Abstract
Wearable devices have been used in pulmonary rehabilitation (PR) programs to monitor and promote physical activity (PA) in patients with chronic respiratory diseases (CRD), such as COPD and asthma. This study aimed to identify the effects of using wearable devices to monitor PA in PR programs for CRD. This systematic review and meta-analysis, registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42024504137), was conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The searches took place in five databases and in the grey literature, with no language or year restrictions. Individuals with COPD or asthma, aged 18 and over, were included. The electronic search identified 3940 references, of which nine articles met the eligibility criteria and were included in the review. Wearable devices promoted a significant increase in the number of daily steps (SMD = 0.35; 95% CI: 0.01 to 0.69; p = 0.04). However, there were no consistent effects on outcomes such as quality of life, functional capacity and anxiety. High heterogeneity and methodological limitations were observed in some studies. Wearable devices promise to increase PA in patients with CRD, especially when integrated into multidisciplinary strategies.
Similar content being viewed by others
Introduction
Pulmonary rehabilitation (PR) is a multidisciplinary approach focused on the care of individuals with chronic respiratory diseases (CRD), such as chronic obstructive pulmonary disease (COPD) and asthma1. This intervention integrates physical exercise, education and the promotion of behavioral changes that are essential for managing these conditions1,2. It is estimated that CRD is responsible for approximately 14% of global deaths, with COPD being the third leading cause of death worldwide3,4. People with COPD and asthma often have a reduction in physical capacity, leading to worsening lung function and increased mortality. This reduction is often due to chronic inflammation, airway obstruction, and muscle deconditioning, which can exacerbate symptoms and contribute to disease progression5,6,7.
In this context, involvement in physical activity (PA) plays a fundamental role in maintaining these patients’ functional capacity and quality of life. PA refers to any bodily movement generated by the contraction of skeletal muscles, which increases energy expenditure beyond the basal level8. To fully understand the benefits of PA in PR, it is important to assess physical performance, which reflects the patients’ ability to carry out daily activities. Traditionally, physical performance is assessed subjectively or objectively, analyzing lower-limb strength, upper-limb strength, and functional mobility, such as walking and sit-to-stand performance. However, behavioral variations can influence these assessments and often do not accurately reflect patients’ daily activity9. In view of this, PA monitors have emerged as more accurate and reliable tools for directly measuring PA compared to self-reported questionnaires10,11.
Technological advances have made it possible to monitor real-time information, capturing data such as PA level, walking patterns and physiological signals through wearable devices using sensors such as accelerometers, gyroscopes and pedometers12,13,14. In the context of PR, these technologies have shown promise for promoting PA, although challenges remain in integrating PA into the daily routines of patients with CRD and supporting its progressive increase over time15,16. While PR is essential for improving exercise capacity and quality of life in people with CRD, incorporating and progressing daily PA into the routine of these patients remains challenging17. In this context, validated activity monitors have been necessary to assess the effects of PR on PA monitoring in these patients16. For this reason, behavioral interventions aimed at increasing PA have been implemented and combined with daily monitoring and feedback through the use of wearable devices integrated into PR protocols17.
Although wearable devices are increasingly used across various health domains, there is still a lack of comprehensive studies evaluating their effectiveness for monitoring PA in PR programs for patients with CRD. To address this gap, the present study systematically reviewed the literature to investigate the effects of using wearable devices on PA monitoring in PR programs for CRD.
Methods
This systematic review and meta-analysis were conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)18. The protocol19 of this review was registered in January 2024 in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42024504137).
Eligibility criteria
The studies were included according to the following PICOS criteria: 1) participants: individuals of both sexes, aged 18 years or over and clinically diagnosed with COPD or asthma; 2) intervention: involved in PR using a wearable device to monitor PA; 3) comparison: PR without the use of the device or usual care; 4) primary outcomes: PA, health-related quality of life (HRQoL), functional capacity (FC) and self-efficacy; 5) secondary outcomes: peripheral muscle strength, respiratory muscle strength, dyspnea/respiratory symptoms and anxiety; 6) study design: randomized controlled trials. There were no restrictions on language or year of publication. Studies were excluded if they were conference abstracts, editorials or if they only dealt with the development of algorithms or applications for assessing diseases. Additional efforts were made to obtain the full text of all studies in order to minimize publication bias.
Search strategy
The searches were carried out on March 7, 2024, in the electronic databases MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, CINAHL and PEDro. The search strategy was constructed by combining terms from the Medical Subject Headings (MeSH) and keywords related to the population (COPD and asthma) and the intervention (PR, exercise therapy, exercise program, wearable devices, smartwatch). The search strategies used for each database are available as supplementary material (supplementary table S1). Grey literature searches were carried out to identify records of unpublished or ongoing studies (ClinicalTrials.gov and Registro Brasileiro de Ensaios Clínicos [REBEC]). In addition, a manual search was carried out on the bibliographic reference lists of the included studies and relevant systematic reviews2,14,16.
