Abstract
To investigate the humoral immunity and clinical characteristics of Chinese college students after experiencing a BA.5/BF.7 and/or XBB.1.5 wave. We enrolled 876 college students who received 2–3 vaccination doses of COVID-19 and followed by BA.5/BF.7 and/or XBB.1.5 breakthrough infections between January 2022 and October 2023. IgG and total antibodies against SARS-CoV-2 were measured by chemiluminescent immunoassay. Neutralizing antibodies were detected using a pseudovirus neutralization assay. Meanwhile, we created an Enterprise WeChat link for college students to self-report SARS-CoV-2 infections and clinical symptoms of COVID-19. We observed that among college students, the most common symptoms upon SARS-CoV-2 infection were fever, fatigue, and sore throat. Moreover, reinfected college students had higher levels of total antibodies and neutralizing antibodies against BA.5, XBB.1.5 and EG.5.1, especially after experiencing the XBB.1.5 wave. Finally, the neutralizing effect against the newly emerged Omicron subvariants XBB.1.5 and EG.5.1 is limited among the college students. Our study demonstrates that hybrid immunity, built from breakthrough infections and reinfections, enhances total antibody levels and bolsters neutralizing activity, contributing to milder clinical presentations upon reinfection. However, neutralization efficacy against newer subvariants, such as XBB.1.5 and EG.5.1, remains compromised.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVD-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a global pandemic. As of September 2024, it has resulted in over 7 million deaths (https://covid19.who.int). Since 2022, Omicron has continuously mutated, producing new subvariants with an increasing ability to evade the host immune system1,2. WHO identified several major subvariants of Omicron in June 2023, including BA.1, BA.5, BQ, XBB, and EG.5 these new subvariants have led to a continuous increase in SARS-CoV-2 breakthrough infection and reinfection3,4. These subvariants can evade immunity acquired through vaccination and previous infections5, leading to increased COVID-19 breakthrough infection rates, reinfection rates, hospitalizations, and severe disease globally6,7,8. Notably, infection rates among children and adolescents are also increasing, including severe COVID-199, which could represent a significant public health burden.
Studies have shown that vaccination is currently one of the most effective measures to reduce disease incidence. Typically, patients are protected by the humoral immune system within months of symptom resolution, with antibody responses peaking shortly after infection but declining over time10,11. Patients with breakthrough infections generally exhibit higher IgG and neutralizing antibody levels compared to those with reinfection12. However, due to the emergence of new variants, the Omicron variants BA.1, BA.2, BA.3, BA.2.12.1, BA.2.75, BA.4/BA.5, BA.4.6, and BF.7 are substantially resistant to vaccine- and infection-induced serum-neutralizing activity13,14,15,16. Unlucky, continuous variation in Omicron has led to persistent infections and reinfections, which have become common among young people, with some severe cases emerging. College students as a young demographic represent a socially active and immunologically distinct group that may experience different exposure risks, immune responses, and recovery trajectories compared to other populations. Therefore, evaluating the clinical symptoms and immune responses of college students during the Omicron epidemic is essential to implementing measures that reduce the risk of reinfection and prevent clustered outbreaks in schools and other settings.
Currently, more research focuses on middle-aged, elderly individuals, and children, with relatively limited data on COVID-19 infections among college students. Therefore, we enrolled 876 college students to analyze the clinical symptoms of SARS-CoV-2 infection. In addition, we assessed the level of humoral immunity, across different infection statuses and vaccination statuses. In particular, we compared changes in neutralizing antibody responses between earlier strains and currently circulating subvariants of Omicron.
Methods
Study design, participants, and sample collection
On October 31, 2023, a questionnaire survey and specimen collection were conducted with 1,065 university students at Hebei University of Economics and Business. The study selection process is shown in Fig. 1. In this study, college students were recruited after BA.5/BF.7 and/or XBB.1.5 wave between January 2022 and October 2023. We created an Enterprise WeChat link for college students to self-report SARS-CoV-2 infections and clinical symptoms of COVID-19. The survey mainly includes the following: baseline information, SARS-CoV-2 infection history, and clinical symptoms experienced at the time of infection. A total of 1065 questionnaires were received, and the number and type of vaccinations administered to the participants were queried through the vaccine inquiry system. After applying the exclusion criteria, 876 individuals were included in the study.
