chang, P. & Fagan, P. Quality of life in vestibular Schwannoma patients managed by surgical or Conservative approaches. Otol Neurotol. 36 (7), 1245–1254 (2015).
Baguley, D. M., Humphriss, R. L., Axon, P. R. & Moffat, D. A. Change in tinnitus handicap after translabyrinthine vestibular Schwannoma excision. Otol Neurotol. 26 (5), 1061–1063 (2005).
Fahy, C., Nikolopoulos, T. P. & O’Donoghue, G. M. Acoustic neuroma surgery and tinnitus. Eur. Arch. Otorhinolaryngol. 259 (6), 299–301 (2002).
Roberts, L. E. Neural plasticity and its initiating conditions in tinnitus. HNO 66 (3), 172–178 (2018).
De Ridder, D. et al. An integrative model of auditory Phantom perception: tinnitus as a unified percept of interacting separable subnetworks. Neurosci. Biobehav Rev. 44, 16–32 (2014).
Kim, C. Y. et al. Tinnitus and Health-Related quality of life after gamma knife radiosurgery for vestibular Schwannoma. J. Int. Adv. Otol. 21 (4), 1–7 (2025).
Govindaraj, R. et al. Tinnitus after treatment of vestibular schwannoma: a systematic review and comparative analysis of microsurgery and stereotactic radiosurgery. J. Neurooncol. 172 (2), 347–359 (2025).
Catalano, P. J. & Post, K. D. Elimination of tinnitus following hearing preservation surgery for acoustic neuromas. Am. J. Otol. 17 (3), 443–445 (1996).
Kanzaki, J., Satoh, A. & Kunihiro, T. Does hearing preservation surgery for acoustic neuromas affect tinnitus? Skull Base Surg. 9 (3), 169–176 (1999).
Acknowledgements
We want to thank all the patients for their participation in this research.
Funding
This study was supported by National Key R&D Program of China (2021YFC2400803).
Author information
Authors and Affiliations
Contributions
1. Haoming Geng: Data collection, Data analysis, Writing—original draft & review & editing & revise, Methodology. 2. Binghan Zhang: Data collection, Data analysis, Writing—original draft & review & editing & revise, Methodology. 3. Yuanchen Tang: Data collection, Methodology. 4. Xiaolong Wu: Data collection, Methodology, Investigation. 5. Yiqiang Zhou: Data collection, Methodology. 6. Gang Song: Conceptualization, Data collection, Methodology, Supervision, Project administration. 7. Jiantao Liang: Conceptualization, Data collection, Methodology, Funding acquisition, Supervision, Project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Geng, H., Zhang, B., Tang, Y. et al. Predictors of long-term postoperative tinnitus outcomes in vestibular schwannoma patients. Sci Rep 15, 44258 (2025). https://doi.org/10.1038/s41598-025-27829-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-27829-6