Selection of studies
The references identified in each database were transferred to the Rayyan QCRI systematic review web application (https://rayyan.qcri.org) to manually remove duplicates. Two pairs of reviewers (TRAO and ATF; TRAO and TAS) screened titles and abstracts, so each abstract was independently assessed by two reviewers. Eligible articles were obtained in full text and analyzed.
Data extraction
Two reviewers (TRAO and FEPdSM) performed data extraction independently using a standardized form (supplementary table S2). Information was collected on study identification (title, authors, year, journal of publication), methods (site, study design, randomization, allocation concealment, blinding, dropouts and intention-to-treat analysis), participant characteristics (age, sex, health, disease severity, setting, inclusion/exclusion criteria), interventions (groups, duration, frequency, physical activity monitor and location on the body) and results (primary and secondary outcomes with pre- and post-intervention measures). Limitations, funding sources, and study registration numbers were also recorded.
Analysis of the risk of bias and the methodological quality of the studies
The PEDro scale evaluation tool was applied by two independent reviewers (TRAO and FEPdSM) to assess methodological quality focused on clinical trials in the field of physiotherapy. The following scale criteria were used: eligibility criteria, random allocation, concealed allocation, baseline comparability, blinding of participants, blinding of therapists, blinding of evaluators, adequate follow-up, intention-to-treat analysis, comparisons between groups and measures of variability. The total scale scores range from 0 to 10, and the risk of bias is interpreted as high (0–3), moderate (5–7), or low (8–10)20.
The analysis of the risk of bias of the included studies was carried out by two independent evaluators (TRAO and ATF), and a third evaluator (TAS) was responsible for resolving conflicts. The tool used was Risk of Bias 2.0 (ROB2), developed by Cochrane, which assesses the risk of bias in five specific domains: randomization process, deviations from the intended intervention, loss of data on the outcome, measurement of outcomes and selection of the reported outcome. Each domain is classified as “low risk of bias”, “some concern” or “high risk of bias”, providing a detailed and standardized assessment for each study21.
Assessing the certainty of the evidence
The assessment of certainty around the evidence was carried out using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. Two authors (TRAO and TAS) used the five GRADE consideration ratings (risk of bias, inconsistency, indirect evidence, imprecision and publication bias) to assess the certainty of the evidence regarding the studies contributing data to the pre-specified outcomes. A third reviewer (ATF) was responsible for resolving conflicts.
Data analysis
The results of the outcomes of interest for each included study were tabulated to produce a quantitative synthesis and then, when possible, grouped into meta-analyses using Review Manager software (RevMan v.5.4; Cochrane Collaboration, Oxford, UK). The analyses were conducted using a random effects model, applying the inverse variance statistical method. The primary and secondary endpoints in this study were continuous variables, so the standardized mean difference (SMD) was considered and the data were analyzed according to the intention-to-treat principle. Subgroup analyses were carried out considering the different methods of measuring the outcomes, including different units of measurement, functional tests and questionnaires. In cases where the questionnaires were interpreted in opposite directions, the results obtained were multiplied by − 1 as suggested by Borenstein et al.22, ensuring standardization of the direction of the effects and the adoption of a single criterion. To assess the effect size (Z), a p-value < 0.05 was considered statistically significant and for heterogeneity (I2) a p-value < 0.1023, with low heterogeneity being considered for I2 values below 25%, moderate for values between 25 and 75% and high for values above 75%.
The study’s corresponding author was contacted by email whenever there was missing or unclear information, such as information related to the outcomes. The Automeris website (https://automeris.io/) was used to identify the mean and standard deviation values for articles that presented data in graph form. For the studies that provided median and interquartile range data, the transformation to mean and standard deviation was carried out using the equations proposed by Hozo et al.24. Studies that did not provide the necessary data, even after contact by email, were excluded from the meta-analysis, but remained included for the other qualitative assessments.
Results
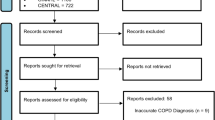
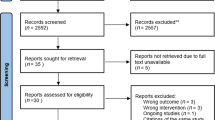
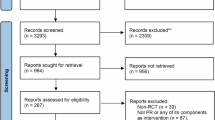
The electronic search generated a total of 3940 references in the aforementioned databases. A total of 2038 duplicates were identified, and after exclusion, 1902 articles remained to be analyzed. Of these, 1879 were excluded by title and abstract, leaving 23 studies for analysis by full text. Based on the eligibility criteria, 18 articles were excluded, and 5 were included. In addition, 4 studies were identified through a manual search. No ongoing studies were found during the search. In the end, 9 articles were included in this review. The PRISMA flow diagram25 shows the results of the search and selection process (Fig. 1).