The inclusion criteria were as follows: (1) participants were over 18 years old; (2) participants had received at least two doses of a COVID-19 vaccine; (3) participants had provided informed consent through a signed document; The exclusion criteria were as follows: (1) incomplete questionnaire information; (2) incomplete or missing vaccination records. Blood samples were collected from all 876 participants, and serum was isolated by centrifugation at 4000 rpm for 10 min, then stored at −80℃ until testing. Total antibodies and IgG antibodies were tested in all participants. Due to the limited sample size, 100 participants with sufficient serum volume were selected to test neutralizing antibodies (NAbs) using the pseudovirus neutralization assay.
This study divided college students into three groups based on their infection status: the first group consisted of students who had not been infected (uninfected); the second group consisted of students who experienced a single breakthrough infection (breakthrough infection); and the third group consisted of students who experienced two or more infections (reinfection). To assess the changes in total antibody and IgG levels in students experiencing different strain waves under various infection statuses, we divided the students into the following four subgroups: the first group consisted of students who experienced a breakthrough infection after the BF.7/BA.5 wave; the second group consisted of students who experienced reinfection after the BF.7/BA.5 wave; the third group consisted of students who experienced a breakthrough infection after the XBB.1.5 wave; and the fourth group consisted of students who experienced reinfection after the XBB.1.5 wave. To further assess the temporal changes in total antibody and IgG levels, we divided the students into six subgroups according to the time of their most recent infection and infection status: the first group consisted of students who had not been infected; the second group consisted of students who experienced a breakthrough infection less than 6 months ago (median interval time = 144 days) (Breakthrough infection < 6 months); the third group consisted of students who experienced a breakthrough infection 6–9 months ago (median interval = 248.5 days) (Breakthrough infection 6–9 months); the fourth group consisted of students who experienced a breakthrough infection more than 9 months ago (median interval = 308 days) (Breakthrough infection > 9 months); the fifth group consisted of students who experienced reinfection less than 6 months ago (median interval = 131.5 days) (Reinfection < 6 months); and the sixth group consisted of students who experienced reinfection 6–9 months ago (median interval = 208 days) (Reinfection 6–9 months).
Reliability and validity analysis of the questionnaire survey
To ensure the reliability and validity of the questionnaire used to assess recent SARS-CoV-2 infections, we conducted data quality control for each study participant based on actual circumstances and experimental results, ensuring that the data remained reasonable within a controllable range. Additionally, we conducted a series of psychometric tests. The internal consistency was evaluated using Cronbach’s α = 0.69 (Tables 1 and 2), indicating acceptable reliability. The Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy was 0.75, and Bartlett’s test of sphericity was significant (P < 0.0001), supporting the suitability of the questionnaire for capturing infection status (Table 3). Additionally, the questionnaire was pilot-tested in a small group of PLWH to verify clarity and the accuracy of self-reported infection data.
Experiment
Chemiluminescent microparticle immunoassay (CLIA)
The SARS-CoV-2 total antibody and IgG antibody against recombinant nucleoprotein (N) and spike (S1) proteins of SARS-CoV-2 in serum specimens were detected using a chemiluminescence method according to the manufacturer’s instructions. Samples were thawed in a refrigerator at 4℃ the day before the testing and then kept at room temperature with the reagents until testing. Gently mix the serum with a pipette and 200 µL of serum was added to the reaction cup. The serum level of 2019-nCoV Ag was measured by an automated chemiluminescence immunoassay analyzer (Caris200) using the accompanying kits (innodx, Xiamen, China) according to the instructions of the manufacturers. The result of the 2019-nCoV Antibody was negative when the sample S/CO < 1.0. The serum level of 2019-nCoV IgG was measured by an automated chemiluminescence immunoassay analyzer (YHLO iFlash 3000) using the 2019-CoV IgG antibody detection kit (YHLO, Shenzhen, China) according to the instructions of the manufacturers. The result of < 10 AU/mL was regarded as no response.
Cell lines
Human embryonic kidney HEK-293T cells were cultured at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS, Gibco, USA) and supplemented with 1% penicillin-streptomycin (Gibco). The cells were disrupted at confluence with 0.25% trypsin in 1 mM EDTA (Solarbio) every 48–72 h.
Spike plasmid pseudovirus production
Pseudovirus particles were generated as previously described by cotransfecting HEK-293T cells (ATCC, CRL-3216) with human immunodeficiency virus backbones expressing firefly luciferase (pNL4-3-R-E-luciferase) and the pcDNA3.1 vector encoding the S protein of the D614G, BA.5, XBB.1.5, EG.5.1, and BA.2.86 plasmids. Codon-optimized, full-length open reading frames of the spike genes of the D614G, BA.5, XBB.1.5, and EG.5.1 strains were synthesized by GenScript (Nanjing, China). The medium was replaced with fresh medium at 24 h, and the supernatants were harvested at 48 h post-transfection and clarified by centrifugation at 300 × g for 10 min before being aliquoted and stored at −80 °C until use.
Pseudovirus neutralization assay
A SARS-CoV-2 pseudovirus neutralization assay (pVNT) was performed17, with the target cell line HeLa overexpressing hACE2 orthologs. All viruses were first titrated to normalize the viral input between assays. Duplicate 3-fold 8-point serial dilutions of heat-inactivated sera (starting at 1:30) were incubated with 500–1000 TCID50 of the SARS-CoV-2 pseudotyped virus for 1 h at 37℃ and 5% CO2. Subsequently, 1 × 104 HeLa-ACE2 cells were added to each well and incubated at 37℃ and 5% CO2 for 48 h. Afterward, the supernatant was removed, and the cells were lysed using a passive lysis buffer (Vazyme) for 3 min at room temperature. The lysates were transferred to an opaque white 96-well plate, reconstituted luciferase assay buffer (Vazyme) was added, and the proteins were mixed with each lysate. Luminescence was measured immediately after mixing using a GloMax 96 Microplate Luminometer (Promega). The neutralization titer (NT50) was determined by luciferase activity with a four-parameter nonlinear regression inhibitor curve in GraphPad Prism 10.1.2 (GraphPad Software). The NT50 was reported as the reciprocal serum dilution causing a 50% reduction in relative light units. A sample with an NT50 value of no more than 30 (the detectable limit) was considered negative for neutralizing antibodies and was assigned a nominal value of 10 in geometric mean titer (GMT) calculations, which is the lowest serum dilution MM factor used in the pseudovirus neutralization assay.
Statistical analysis
This study is a cross-sectional survey aimed at estimating the COVID-19 positivity rate in a specific population. To ensure the reliability and representativeness of the statistical results, we calculated the sample size. According to the research by Sijia Zhou et al., the COVID-19 positivity rate is 90%18, and the sample size calculation uses the following formula:
Where Z is the confidence level, set to 1.96 (corresponding to 95% confidence); p is the expected positivity rate, set to 0.84 (i.e., 84% positivity rate); E is the margin of error, set to 0.02. By substituting into the formula, the minimum sample size is calculated to be approximately 864.
All statistical analyses were performed using GraphPad Prism 10.0 (GraphPad Software Inc., CA, USA) and SPSS 26.0 (SPSS Inc., IBM, New York, United States). The normality of data distribution was assessed using the Shapiro-Wilk test. Continuous variables were expressed as mean ± SD for normal, and non-normally distributed data were expressed as medians and interquartile ranges [IQR]. Categorical data were reported as n (%) and comparisons were analyzed by chi-square test or Fisher’s exact test. The Wilcoxon rank-sum test or Mann-Whitney U test was used for comparison between the two groups. The Kruskal–Wallis tests with the false discovery rate method were used for multiple comparisons where it appreciates.
Results
Demographic characteristics of college students
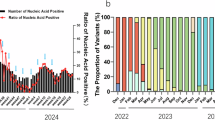
In this study, data were collected from 876 college students using a questionnaire. Among these participants, 599 experienced breakthrough infections after exposure to the BA.5/BF.7 and/or XBB.1.5 variants between January 2022 and October 2023. Of these, 97 individuals experienced reinfections between December 2022 and October 2023 after initial exposure to the BA.5/BF.7 and/or XBB.1.5 variants. The prevalence of SARS-CoV-2 was 79.5%, with 68.4% of participants experiencing a breakthrough infection and 11.1% experiencing reinfection. A significant increase in infections was observed in December 2022, peaking at that time. A secondary peak in reinfections occurred in May 2023, followed by a gradual decline (Fig. 2B).
Description of symptoms and prevalence of the subvariant. (A) Percentage of symptoms compared to breakthrough infection and reinfection during SARS-CoV-2 infection. (B) The prevalence of SARS-CoV-2 at different times for all individuals. “Others” represents the occurrence of symptoms not shown in the figure. *: <0.05, **: <0.01, ***: <0.001, ****: <0.0001.
We investigated the male-to-female ratio was 2.2:1, and the interval between breakthrough infection and reinfection was 154 days. Antibody evaluations were conducted after breakthrough infections (median 304 days) and reinfections (median 158 days). Furthermore, three individuals (0.5%) with breakthrough infections were hospitalized after COVID-19, which was not observed in reinfected patients (Table 3). We found that 845 college students (96.5%) received the inactivated booster vaccine, and all these students received the vaccine at the same time point. As a result, the median interval between the last dose of the vaccine and sampling for each cohort is 678 days. And vaccination information is shown in Table 3.
Clinical symptoms in college students against SARS-CoV-2
According to the subjective description of college students, the clinical symptoms during infection were recorded, and the occurrence of symptoms in the breakthrough infection and reinfection cohorts was compared (Table S1). First, the main symptom in both breakthrough infection and reinfection was fever (Breakthrough infection vs. Reinfection: 89.5% vs. 80.4%), fatigue (Breakthrough infection vs. Reinfection: 60.6% vs. 51.5%), and sore throat (Breakthrough infection vs. Reinfection: 56.9% vs. 34.0%). Our results indicated that the incidence of symptoms in reinfection was found to be lower than that observed in the breakthrough infection. Among these, the differences in fever (P = 0.009), sore throat (P < 0.0001), headache (P = 0.039), cough (P = 0.005), expectoration (P = 0.041), loss of taste/smell (P = 0.003) were statistically significant (Fig. 2A).
Total and IgG antibody responses in college students
The college students were classified into three cohorts based on their SARS-CoV-2 infection status: uninfected, breakthrough infection, and reinfection. All antibody levels are expressed as median [IQR]. The seropositivity of total antibodies and IgG antibodies were 100% in all cohorts. Antibody levels were lower in the uninfected group compared to those who had been infected with SARS-CoV-2 (Fig. 3A and D). Specifically, the total antibody levels in the reinfection group were significantly higher than those in the breakthrough infection group (P = 0.0142) and significantly higher than in the uninfected group (P = 0.0112) (Fig. 3A).
The responses of total antibody and IgG antibody against SARS-CoV-2 among college students. (A, D) Total antibody and IgG antibody responses during the uninfected, breakthrough infection and reinfection among the 876 fully vaccinated college students. (B, E) Total antibody and IgG antibody responses against SARS-CoV-2 who experienced breakthrough infections and/or reinfections after the BA.5/BF.7 wave and/or the XBB.1.5 wave. (C, F) Total antibody and IgG antibody responses for uninfected, breakthrough infection < 6 months, 6–9 months, > 9 months, and reinfection < 6 months, 6–9 months according to the time of infection. All values are presented as the median and interquartile range. The black dotted line represents the positivity cutoff for neutralizing antibodies (total antibody: <10 AU/ml is negative; IgG: S/CO < 1.0 COI is negative). IgG, immunoglobulin G.
According to the report from the China Center for Disease Control and Prevention, the BA.5/BF.7 wave prevailed in China from January to April 2023, while the XBB.1.5 wave occurred from April to October 2023. Therefore, we categorized patients based on different variant waves and their infection status during these periods. Specifically, we compared students who experienced breakthrough infections or reinfections during the BA.5/BF.7 wave with those who experienced breakthrough infections or reinfections during the XBB.1.5 wave. Our findings show that the total antibody and IgG levels of college students who experienced reinfections during the BA.5/BF.7 wave were significantly higher than those of college students who experienced breakthrough infections during the XBB.1.5 wave (P < 0.05) (Fig. 3B and E).
We grouped students based on the time interval between infection and sample collection to compare their antibody concentrations. The groups were as follows: uninfected group, students with a single breakthrough infection (< 6 months, 6–9 months, > 9 months post-infection), and students with reinfection (two or more infections) (< 6 months, 6–9 months post-reinfection). We found that the total antibody levels of uninfected college students were significantly lower than those who had experienced a breakthrough infection 6–9 months prior (breakthrough infections 6–9 months) (P = 0.0433) and less than 6 months prior (P = 0.0081). The total antibody levels of college students with a breakthrough infection less than 6 months prior were significantly lower than those in students who experienced reinfection within 6 months (reinfection < 6 months) (P = 0.0249). Additionally, the IgG antibody levels of uninfected college students were significantly lower than those in students who experienced reinfection < 6 months (P = 0.0266). Furthermore, the total antibody and IgG levels in students 9 months after a breakthrough infection showed a declining trend, while a similar decline began to appear in students 6 months after reinfection (Fig. 3C and F), although no statistical significance was observed.
Neutralizing capacity
We investigated neutralizing antibody responses in a cohort of 100 individuals who were vaccinated and then uninfected (n = 29) or experienced breakthrough infection (n = 61) and reinfection (n = 10), selected at random. Firstly, we characterized the neutralization chart of the ancestral D614G strain and the BA.5, XBB.1.5, EG.5.1 variants from all individuals (Fig. 4). Across all cohorts, the highest titers were observed for D614G, while substantially lower titers were evident for BA.5, XBB.1.5 and EG.5.1 (Fig. 4). Specifically, all individuals in the uninfected cohort had neutralizing antibodies against D614G, BA.5, XBB.1.5 and EG.5.1, with GMTs of 4344, 1430, 344.2, and 331.9, respectively. Additionally, a comparable neutralizing GMT against XBB.1.5 and EG.5.1 subvariant, which were significantly lower than the GMTs against BA.5 (P = 0.0015), with a 4.2–4.3.2.3-fold decrease. We observed the highest GMT against D614G (GMT = 4344, 95% confidence interval [CI] 3286–5776), which was higher than BA.5 (GMT = 1430, 95% CI 1001–2044, P = 0.0160), XBB.1.5 (GMT = 344.2, 95% CI 226.5–522.9.5.9, P < 0.0001), EG.5.1 (GMT = 331.9, 95% CI 209.6–525.6.6.6, P < 0.0001), exhibiting a 3.0 to 13.1-fold decrease in GMT compared to D614G (Fig. 4C). In the breakthrough infection cohorts, we observed that all individuals had neutralizing antibody titers of more than 30 against D614G and BA.5, with GMTs of 3825 (95% CI 2989–4895) and 2299 (95% CI 1733–3049). Whereas only 98% and 97% of sera neutralized XBB.1.5, EG.5.1 subvariants with GMTs of 733.1 (95% CI 513.9–1046) and 640.3 (95% CI 435.3–941.8.3.8). Furthermore, the GMTs against the XBB.1.5 and/or EG.5.1 subvariants were significantly lower than the GMTs against D614G and/or BA.5 (P < 0.0001). The reduction of GMT against BA.5, XBB.1.5, and EG.5.1 were 1.7-, 5.2-, and 6.0-fold compared to D614G, respectively (Fig. 4B). In the reinfection cohorts, almost all individuals had detectable neutralizing antibodies for the XBB.1.5 (GMT = 887.1, 95% CI 626.4–1256), EG.5.1 (GMT = 843.1, 95% CI 565.6–1257) variants, but in line with other cohorts, the GMTs against these two variants were significantly lower than the GMTs against D614G (GMT = 3331, 95% CI 2472–4490) and/or BA.5 (GMT = 2279, 95% CI 1523–3411). The reduction of GMT against BA.5, XBB.1.5, and EG.5.1 were 1.5-, 3.8-, and 4.0-fold compared to D614G, respectively (Fig. 4A).
Comparisons of neutralizing antibody titers against SARS-CoV-2 D614G, BA.5, XBB.1.5, EG.5.1 subvariants. (A) Neutralization of SARS-CoV-2 Omicron subvariants of D614G, BA.5, XBB.1.5, EG.5.1 by 29 sera collected from individuals uninfected. (B) Neutralization of D614G, BA.5, XBB.1.5, EG.5.1 subvariants by 61 sera collected from individuals with breakthrough infection. (C) Neutralization of D614G, BA.5, XBB.1.5, EG.5.1 subvariants by 10 sera collected from individuals with reinfection. (D, E, F, G) Comparisons of neutralizing antibody titers against SARS-CoV-2 D614G, BA.5, XBB.1.5, EG.5.1 among breakthrough infection, reinfection, uninfected. The black dotted line represents the positivity cutoff for neutralizing antibodies (NT50 of 30). The GMTs are shown under each column along with the percentage of individuals with NT50 values above the 30. And the fold change of GMT is also denoted after P value.
Subsequently, we assessed the neutralizing activity of D614G, BA.5, XBB.1.5, and EG.5.1 in different infection statuses. The highest titers were observed in reinfection cohorts for BA.5, XBB.1.5, and EG.5.1 variants (Fig. 4D, E, F). Specifically, the GMTs of the breakthrough infection cohort were significantly higher than the GMT of the uninfected cohort against BA.5 (P = 0.0292), with a 1.6-fold increase. The GMT of the XBB.1.5 variant was lowest in the uninfected cohort (Fig. 4F), which was significantly lower than breakthrough infection (P = 0.0070) and reinfection cohorts (P = 0.0435), with a 2.1–2.6.1.6-fold decrease. The EG.5.1 variant exhibited a similar trend to the XBB.1.5 variant, though it was not statistically significant (Fig. 4G). In summary, reinfection with the new variants resulted in higher neutralizing activity compared to breakthrough infection and uninfected individuals, but they showed lower GMT levels relative to the D614G strain and BA.5 variant.
Discussions
In this study, we conducted a cross-sectional study aimed to reveal the symptoms of various variants against SARS-CoV-2 infection among college students and the degree of protection provided by humoral immunity. This is crucial for assessing immunity and the potential risk of subsequent infections. We conducted a characteristic analysis of 876 vaccinated college students after the reopening of China. Additionally, we focused on observing the changes in neutralizing responses of the new Omicron subvariants.
Before the emergence of the Omicron strain, COVID-19 reinfections were rare. However, with the appearance of new subvariants, breakthrough infections and reinfections have become more frequent3. On December 7, 2022, following the termination of the zero-COVID-19 policy, peaks emerged19. Our study is consistent with the reported findings, a significant increase in infections was observed in December 2022, peaking at that time. A secondary peak in reinfections occurred in May 2023. Generally, the reinfection rate of SARS-CoV-2 ranges from 3% to 12%20,21,22. Our study found that the COVID-19 incidence in college students, breakthrough infection rate, and reinfection rate were 79.5%, 68.4%, and 11.1%, respectively. Compared to the reinfection rate reported among healthcare workers23, the rate among college students is relatively lower and led to relatively mild symptoms. We attribute this to the more controlled environment in universities, which limited students’ exposure to external contacts during the pandemic. Our questionnaire survey showed that three individuals reported hospitalization due to infection, suggesting that with the emergence of new Omicron variants, they possess the ability to escape the immunity previously acquired through vaccination and post-infection5,7,8. Notably, the average interval between reinfection and breakthrough infection in our cohort was 154 days, shorter than the 180-day interval typically seen in previous studies2,24. We speculate that this shortened interval is also related to immune escape.
We further evaluated the symptoms of college students with SARS-CoV-2 infection to observe the risks posed by COVID-19. We found that consistent with the symptoms reported in general population25. In our study, fever, fatigue, and sore throat were found at a higher rate of occurrence among college students. Interestingly, we found that patients with reinfection experienced fewer clinical symptoms upon SARS-CoV-2 infection compared to those with breakthrough infections26, a similar conclusion was reached in our investigation, we observed the incidence of symptoms in reinfection was found to be lower than that observed in the breakthrough infection. Furthermore, only up to 11.1% of participants experienced reinfection after the XBB.1.5 wave. Thus, breakthrough infections with Omicron BA.5 may provide a certain level of protection for the college student population, reducing the severity of reinfection, which demonstrates the efficacy of antibodies in providing long-lasting protection against infection in a population of college students.
Higher levels and increased avidity of antibodies are associated with a lower probability of reinfection and milder disease upon reinfection in COVID-19 patients. The persistence and maturation of these antibodies play a crucial role in long-term immunity27,28. Consistent with previous studies29,30, we found that individuals with reinfection had higher total antibody levels compared to those with breakthrough infections and those who had uninfected. Furthermore, college students who experienced reinfection exhibited elevated total antibody and IgG levels compared to those who experienced breakthrough infection after experiencing XBB.1.5 wave. And the seropositivity of total antibodies and IgG was 100%. This finding suggests that hybrid immunity, involving both infection and vaccination, provides enhanced protection, which could explain the milder symptoms observed in reinfected individuals. However, college students should also consider self-administering booster vaccines at the appropriate time to prevent the impact of clustered infections on their lives and studies.
Furthermore, the decrease in booster immunizations and breakthrough infections aligns with the reduction in neutralizing antibodies, supporting their strong correlation with protection against COVID-19 infection31,32,33. So we next investigated neutralizing antibody responses in a cohort of 100 individuals who were vaccinated and then uninfected and experienced breakthrough infection and reinfection. Our results are consistent with previous studies34,35, D614G had the highest neutralizing activity across the uninfected, breakthrough infection, and reinfection cohorts, followed by BA.5. The newly emerged XBB.1.5 and EG.5.1 strains showed limited neutralizing activity in all cohorts. This also indicates that the neutralizing activity against the new Omicron subvariants was reduced in college students. Our study found that the GMT decline was least pronounced in reinfected individuals, followed by those with breakthrough infections. In contrast, the GMT level in uninfected individuals was significantly lower and declined more rapidly. This suggests that infection-induced immune responses demonstrate a broader ability to neutralize compared to those induced by vaccines15. Noteworthy three individuals who had breakthrough infections lost neutralizing activity against XBB.1.5 or EG.5.1. Moreover, stronger neutralizing activity against XBB.1.5 and EG.5.1 was found in reinfected individuals compared to those with breakthrough infections and those uninfected. We believe that this phenomenon is due to the median time from sampling to breakthrough infection being 304 days, much higher than the median time of 158 days from sampling to reinfection, leading to a decline in neutralizing ability among those with breakthrough infections. These finding highlights the potential benefit of hybrid immunity in enhancing neutralizing responses, especially against new variants. College students exhibit stronger immunity after infection compared to other specific groups. However, the neutralizing effect against new subvariants remains compromised, highlighting the need for continued vaccination and antibody monitoring, particularly with vaccines targeting emerging SARS-CoV-2 subvariants, to maintain immunity.
To the best of our knowledge, current research is primarily focused on the prevalence, risk factors, and immunity of children and adolescents (aged 0–19) following SARS-CoV-2 infection36,37,38. However, there is limited research on comprehensive humoral immunity testing in college students. This study investigates the prevalence and changes in humoral immunity in college students (aged 22–24) following the emergence of new Omicron subvariants, particularly by using pseudovirus neutralization assays to assess the neutralizing activity against the circulating XBB.1.5 and EG.1.5 variants. This is crucial for evaluating the protective status of antibodies in the college student population and formulating vaccination strategies for this group. Due to their high-density living and learning environments, understanding humoral responses in this group is critical for designing targeted public health interventions, especially in the context of future outbreaks in educational institutions. We found hybrid immunity provides strong protection for college students. However, in the context of ongoing Omicron mutations, the neutralizing ability against new variants offers weaker protection for this group. Based on this study, we recommend that college students receive vaccines targeting specific variants when necessary to provide long-term immune protection and prevent reinfection. Although our study did not find particularly severe cases of COVID-19, the continuous cross-mutation of strains and the decline in neutralizing antibodies contribute to a decrease in herd immunity. We also refute the common belief that young people have a better ability to resist viruses, emphasizing that every group should strengthen their awareness of virus resistance to protect themselves and prevent more severe and widespread infections.
The study has several limitations. Firstly, our study is a cross-sectional study, which limits our ability to monitor disease progression and accurately assess antibody titers over time. Longitudinal data are crucial for understanding the temporal dynamics of immunity and the impact of emerging subvariants. Therefore, we hope to incorporate longitudinal analysis in future studies to explore these aspects more comprehensively. Secondly, our cohort consisted entirely of college students aged 22–24 from a single location, representing a relatively homogeneous age group. Students within this group tend to share certain socioeconomic and behavioral characteristics, which makes it challenging to generalize their immune protection status to other populations. Therefore, these findings should be interpreted with caution when applied to different regions or populations. Future studies will aim to include more diverse and representative cohorts to better evaluate humoral immunity across broader demographic contexts. Thirdly, we did not evaluate cellular immunity. Cellular immunity is fundamental in determining disease severity. Finally, this study relies on self-reported data, which is potentially biased, however, we have performed data quality control based on experimental results and the actual situation. While these measures help mitigate bias, we emphasize that the questionnaire results from this study should be interpreted with caution due to the inherent limitations of self-reported data.
Conclusions
Our data demonstrate that the primary symptoms of breakthrough infections and reinfections are fever, fatigue, and sore throat. In the college student population, hybrid immunity (formed through both prior infection and vaccination) results in higher total antibody and neutralizing antibody responses during reinfection. This leads to milder symptoms. However, 2% to 3% of college students exhibit a lack of neutralizing capacity against XBB.1.5 and EG.5.1. