Abstract
Utilization of proton pump inhibitors (PPIs) in patients with overt gastrointestinal bleeding (GIB) is poorly characterized and its impact on clinical outcomes remains unclear. This study determined PPIs utilization in patients with GIB and its impact on short- and long-term mortality and rebleeding. A prospective cohort of patients admitted with GIB from January 2013 to August 2023 at a tertiary referral center was examined. Appropriate use of PPIs was based on AGA guidelines. 846 patients admitted with GIB were followed up until death or August 2023; 462 had upper GIB including 243 from acid-related lesions. Overutilization rate of PPIs was 51% upon admission and 27% upon discharge. The underutilization rate was 25% upon discharge. Compared to PPI non-users, PPI users had less endoscopic stigmata of recent hemorrhage (SRH) and decreased need for endoscopic therapy. However, they had similar 1-month and end of follow-up mortality and rebleeding. Independent predictors of 1-month and end of follow up rebleeding were SRH [OR 2.03 (1.16–3.54), and 1.78 (1.21–2.64)], and severe bleeding. Being admitted on anticoagulants was predictive of 1-month rebleeding [OR 2.3 (1.2, 4.4)], while being discharged on antiplatelets was protective against long term rebleeding [OR 0.45 (0.28–0.72)]. Blood transfusion and Charlson Comorbidity Index were predictors of 1-month and long-term mortality. Being discharged on anticoagulants predicted long-term mortality [OR 2.51(1.45–4.36)]. There is significant over- and underutilization of PPIs in patients with GIB without a significant effect on outcomes. PPI stewardship would likely reduce healthcare costs and minimize side effects.
Similar content being viewed by others
Introduction
Gastrointestinal bleeding (GIB) is a serious medical condition that is associated with significant mortality and morbidity. PPIs are commonly used in clinical practice and particularly in patients admitted with GIB. However, their appropriateness in this setting remains uncertain. Further, the effect of their utilization on clinical outcomes has not been well studied. Appropriate indications for short-and long-term PPI use were recently stated in a American Gastroenterology Association (AGA) expert review 20211. Short-term PPI therapy (≤ 8 weeks) is appropriate for conditions such as eradication of Helicobacter pylori infection as part of combination therapy, stress ulcer prophylaxis for patients in the intensive care unit with risk factors for upper GIB (UGIB), uninvestigated gastroesophageal reflux disease or dyspepsia and treatment of NSAID related gastric or duodenal peptic ulcers. On the other hand, long-term PPIs use (> 8 weeks) is appropriate for Barrett’s esophagus, Los Angelos classification grade C or D erosive reflux esophagitis, esophageal peptic strictures, Zollinger-Ellison syndrome, eosinophilic esophagitis, gastroprotection in Aspirin or NSAID users at high-risk for UGIB, and prevention of progression of idiopathic pulmonary fibrosis. The AGA emphasized the role of PPIs as prophylaxis for patients at high risk of GIB but did not make a recommendation on their use in patients presenting with GIB.
The AGA defines overutilization as habitual PPI use without an appropriate indication or inappropriate continuation of PPI after hospital discharge1. Underutilization is defined as lack of PPI use despite the presence of an indication1.
PPIs are overutilized both in the inpatient and outpatient settings at a rate ranging from 33% to 67%2,3,4,5,6,7. A recent large systematic review of 28 million PPI users revealed that 25% of the world’s adult population uses PPIs. Among those, 25% are long-term users (for more than 1 year), and 40% of them have uncertain or no indication. Moreover, 60% of patients who were started on PPIs during hospitalization, continued PPIs inappropriately after discharge3. Examples of overutilization include administration of PPIs to patients with lower GIB (LGIB)8, and patients who use aspirin or NSAIDs but do not meet criteria for gastroprotection.
PPIs overutilization has potentially adverse outcomes. It may increase the risk of small intestinal and colorectal bleeding, particularly among aspirin or NSAIDs users9,10. Moreover, long-term suppression of gastric acid can lead to dysbiosis and small intestinal bacterial overgrowth9. Furthermore, PPIs overutilization imposes an economic cost11 with more than 2 billion dollars being spent unnecessarily on PPIs yearly12. In addition it contributes to polypharmacy and side effects1,13 such as chronic kidney disease, bone fractures, enteric infections14, and SBP in cirrhotic patients15,16. Thus, it is essential to evaluate the benefits versus the risks of PPI therapy in managing GIB to optimize patient outcomes.
On the other hand, PPIs are underutilized in the prophylaxis of high-risk patients who are on dual antiplatelet therapy or chronic NSAID users, despite their proven benefit in reducing the risk of UGIB17,18,19. High-risk patients included those with a history of UGIB or multiple risk factors such as advanced age (> 65 years), concurrent use of NSAIDs, steroids, or anticoagulants, and H. pylori infection18.
All current guidelines endorse PPI use in patients with endoscopically diagnosed peptic ulcer bleeding (PUB)20,21,22. Pre-endoscopic PPI therapy in patients with UGIB, has no impact on mortality and rebleeding rates within 1 month23. However, it downstages stigmata of recent hemorrhage (SRH) and decreases the need for endoscopic hemostatic treatment23. PPI therapy after successful endoscopic hemostasis of a bleeding ulcer significantly reduces1-month rebleeding rate but has no effect on 1-month mortality rate24,25,26. However, some studies suggested that PPI use post-endoscopic therapy in PUB may be associated with reduced 1-month mortality rate24.
Nevertheless, the mortality rate among patients with PUB has remained around 5–10% for the past 50 years27. The unclear role and the gap of knowledge surrounding PPI use in overt GIB and its effects on long-term mortality and rebleeding remain substantial. Limited data exists on the pattern of PPIs utilization in all comers with GIB, and evidence regarding their effect on short-term mortality or rebleeding is scarce. Additionally, there is no data about the effect of PPIs use on long-term mortality or rebleeding. Furthermore, controversies and uncertainty persist regarding the effect of concomitant antithrombotic use along with PPIs on major clinical outcomes.
This study aims to evaluate the utilization pattern of PPIs in patients with overt GIB and to determine their impact on short- and long-term mortality and rebleeding, with the goal of informing better clinical decision-making in GIB management. The hypothesis was that PPIs are overutilized in patients with overt GIB and do not provide a significant protective effect on short- and long-term mortality or rebleeding.
Methods
This was a prospective observational cohort study of patients admitted with overt GIB at a tertiary referral center over a decade. The study population included adult patients (aged 18 years and above) admitted to the American University of Beirut Medical Center (AUBMC) with a confirmed diagnosis of overt GIB between January 2013 and August 2023. Patients aged under 18 years, those with known history of inflammatory bowel disease, pregnant women, and individuals with occult GIB were excluded from the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. A written or verbal informed consent was obtained from each patient included. This study was approved by the Institutional Review Board at AUBMC (Protocol number: IM.KB.12).
Overt GIB was defined as either witnessed or reported coffee-ground emesis, hematemesis, melena, or hematochezia. All cases of overt GIB were confirmed by AUBMC gastroenterologists. UGIB was determined by the presence of coffee-ground emesis, hematemesis, and/or melena and/or the presence of SRH on upper gastrointestinal endoscopy. Bleeding from acid-related lesions (ARL) was defined as GIB originating from reflux related esophageal erosions or ulcers, gastric erosions or ulcers, or duodenal erosions or ulcers. Bleeding was considered from the LGI tract when hematochezia was reported and/or when SRH was present in the colon by colonoscopy in the absence of an UGI source. Bleeding was considered of small bowel origin (SIB) when SRH was detected in the small bowel through endoscopy and/or other procedures (capsule endoscopy, balloon enteroscopy) after excluding UGIB and LGIB sources. All other events without a clearly identified bleeding site were considered unspecified GIB events. Severe GIB was defined by the presence of any of the following: systolic blood pressure (SBP) < 100 mm Hg, > 2 units of red blood cells transfused, or ≥ 2 g/dl drop in hemoglobin (Hb) level. SRH was defined as the presence of any of the following during endoscopy: spurting or oozing blood, visible vessel, adherent blood clot, or a pigmented raised spot. PPIs over- and underutilization were defined as per the latest AGA guideline document1.