Flowchart of the study selection process25.
Characteristics of the included studies
The studies included were published between 2005 and 2021 and involved a total sample of 743 individuals (485 men and 258 women) aged between 45 and 75 years. With regard to the CRD investigated, seven17,26,27,28,29,30,31 studies evaluated people with COPD and two32,33 studies people with asthma. The duration of the programs varied between eight and 52 weeks, with an average of three sessions per week, each lasting between 30 and 60 min. The detailed characteristics of each study are described in Table 1.
Characteristics of the interventions
The interventions analyzed in the studies included a variety of approaches combining PR, monitored PA and behavioral modification strategies using wearable devices. Two studies28,33 used pedometers, three studies26,29,32 used accelerometers, and four studies17,27,30,31 used both pedometers and accelerometers to track PA and provide feedback to participants. Some studies integrated diaries to record steps or symptoms17,27,28,31,33, follow-up calls33 and educational sessions for psychosocial support or behavioral guidance26,27,28,32. The characteristics of the interventions are described in Table 1.
CG, control group; FC, functional capacity; HR, heart rate; HRQoL, health-related quality of life; IG, intervention group; N, number of participants; NR, not reported; PA, physical activity; PR, pulmonary rehabilitation.
Characteristics of the outcomes
The outcomes of the included studies and the ways in which they were measured are detailed in Table 2. PA was assessed by the number of steps17,26,27,28,29,31,33 and daily time in minutes26,27,30,31,32. HRQoL was measured using the Clinical COPD Questionnaire (CCQ)17, Chronic Respiratory Questionnaire (CRQ)26,30,31, Asthma Quality of Life Questionnaire (AQLQ)33 and St. George’s Respiratory Questionnaire (SGRQ)27,28,29 questionnaires. FC was measured using the six-minute walk test (6MWT)17,26,27,29,30,33, the two-minute step test (2MST)28 and the incremental shuttle walk test (ISWT)31. Quadriceps strength was assessed using one-repetition maximum (1RM) on a dynamometer, reported in kilograms17,27,30 and as a percentage26. Disease-related symptoms were measured using the CAT17,29, ACQ32,33 and mMRC30 instruments. Self-efficacy was assessed using satisfaction17 and self-efficacy27,28 questionnaires. Finally, respiratory muscle strength was measured using a respiratory dynamometer30.
ACQ, Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; CAF, COPD-Anxiety Questionnaire; CAT, COPD Assessment Test; CCQ, Clinical COPD Questionnaire; CRQ, Chronic Respiratory Questionnaire; 6MWT, Six-minute Walk Test; 2MST, Two-minute Step Test; HADS, Hospital Anxiety and Depression Scale; ISWT, Incremental Shuttle Walk Test; mMRC, Modified Medical Research Council; QS, Quadriceps Strength; SGRQ, St. George’s Respiratory Questionnaire.
Methodological quality
The methodological quality of the included studies, assessed using the PEDro scale is shown in supplementary table S3. Five studies26,27,29,31,33 obtained low methodological quality and four17,28,30,32 studies moderate risk. The lowest scoring domains were: blinding (subject); blinding (therapist); blinding (evaluator); intention to treat.
The analysis of the risk of bias of the studies included in this review showed a predominance of “low risk” in the domains related to the randomization process (D1) and absence of outcome data (D3), while for the domain deviations from the intended intentions (D2) there was a predominance of “some concern”. Additionally, there was variability in other domains influenced by the nature of the outcomes assessed. Studies with objective outcomes, such as PA, FC and quadriceps strength, showed greater methodological consistency in the domains of measurement of outcomes (D4) and selection of reported results (D5), being mostly classified as “low risk” and reflecting greater rigor in the collection and measurement criteria. On the other hand, studies with subjective outcomes assessed by questionnaires, such as HRQoL, symptoms and anxiety, were more often classified as “high risk” for these same domains, showing a greater susceptibility to observation bias, since the lack of blinding of the evaluator may have influenced the assessment of the result, highlighting challenges inherent in the collection of subjective data. The analysis of the outcome of each study and the percentage obtained per domain are detailed in supplementary figures S1 and S2, respectively.
Effects of using wearable devices to monitor physical activity
The PA outcome was reported by nine studies, being described as number of steps by seven studies17,26,27,28,29,31,33 and time in PA by five studies26,27,30,31,32, a result of which a subgroup analysis was carried out (Fig. 2). Despite the moderate heterogeneity, the meta-analysis for this outcome indicated a significant effect of increasing the number of steps in the experimental group compared to the control group (SMD = 0.35; 95% CI: 0.01 to 0.69; p = 0.04; I2 = 62%). With regard to time, the results indicate a non-significant effect (SMD = 0.53; 95% CI: − 0.33 to 1.39; p = 0.23; I2 = 81%), with high heterogeneity. The overall analysis showed a significant effect in favor of the experimental group (SMD = 0.38; 95% CI: 0.08 to 0.68; p = 0.01; I2 = 66%), reinforcing the potential of the devices to promote increased PA in CRD patients. Two studies26,32 were not included in this meta-analysis because post-intervention data were not available.
HRQoL was analyzed by subgroups according to the questionnaire used in the study (supplementary figure S3). The questionnaires used were the SGRQ27,28,29, CRQ26,30,31, CCQ17 and AQLQ33. To ensure the same direction of analysis for all the studies, the results obtained in the CRQ and AQLQ questionnaires were multiplied by − 1 as suggested by Borenstein et al. (2021)22. The results showed that there were no significant differences between the experimental and control groups in all subgroups. For the SGRQ (SMD: 0.27; 95% CI: − 0.43 to 0.98; p = 0.45), CRQ (SMD: 0.00; 95% CI: − 0.27 to 0.27; p = 0.99), CCQ (SMD: − 0.16; 95% CI: − 0.73 to 0.40; p = 0.57) and AQLQ (SMD: − 0.37; 95% CI: − 1.02 to 0.29; p = 0.27). Heterogeneity was only moderate for the SGRQ (I2 = 69%). The combined effect (SMD: 0.00; 95% CI − 0.20 to 0.19; p = 0.96) indicated that there was no statistically significant difference between the experimental and control groups.
Subgroup analysis was used for the FC outcome, considering the different tests performed: 6MWT17,27,30,32,33, 2MST28 and ISWT31 (supplementary figure S4). The overall result indicated a non-significant difference between the experimental and control groups (SMD: 0.18; 95% CI: − 0.03 to 0.40; p = 0.10), with low heterogeneity between the studies (I2 = 0%). None of the subgroups showed statistical significance: 6MWT (SMD: 0.20; 95% CI: − 0.08 to 0.48; p = 0.16), TD2 (SMD: 0.16; 95% CI − 0.82 to 1.14; p = 0.75) and ISWT (SMD: 0.16; 95% CI: − 0.20 to 0.51; p = 0.39). The study by Geidl et al. (2021)29 was not included in this meta-analysis because post-intervention data were not available.
The outcome peripheral muscle strength was assessed using quadriceps strength measured in kilograms17,27,30 and as a percentage32 (supplementary figure S5). The combined effect was not statistically significant (SMD: 0.11; 95% CI: − 0.30 to 0.52; p = 0.60) and heterogeneity was moderate (I2 = 39%), suggesting variability between studies. The subgroups were also not statistically significant: kilograms (SMD: 0.22; 95% CI: − 0.31 to 0.75; p = 0.42) and percentage (SMD: − 0.13; 95% CI: − 0.64 to 0.37; p = 0.60).
The symptoms outcome was analyzed by subgroups according to the questionnaire used in each study (supplementary figure S6). The questionnaires used were CAT17,29, ACQ32,33, mMRC30. The overall results showed no statistical significance (SMD: − 0.07; 95% CI − 0.26 to 0.11; p = 0.43), indicating no relevant differences between the groups. Total heterogeneity was low (I2 = 0%). There were no statistically significant differences for the subgroups: CAT (SMD: − 0.15; 95% CI − 0.62, 0.32; p = 0.53; I2 = 61%), ACQ (SMD: − 0.29; 95% CI − 0.85 to 0.27; p = 0.31) and mMRC (SMD: − 0.22; 95% CI − 0.98 to 0.55; p = 0.58).
The anxiety outcome was analyzed in two subgroups: HADS17,33 and CAF29 (supplementary figure S7). No statistical significance was observed in the overall result (SMD: − 0.16; 95% CI − 0.35 to 0.04; p = 0.11), as well as in the subgroup analyses considering the HADS questionnaire (SMD: − 0.28; 95% CI − 0.71 to 0.15; p = 0.20) and CAF (SMD: − 0.13; 95% CI − 0.34 to 0.09; p = 0.25). Overall heterogeneity was low (I2 = 0%).
It was not possible to carry out a meta-analysis for the outcomes self-efficacy and respiratory muscle strength due to inconsistencies in the assessment methods. Three studies17,27,28 assessed self-efficacy, of which Armstrong et al17 used an unvalidated instrument and Cruz et al. (2016)27 used a generic self-efficacy scale. Thus, only the study by De Blok et al.28 reported the self-efficacy outcome adequately, using the 10-point adapted version of the Perceived Physical Ability Subscale of the Physical Self-Efficacy Scale34, indicating a non-significant effect for the intervention (p = 0.31). For the purposes of analyzing the quality of the evidence for this outcome, the individual data from this study are shown in supplementary figure S8. On the other hand, respiratory muscle strength was only assessed by Kawagoshi et al. (2015)30, who used a respiratory dynamometer to measure it, diverging from the outcomes of interest described in this protocol19, which provided for the use of manovacuometry, in accordance with the recommendations of the American Thoracic Society and European Respiratory Society (ATS/ERS)35.
Assessing the certainty of the evidence
For the outcomes analyzed, the certainty of the evidence ranged from low to moderate. The main limitation found was the low methodological quality of the studies included, which led to downgrades in the certainty of the evidence due to concerns such as inconsistency of results and risk of bias due to lack of blinding in the assessment of outcomes. For the FC outcome, the certainty of the evidence was considered moderate, given that consistent and homogeneous results were found between the studies, with a downgrade only due to some concerns identified in the methodological analysis. For the other outcomes, such as PA, HRQoL, peripheral muscle strength, symptoms and anxiety, the certainty of the evidence was low, due to factors such as high heterogeneity between the studies, methodological and sampling limitations. The detailed analysis for each outcome is shown in supplementary figure S9.
Discussion
This systematic review and meta-analysis aimed to investigate the effects of using wearable devices to monitor PA in PR programs for CRD. The results found reinforce the potential of wearable devices as auxiliary tools in the context of PR. The use of these devices promoted an increase in the number of daily steps compared to the control but had no influence on other outcomes such as HRQoL, FC, peripheral muscle strength, symptoms and anxiety. Our study provides an updated synthesis on the use of wearable devices in PR for COPD and asthma, assessing multiple outcomes, including HRQoL, and comparing step count versus activity time, highlighting the value of integrating behavioral strategies with device use.
The present study shows that wearable devices during PR increase the number of steps, positively impacting the amount of PA that this patient performs using the device. Previous studies14,36,37 carried out corroborate these findings, highlighting the effectiveness of behavioral feedback integrated with monitoring to promote PA in people with COPD and asthma. Shah et al.38 pointed out that the use of wearable devices consistently increased daily steps, but PA time showed no significant changes, reinforcing the findings of our study that the increase in the number of steps is a more sensitive indicator of physical engagement than the total time spent. Other studies39,40 have highlighted the importance of combining wearable devices with educational strategies and integrating behavioral counseling, showing that educational and support programs can contribute to greater consistency.
In this sense, the results of the analysis show that the number of steps is a more consistent and clinically relevant outcome than PA time when assessing the effects of device-based interventions in people with COPD. The significant increase in the number of steps suggests that the devices have the potential to promote an increase close to the minimally important clinical difference (MCID), estimated at between 350 and 1100 steps per day41. On the other hand, although PA time showed a positive effect, this was not statistically significant and showed high heterogeneity. A sensitivity analysis was carried out removing the study by Kawagoshi et al.30, with no changes in the results of the meta-analysis and indicating no substantial changes. Considering the MCID for time spent in moderate to vigorous activity, which varies between 8 and 11 min a day42, the SMD observed represented an increase that was close to the MCID, indicating potential clinical relevance. However, the lack of statistical significance and the variability between studies limit the reliability of this result. It is important to note that time spent in PA, measured as daily minutes, may include low-intensity activities such as standing, whereas the number of steps more directly reflects active movement, making step count a more sensitive and reliable indicator of physical engagement. These findings reinforce that the number of steps is a more reliable and sensitive indicator for measuring changes in PA, while PA time, although complementary, requires greater standardization in future studies to generate more robust evidence.
The FC assessed by the 6MWT, 2MST and ISWT tests was not statistically significant. This can be attributed to factors such as differences in the intervention protocols, which varied in terms of intensity, duration and type of exercise. These findings are consistent with the study by Qiu et al.39 which showed that PR programs combined with wearable devices offer significant results with functional tests, in line with improved exercise tolerance in the short term, with no improvement in intervention durations > 6 months. Another study38 reported that the improvement in 6MWT performance was limited when wearable devices are used alone, suggesting that monitoring alone may not be enough to substantially improve functional capacity.
Despite occasional improvements observed in some studies, especially in patients who received educational support and regular feedback, the overall analysis revealed no significant impact on HRQoL. This discrepancy may be influenced by several factors. First, improvements in HRQoL often require longer intervention periods to become perceptible, as they reflect broader lifestyle and psychological changes rather than immediate physical outcomes. Second, differences between the HRQoL questionnaires used may lead to variability in the domains assessed, some focusing more on physical limitations, others on emotional or social aspects. Finally, variations in the mode of questionnaire administration (self-reported vs. interviewer-administered) may also affect participant responses. These findings are confirmed by other studies36,37,38, which suggest that the isolated use of wearable devices may not be enough to significantly impact quality of life.
Wearable devices combined with general aerobic and resistance exercise programs were not associated with statistically significant increases in quadriceps strength. This may be because not all interventions included exercises specifically targeting this muscle group, which are necessary to elicit measurable strength gains. Hornikx et al.43 similarly found no significant improvements, reporting that telephone counseling with pedometer feedback was insufficient to promote peripheral muscle strength. In contrast, the authors affirm programs that incorporate exercises specifically designed to strengthen the quadriceps tend to produce superior results, as gains in muscle strength depend on the specificity and intensity of the exercises performed.
The evaluation of the effects of PR using wearable devices on symptom reduction showed no difference between the groups evaluated. Other studies36,37,38 have shown similar results, highlighting that these subjective outcomes are more sensitive to behavioral and contextual factors. In this context, the treatable traits approach has been proposed in CRD as a way to personalize disease management. Treatable traits are individual, clinically relevant characteristics, such as symptoms, behaviors, or physiological parameters, that are measurable, modifiable, and can be specifically targeted by interventions44,45,46. This approach can help to explain why some interventions do not have homogeneous effects among different patients, reinforcing the need for more targeted therapeutic strategies. Silva et al.47 found that physical training combined with PA counseling showed significant improvements in the perception and control of disease-related symptoms, but the effects were more evident in long-term programs > 12 weeks. Most of the included studies17,29,30,32,33 that evaluated this outcome in this review show interventions lasting up to 12 weeks, and the length of PR may influence symptom reduction.
For the anxiety outcome, no significant effect was identified for the interventions evaluated, suggesting that more specific and intensive interventions may be necessary for significant changes in this domain44. Considering that anxiety is a behavioral factor and part of the treatable traits in CRD, interventions aimed specifically at mental health, such as cognitive-behavioral therapy or psychological support programs, may be fundamental to optimizing results in this aspect44,45,46. Armstrong et al.48 implemented a combined intervention of cognitive-behavioral therapy and PA behavior modification strategies during PR in people with COPD. The results showed a clinically significant reduction in HADS anxiety scores, with an average reduction of 2 units. These discrepancies suggest that interventions combining psychological and behavioral components may be more effective in reducing anxiety in people with COPD, compared to interventions focused solely on monitoring PA through wearable devices. Furthermore, considering that symptoms such as dyspnea, fatigue and exercise limitation can also be influenced by emotional and psychological factors, a broader approach, including mental health support, may be essential to optimize outcomes in the management of the disease48.
The heterogeneity observed in the results is not only due to the size of the sample, but also to the differences between the populations studied, such as the age, severity of the disease and sex, more men than women, among the participants. In addition, factors such as variations in the protocols used, including frequency, duration and types of exercise, may have influenced the outcomes. It is worth noting that, despite the wide variation in intervention time, only one study30 had a duration of more than 12 weeks. It is also important to consider that each outcome assessed may have been affected by individual patient characteristics, such as level of adherence to the intervention, behavioral aspects and specific symptoms of the disease. Thus, the diversity of responses found reinforces the importance of personalized approaches, such as treatable traits, which take into account the particularities of each patient in order to optimize the effects of PR.
In addition, the variability in assessment methods and the lack of adequate blinding limit the reliability of the findings. Future strategies should explore more personalized interventions, integrating telemonitoring and digital health approaches to maximize the benefits observed. This integration highlights the potential of wearable devices as complementary tools in PR, amplifying the impact on the outcomes analyzed.
Conclusion
The results of this study show that wearable devices fulfilled their role in increasing PA in patients with CRD. However, this increase was not sufficient to generate consistent improvements in outcomes such as HRQoL and FC. This suggests that while devices are effective in monitoring and encouraging PA, their impact is limited when it comes to promoting broader clinical changes.
For these devices to be more effective, it is essential that their use is integrated with complementary strategies, such as behavioral support, individualized interventions and structured PR programs that take into account the treatable traits of each patient. Thus, just quantifying PA is not enough; it needs to be associated with approaches that ensure that the increase in activity generates relevant clinical effects. In addition, the methodological heterogeneity and limitations in the studies analyzed reinforce the need for more standardized randomized controlled clinical trials to better understand the real benefits of these technologies.
Thus, although wearable devices represent a promising tool for monitoring and promoting PA, for their impact to be optimized, it is essential that they be incorporated into multidisciplinary strategies that comprehensively address the challenges of PR. The integration of technology and healthcare can expand the personalization of therapies and offer efficient remote monitoring alternatives, especially in out-of-hospital rehabilitation programs. Thus, wearable devices not only complement clinical practice, but can also contribute to improving treatment adherence and self-care, provided they are used within a structured therapeutic context.
Data availability
All data generated or analysed during this study are included in this published article.
References
Spruit, M. A. Pulmonary rehabilitation. Eur. Respir. Rev. 23(131), 55–63 (2014).
Souto-Miranda, S., Rodrigues, G., Spruit, M. A. & Marques, A. Pulmonary rehabilitation outcomes in individuals with chronic obstructive pulmonary disease: A systematic review. Ann. Phys. Rehabil. Med. 65(3), 101564 (2022).
Soriano, J. B. et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Respir. Med. 8(6), 585–596 (2020).
Ambrosino, N. & Fracchia, C. Strategies to relieve dyspnoea in patients with advanced chronic respiratory diseases. Narrat. Rev. Pulmonol. 25(5), 289–298 (2019).
Xiong, T. et al. Exercise rehabilitation and chronic respiratory diseases: effects, mechanisms, and therapeutic benefits. Int. J. Chron. Obstruct. Pulmon. Dis. 18, 1251–1266 (2023).
Santino, T. A. et al. Patient- and proxy-reported outcome measures instruments for the assessment of asthma control among adult and pediatric population. Medicine 99(19), e20078 (2020).
Nunes, C., Pereira, A. M. & Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pract. 3(1), 1 (2017).
Caspersen, C. J., Powell, K. E. & Christenson, G. M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 100(2), 126–131 (1985).
Sylvia, L. G., Bernstein, E. E., Hubbard, J. L., Keating, L. & Anderson, E. J. Practical guide to measuring physical activity. J. Acad. Nutr. Diet 114(2), 199–208 (2014).
Pitta, F., Troosters, T., Spruit, M. A., Decramer, M. & Gosselink, R. Activity monitoring for assessment of physical activities in daily life in patients with chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 86(10), 1979–1985 (2005).
Rabinovich, R. A. et al. Validity of physical activity monitors during daily life in patients with COPD. Eur. Respir. J. 42(5), 1205–1215 (2013).
Dias, D. & Cunha, J. P. S. Wearable health devices—vital sign monitoring, systems and technologies. Sensors 18(8), 2414 (2018).
Majumder, S., Mondal, T. & Deen, M. Wearable sensors for remote health monitoring. Sensors 17(12), 130 (2017).
Armstrong, M. et al. Use of pedometer as a tool to promote daily physical activity levels in patients with COPD: A systematic review and meta-analysis. Eur. Respir. Rev. 28(154), 190039 (2019).
Sasaki, J. E., da Silva, K. S., Gonçalves, B. & John, D. Chapter 2—measurement of physical activity using accelerometers. In Computer-assisted and web-based innovations in psychology, special education and health (eds Lusielli, J. K. & Fischer, A. J.) 33–60 (Academic Press, 2016).
Reilly, C. et al. Physical activity promotion interventions in chronic airways disease: A systematic review and meta-analysis. Eur. Respir. Rev. 32(167), 220109 (2023).
Armstrong, M. et al. Behavioural modification interventions alongside pulmonary rehabilitation improve COPD patients’ experiences of physical activity. Respir Med 180, 106353 (2021).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Oliveira, T. R. A., Fernandes, A. T. D. N. S. F., Santino, T. A., Menescal, F. E. P. D. S. & Nogueira, P. A. D. M. S. Effects of using wearable devices to monitor physical activity in pulmonary rehabilitation programs for chronic respiratory diseases: a systematic review protocol. PLoS ONE 19(7), e0308109 (2024).
Shiwa, S. R. et al. PEDro: A base de dados de evidências em fisioterapia. Fisioterapia em Movimento 24, 523–533 (2011).
Sterne, J. A. et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, 14898 (2019).
Borenstein, M., Hedges, L. V., Higgins, J. P. T. & Rothstein, H. R. Introduction to Meta-Analysis (John Wiley & Sons, 2021).
Deeks, J. J., Higgins, J. P. T. & Altman, D. G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (eds Higgins, J. P. T. et al.) (Cochrane, 2023).
Hozo, S. P., Djulbegovic, B. & Hozo, I. Estimating the mean and variance from the median, range and the size of a sample. BMC Med. Res. Methodol. 5, 1–10 (2005).
McKenzie, J. E. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Burtin, C. et al. Physical activity counselling during pulmonary rehabilitation in patients with COPD: A randomised controlled trial. PLoS ONE 10(12), e0144989 (2015).
Cruz, J., Brooks, D. & Marques, A. Walk2Bactive: A randomised controlled trial of physical activity-focused behavioural intervention beyon pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron. Respir. Dis. 13(1), 55–66 (2016).
De Blok, B. M. J. et al. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: A pilot study. Patient Educ. Couns. 61(1), 48–55 (2005).
Geidl, W., et al. Long-term benefits of adding a pedometer to pulmonary rehabilitation for COPD: The randomized controlled STAR trial. Int. J Chronic Obstr. Pulm. Dis. 1977–1988.
Kawagoshi, A. et al. Effects of low-intensity exercise and home-based pulmonary rehabilitation with pedometer feedback on physical activity in elderly patients with chronic obstructive pulmonary disease. Respir. Med. 109(3), 364–371 (2015).
Nolan, C. M. et al. Pedometer step count targets during pulmonary rehabilitation in chronic obstructive pulmonary disease: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 195(10), 1344–1352 (2017).
Boyd, A. et al. Feasibility of exercising adults with asthma: A randomized pilot study. Allergy Asthma Clin. Immunol. 8, 1–9 (2012).
Coelho, M. C. et al. Effects of an unsupervised pedometer-based physical activity program on daily steps of adults with moderate to severe asthma: A randomized controlled trial. J. Sports Sci. 36(10), 1186–1193 (2017).
Bosscher, R. J., Laurijssen, L. & de Boer, E. Measuring physical self-efficacy in old age. Percept. Mot. Skills 77, 470 (1993).
Society ER ATS, ERS. Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 166(4), 518–524 (2002).
Burge, A.T., Cox, N.S., Abramson, M.J. and Holland, A.E.,. Interventions for promoting physical activity in people with chronic obstructive pulmonary disease (COPD). Cochrane Database Systematic Review 2020; (4):CD012626.
Osadnik, C. R., McDonald, V. M., Jones, S., Clark, K., & Gibson, P. G. Pulmonary rehabilitation versus usual care for adults with asthma. Cochrane Database Systematic Review 2022; (8), CD013607.
Shah, A. J. et al. Wearable technology interventions in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. NPJ Digit. Med. 6(1), 222 (2023).
Qiu, S. et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: A meta-analysis. Ther. Adv. Respir. Dis. 12, 1753466618787386 (2018).
Lahham, A., McDonald, C. F. & Holland, A. E. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: A systematic review and meta-analysis of randomized controlled trials. Int. J. Chron. Obstruct. Pulmon. Dis. 11, 3121–3136 (2016).
Teylan, M. et al. Physical activity in COPD: Minimal clinically important difference for medical events. Chron. Respir. Dis. 16, 1479973118816424 (2018).
Rebelo, P. F. S. et al. Minimal clinically important difference for time spent in moderate to vigorous physical activities in COPD after pulmonary rehabilitation. Eur. Res. J. 64(suppl 68), OA3689 (2024).
Hornikx, M., Demeyer, H., Camillo, C. A., Janssens, W. & Troosters, T. The effects of a physical activity counseling program after an exacerbation in patients with chronic obstructive pulmonary disease: A randomized controlled pilot study. BMC Pulm. Med. 15, 1–8 (2015).
McDonald, V. M. & Gibson, P. G. Treatable traits in asthma and COPD. Arch. Bronconeumol. 58(8), 583–585 (2022).
McDonald, V. M, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. European Respiratory Journal 2019; 53(5).
Cardoso, J. et al. Treatable traits in COPD—A proposed approach. Int. J. Chronic Obstruct. Pulm. Dis. 53, 3167–3182 (2021).
Silva, L., Paixão, C., Nogueira, P., Rodrigues, D. & Marques, A. A meta-analysis on the structure of pulmonary rehabilitation maintenance programmes on COPD patients’ functional capacity. NPJ Primary Care Respir. Med. 32(1), 38 (2022).
Armstrong, M. et al. Cognitive behavioural therapy combined with physical activity behavioural modification strategies during pulmonary rehabilitation in patients with COPD. ERJ Open Res. 9(5), 00074–02023 (2023).
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
TRAO: Conceptualization, Methodology, Investigation, Data Curation, Writing—Original Draft. ATdNSFF: Conceptualization, Data Curation, Writing—Original Draft, Writing—Review & Editing. TAS: Conceptualization, Data Curation, Writing—Original Draft, Writing—Review & Editing. FEPdSM: Data Curation, Writing—Review & Editing. PAdMSN: Conceptualization, Methodology, Supervision, Writing—Review & Editing. All authors contributed to editing the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oliveira, T.R.A., do Nascimento Sales Figueiredo Fernandes, A.T., Santino, T.A. et al. Effects of wearable devices on physical activity monitoring in pulmonary rehabilitation programs for chronic respiratory diseases: a systematic review and meta-analysis. Sci Rep 15, 44767 (2025). https://doi.org/10.1038/s41598-025-28554-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28554-w