Patients were initially interviewed by a member of the research team during index admission as well as during follow-up phone calls after discharge. Interview data were supplemented with information retrieved from the medical records. Patient demographics (including age, sex and social history) and clinical variables [presenting symptoms and vital signs, severity of GIB, age-adjusted Charlson Comorbidity Index (CCI)] and laboratory results, imaging results, endoscopic findings and subsequent endoscopic interventions, resuscitative measures and blood transfusions were collected. Complete medication reconciliation including use of PPIs, NSAID, steroids, antiplatelet (AP) only, anticoagulant (AC) only, or both, was performed upon admission and upon discharge. For exposure, drug use was considered as “current” when the drug was taken up to 7 days before hospitalization. Patients were divided into two groups based on PPI intake (PPI users versus PPI non-users) upon admission and upon discharge. In addition, indications for PPI use were documented according to latest AGA guideline document1. Follow-up was made through phone calls (with patient or family members if the patient was deceased) at 1 month and 1 year after discharge, and then yearly thereafter until the end of follow-up date (August 2023).
Study outcomes included 1-month and end of follow up mortality and rebleeding. Outcomes were recorded by review of medical records and/or by phone interviews with the patient and/or family members. The causes of death were grouped into sepsis, multi-organ failure, systemic cancer, cardiovascular or thromboembolic, and uncontrolled GIB (defined as death when GIB could not be controlled). Rebleeding was defined as a recurrence of overt GIB occurring 24 h or longer after the initial endoscopic evaluation and/or hemostatic therapy and stabilization, accompanied by either a change in vital signs or a decrease in Hb concentration by 2 g/dL or more. Rebleeding events during index hospitalization or requiring readmission were combined into a single variable.
The Statistical Package for the Social Sciences (SPSS) version 29.0 was used for data entry, management, and analyses. Continuous variables were expressed as means ± standard deviations. Categorical variables were described as numbers and percentages. In the bivariate analysis, the association between PPIs use upon admission or discharge and the other categorical variables was assessed using the chi-squared test, whereas the Student’s t-test was used for the association with continuous variables. Patients who died during index hospitalization were excluded from the analysis comparing PPI users versus non-users upon discharge. Subgroup analysis was performed for bleeders from ARL while also excluding patients who died during the index hospitalization. Multivariate logistic regression analysis was performed to determine the independent predictors of outcomes after adjusting for confounders. The independent variables selected for modeling included those that were found to be significant in the bivariate analysis as well as those deemed clinically relevant. The results were described as odds ratios (OR) and their corresponding 95% confidence intervals (CI). A p-value < 0.05 was used to indicate statistical significance.
Results
Study population and PPIs utilization pattern
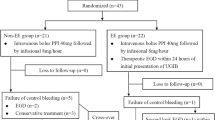
893 patients were admitted with overt GIB, 47 patients were lost to follow-up, and 846 patients had follow-up until death or August 2023. This study included only the 846 patients who had complete follow-up until the end of Aug. 2023. The mean age was 68 ± 16 years with male gender predominance (64%). Approximately half of the patients (n = 462; 55%) had UGIB, 296 had LGIB, 15 had SIB and the source of GIB was unspecified in 73 patients (Fig. 1). 243 of the UGIB patients had bleeding from ARL (53%) and 187 (43%) bled from peptic ulcer disease. The second most common cause of UGIB was variceal bleeding (n = 49; 11%). The most common cause of LGIB was diverticular disease (n = 80; 27%) followed by hemorrhoids (n = 47; 16%). 385 patients were admitted already taking PPIs at home. 73 patients (8.6%) died during the index hospitalization. 711 patients were prescribed PPIs during hospitalization. 623 patients (80.6%) were discharged on PPIs, and 150 (19.4%) were discharged off PPIs (Fig. 1). 75.3% of the patients who were admitted on PPIs and 84.4% of those who were administered PPIs during hospitalization, continued using them after hospital discharge. The overall PPI overutilization rate was 36%. PPIs were overutilized in 195 patients (51%) upon admission and 169 (27%) upon discharge. The underutilization rate was 25% upon discharge.
Comparison of clinical characteristics and outcomes
PPI users versus non-users at admission
Compared to patients admitted with GIB who were not taking PPIs, those who were already taking PPIs were older, had higher CCI, were more likely to be on antithrombotic therapy, and to require ≥ 3 units of packed red cells transfusion. However, PPI users were less likely to have SRH and were less likely to require endoscopic therapy (Table 1). Regarding the source of GIB, PPI use upon admission was associated with 24.3% lower risk of UGIB and particularly 54.9% lower risk of bleeding from ARL. However, it was associated with 33.2% higher risk of LGIB, while the risk of SIB remained nearly the same (Table 1). On bivariate analysis, patients who were admitted on PPIs had higher rates of in-hospital, 1-month, 1-year and end of follow-up mortality (Table 2). In contrast, there was no difference between the two groups in the risk of rebleeding at various time point intervals (Table 2).
PPIs users versus non-users upon discharge
Compared to patients discharged off PPIs, those who were discharged on PPIs had similar baseline demographics and clinical characteristics. However, they were more likely to have experienced in-hospital bleeding and to have been discharged on aspirin (Table 3). All bleeders from ARL (n = 225) were discharged on PPIs. Additionally, 198 patients (31.8%) with LGIB or SIB were inappropriately discharged on PPIs (Table 3). Regarding mortality and rebleeding, no significant difference was observed between the two groups at 1-month, 1-year and by the end of follow-up (Table 4). Similarly, there was no significant difference in terms of UGIB as source of rebleeding [43 (6.9%) in the PPIs group vs. 13 (8.7%) in the no PPIs group; P = 0.242).
Cause of death analysis
By the end of follow-up, 405 patients had died, of whom 332 passed away after discharge from the index hospitalization. Sepsis was the most common cause of death [90 (11.6%)] followed by cardiovascular and/or thromboembolic causes [68 (8.8%)]. Being discharged on PPIs did not significantly impact mortality from either cause [(12.2% vs. 9.3% P = 0.326) and (8.5% vs. 10.0% P = 0.562) respectively]. Similarly, there was no significant difference in “uncontrolled GIB” as a cause of death [12 (1.9%) vs. 2 (1.3%); P = 1.000).
Outcomes of patients with GIB from acid-related lesions (ARL)
252 patients had bleeding from ARL (Table 5). 183 patients (72.6%) were admitted on PPIs while 69 (27.4%) were admitted off PPIs. Endoscopy revealed SRH in 103 patients (40.9%), while 149 patients (59.1%) had no SRH. Of the patients with SRH, 41 patients were admitted on PPIs (22.4%), while 62 patients were admitted off PPIs (77.6%). In-hospital mortality occurred in 27 patients (10.7%), with a higher incidence in the PPIs group compared to the non-PPIs group [18 (26.1%) vs. 9 (4.9%); P < 0.001]. However, no significant differences were observed between the two groups in terms of 1-month mortality [8 (11.6%) vs. 22 (12.0%); P = 0.925] or 1-month rebleeding [6 (8.7%) vs. 18 (9.8%); P = 0.783]. All patients (n = 225) who had GIB from ARL were discharged on PPIs. When comparing these patients to the subgroup of patients who had UGIB from non-ARL who were also discharged on PPIs (n = 148), PPIs use upon discharge in bleeders from ARL was associated with a lower 1-month rebleeding rate [19 (8.4%) vs. 27 (18.2%); P = 0.005] but a similar 1-month mortality rate [30 (13.3%) vs. 19 (12.8%); P = 0.890] and a similar rebleeding rate by the end of follow-up [40 (17.8%) vs. 30 (20.3%); P = 0.546].
Independent predictors of outcomes
Short- and long-term rebleeding
PPI use upon admission was not an independent predictor of 1-month rebleeding. Predictors of 1-month rebleeding were: being admitted on AC only, severe bleeding and SRH (Table 6). Similarly, PPI use upon discharge was not an independent predictor of end of follow-up rebleeding. Predictors of end of follow-up rebleeding were severe bleeding and SRH, whereas being discharged on AP only was strongly protective against long-term rebleeding (Table 6).
Short- and long-term mortality
On multivariate analysis, PPI use upon admission was not an independent predictor of 1-month mortality. Predictors of 1-month mortality were age-independent CCI, severe bleeding, blood transfusion and SRH (Table 7). Similarly, PPIs use upon discharge was not an independent predictor of end of follow-up mortality. Predictors of end of follow-up mortality were age-related CCI, blood transfusion and being discharged on AC only (Table 7).
Discussion
This study revealed a significant overutilization of PPIs in 51% of patients admitted with overt GIB and in 27% of patients upon discharge. Importantly, PPI utilization did not result in any significant impact on short- or long-term mortality or rebleeding rates. To our knowledge, this is the first study to comprehensively evaluate PPI utilization patterns in all comers with GIB. The observed overutilization rate is consistent with previously reported figures in the literature2,3,4,5,6,7, although earlier studies primarily examined all types of inpatient hospitalizations and, in some cases, the outpatient setting, whereas our study focuses solely on patients hospitalized with GIB. Additionally, overutilization upon discharge in our study was observed in 198 patients admitted with LGIB or SIB who were inappropriately discharged on PPIs. A similar finding was previously reported by Quinn et al.8 This overutilization is taking place at a tertiary referral center where GI experts prescribe PPIs. This highlights the potential for guideline deviations even among specialized providers, emphasizing the need for greater adherence to evidence-based practices.
On the other hand, underutilization of PPIs was observed in 24.7% of patients upon discharge. However, this was not the case for patients with bleeding from ARL, as all were discharged on PPIs in accordance with established guidelines1,20,21,22. The observed rate of underutilization is notably lower than that reported in a Korean study18 (50%), likely due to differences in the study populations. Our study focused on patients with GIB, whereas the Korean study involved patients with myocardial infarction, where PPI use was primarily for prophylaxis. Despite this, the category of patients in whom PPIs were underutilized appeared similar, predominantly involving high-risk individuals who required gastroprotection.
Moreover, our data showed that PPIs use upon admission was associated with less SRH and less need for endoscopic therapy. This effect was particularly evident in patients who bled from ARL, where those on prior PPI therapy had about a 68% lower chance of having SRH, reaffirming the established role of pre-endoscopic PPIs therapy in UGIB as highlighted in the most recent meta-analysis by Kanno et al.23. Our findings also align with the results of the same meta-analysis23, demonstrating that PPIs use upon admission (i.e. pre-endoscopy) had no impact on 1-month mortality or rebleeding rates, even in the subgroup of patients who had bleeding from ARL. This finding was further clarified by the multivariate analysis, which demonstrated that short-term outcomes were predominantly influenced by factors such as patient comorbidities, the need for blood transfusions, anticoagulant use upon admission, the severity of bleeding, and the effectiveness of endoscopic therapy. Conversely, PPIs use upon admission was associated with higher risks of LGIB and SIB, a finding consistent with the results of the meta-analysis by Jung et al.9 This further highlights the paradoxical effect of PPIs use, depending on the GIB location and patient profile.
Interestingly, a substantial number of patients presented with UGIB from ARL despite having been on PPIs. Furthermore, being discharged on PPIs did not eliminate entirely the risk of rebleeding in this subgroup of patients. The reasons for this could be due to poor compliance, inadequate dose, or resistance to PPIs. Nonetheless, our data demonstrated that PPIs use upon discharge (i.e. post-endoscopy) in the subgroup of bleeders from ARL was associated with a lower 1-month rebleeding rate but had no effect on the 1-month mortality rate. These findings align with the established guidelines on the role of post-endoscopic hemostasis PPI therapy24,25,26. However, the added value in our study lies in the long-term follow-up, in contrast to previous studies that reported outcomes limited to a 1-month timeframe. Nonetheless, PPI use upon discharge had no impact on end of follow-up mortality or rebleeding rates. This finding can be explained by multivariate analysis, which demonstrated that long-term outcomes were predominantly influenced by factors such as age, comorbidities, the need for blood transfusions, the severity of bleeding and anticoagulant use upon discharge.
Our previously published studies in 2016 and 202128,29 showed that antiplatelet use was protective against long-term rebleeding. Our current study reproduces those observations. Additionally, a recent study published in 2023 by Marmo et al. demonstrated a protective effect of aspirin use on rebleeding in patients with UGIB30. While the exact mechanism remains unclear, this consistent observation warrants further investigation, which we plan to explore in future studies. One possible explanation is that the initial bleeding may have been caused by antiplatelet-induced lesions, and the subsequent prescription of gastroprotective therapy, such as PPIs, significantly reduced the risk of long-term rebleeding.
While this study provides valuable insights, several limitations must be considered. First, its observational nature prevents us from establishing causal relationships between PPIs use and clinical outcomes. Second, the study was conducted at a single medical center and involved only a Lebanese population, which may limit the generalizability of the findings. Additionally, the non-randomized design introduces potential biases. Finally, details regarding PPI use, such as dose, duration of administration, and patient adherence, were not collected, which could have influenced the observed outcomes. Further experimental and multicenter studies are required to validate our findings and explore the underlying mechanisms, particularly in relation to antithrombotic therapy.
In conclusion, there is significant over- and underutilization of PPIs in patients admitted with overt GIB without any significant effect on short- or long-term mortality or rebleeding. Hence, a PPI utilization stewardship program with regular reassessment of PPI indications, particularly at discharge, is likely to optimize PPI usage, reduce healthcare costs, and minimize potential PPI-related adverse effects.
Data availability
The data that support the findings of this study are available from the corresponding author (Kassem Barada), upon reasonable request.
References
Targownik, L. E., Fisher, D. A., Saini, S. D. & AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors. Expert Rev. Gastroenterology 162, 1334–1342 https://doi.org/10.1053/j.gastro.2021.12.247 (2022).
D’Angelo, N., Sigarchy, R., Esswein, A. & Asrar, S. Proton pump inhibitor use and adverse effects in South Atlantic hospitals. HCA Healthc. J. Med. 4, 353–358. https://doi.org/10.36518/2689-0216.1450 (2023).
Shanika, L. G. T., Reynolds, A., Pattison, S. & Braund, R. Proton pump inhibitor use: systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 79, 1159–1172. https://doi.org/10.1007/s00228-023-03534-z (2023).
Gamelas, V., Salvado, V. & Dias, L. Prescription pattern of proton pump inhibitors at hospital admission and discharge. GE Port J. Gastroenterol. 26, 114–120. https://doi.org/10.1159/000488506 (2019).
Hálfdánarson, Ó. et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Th. Adv. Gastroenterol. 11, 1756284818777943. https://doi.org/10.1177/1756284818777943 (2018).
Wallerstedt, S. M., Fastbom, J., Linke, J. & Vitols, S. Long-term use of proton pump inhibitors and prevalence of disease- and drug-related reasons for gastroprotection-a cross-sectional population-based study. Pharmacoepidemiol Drug Saf. 26, 9–16. https://doi.org/10.1002/pds.4135 (2017).
Dharmarajan, T. S. The use and misuse of proton pump inhibitors: an opportunity for deprescribing. J. Am. Med. Dir. Assoc. 22, 15–22. https://doi.org/10.1016/j.jamda.2020.09.046 (2021).
Quinn, A. J. et al. Inappropriate use of proton pump inhibitors in hospitalized patients with lower gastrointestinal bleeding. Hosp Pract (1995) 52, 19–22 https://doi.org/10.1080/21548331.2024.2321824 (2024).
Jung, Y. S., Park, J. H. & Park, C. H. Impact of proton pump inhibitors on the risk of small bowel or colorectal bleeding: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 11, 861–873. https://doi.org/10.1002/ueg2.12448 (2023).
Zhou, M. et al. Proton pump inhibitors and In-Hospital Gastrointestinal bleeding in patients with acute coronary syndrome receiving dual antiplatelet therapy. Mayo Clin. Proc. 97, 682–692. https://doi.org/10.1016/j.mayocp.2021.11.037 (2022).
Savarino, V. et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev. Clin. Pharmacol. 11, 1123–1134. https://doi.org/10.1080/17512433.2018.1531703 (2018).
Metaxas, E. S. & Bain, K. T. Review of proton pump inhibitor overuse in the US veteran population. J. Pharm. Technol. 31, 167–176. https://doi.org/10.1177/8755122515575177 (2015).
Lo, C. H. et al. Association of proton pump inhibitor use with All-Cause and Cause-Specific mortality. Gastroenterology 163, 852–861e852. https://doi.org/10.1053/j.gastro.2022.06.067 (2022).
Moayyedi, P. et al. Safety of proton pump inhibitors based on a Large, Multi-Year, randomized trial of patients receiving Rivaroxaban or aspirin. Gastroenterology 157, 682–691e682. https://doi.org/10.1053/j.gastro.2019.05.056 (2019).
Wong, Z. Y. et al. Proton pump inhibitors increases longitudinal risk of Mortality, Decompensation, and infection in cirrhosis: A Meta-Analysis. Dig. Dis. Sci. 69, 289–297. https://doi.org/10.1007/s10620-023-08150-6 (2024).
Boustany, A. et al. Cirrhotic patients on proton pump inhibitors are at a twofold risk of spontaneous bacterial peritonitis independently of Gastrointestinal bleeding: a population-based retrospective study. Ann. Gastroenterol. 36, 327–332. https://doi.org/10.20524/aog.2023.0794 (2023).
El Hajj, W. et al. Prophylactic proton pump inhibitors in upper Gastrointestinal bleeding: impact and underprescription in a French multicentric cohort. Dig. Dis. Sci. 69, 4053–4062. https://doi.org/10.1007/s10620-024-08663-8 (2024).
Baik, M., Jeon, J., Kim, J. & Yoo, J. Proton pump inhibitor for Gastrointestinal bleeding in patients with myocardial infarction on Dual-Antiplatelet therapy: A nationwide cohort study. J. Epidemiol. Global Health. https://doi.org/10.1007/s44197-024-00267-9 (2024).
Kim, W. Y., Lee, S., Jun, K., Ah, Y. M. & Lee, J. Y. Underutilization of Gastrointestinal prophylaxis in high-risk chronic nonsteroidal anti-inflammatory drug users in Korea. Int. J. Clin. Pharm. 43, 645–653. https://doi.org/10.1007/s11096-020-01176-0 (2021).
Gralnek, I. M. et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy 53, 300–332 https://doi.org/10.1055/a-1369-5274 (2021).
Laine, L., Barkun, A. N., Saltzman, J. R., Martel, M. & Leontiadis, G. I. ACG clinical guideline: upper Gastrointestinal and ulcer bleeding. Official J. Am. Coll. Gastroenterol. | ACG. 116, 899–917. https://doi.org/10.14309/ajg.0000000000001245 (2021).
Siau, K. et al. British society of gastroenterology (BSG)-led multisociety consensus care bundle for the early clinical management of acute upper Gastrointestinal bleeding. Frontline Gastroenterol. 11, 311–323. https://doi.org/10.1136/flgastro-2019-101395 (2020).
Kanno, T. et al. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper Gastrointestinal bleeding. Cochrane Database Syst. Rev. 1, Cd005415. https://doi.org/10.1002/14651858.CD005415.pub4 (2022).
Leontiadis, G. I., Sharma, V. K. & Howden, C. W. Proton pump inhibitor therapy for peptic ulcer bleeding: Cochrane collaboration meta-analysis of randomized controlled trials. Mayo Clin. Proc. 82, 286–296. https://doi.org/10.4065/82.3.286 (2007).
Lau, J. Y. et al. Omeprazole before endoscopy in patients with Gastrointestinal bleeding. N Engl. J. Med. 356, 1631–1640. https://doi.org/10.1056/NEJMoa065703 (2007).
Sung, J. J. et al. Intravenous Esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized trial. Ann. Intern. Med. 150, 455–464. https://doi.org/10.7326/0003-4819-150-7-200904070-00105 (2009).
Leontiadis, G. I. et al. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess 11 iii-iv, 1-164 https://doi.org/10.3310/hta11510 (2007).
Souk, K. M., Tamim, H. M., Abu Daya, H. A., Rockey, D. C. & Barada, K. A. Aspirin use for primary prophylaxis: adverse outcomes in non-variceal upper Gastrointestinal bleeding. World J. Gastrointest. Surg. 8, 501–507. https://doi.org/10.4240/wjgs.v8.i7.501 (2016).
Hosni, M. et al. Increased rebleeding and mortality in patients with Gastrointestinal bleeding treated with anticoagulant drugs compared to antiplatelet drugs. Eur. J. Gastroenterol. Hepatol. 33, e490–e498. https://doi.org/10.1097/meg.0000000000002148 (2021).
Marmo, R., Occhipinti, V., Zullo, A. & Soncini, M. Improved survival for patients with acute upper Gastrointestinal bleeding while on antithrombotic therapy: A multicenter prospective cohort study. J. Clin. Gastroenterol. 57, 278–284. https://doi.org/10.1097/mcg.0000000000001674 (2023).
Author information
Authors and Affiliations
Contributions
J.M. and A.E.Z. contributed equally to study design, data collection, analysis, and drafting of the manuscript. C.R., R.M., and W.E. assisted in data acquisition and verification. H.T. conducted and supervised the statistical analysis. A.I.S., F.H.M., F.D., A.S., Y.H.S., and N.A. critically revised the manuscript for important intellectual content. D.C.R. provided methodological input and reviewed the manuscript. K.A.B. conceived and supervised the study, interpreted the findings, and finalized the manuscript. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mrad, J., Zreik, A.E., Rizk, C. et al. Proton pump inhibitors have no effect on short or long term rebleeding or mortality in patients with overt gastrointestinal bleeding. Sci Rep 15, 44728 (2025). https://doi.org/10.1038/s41598-025-28406-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28406-7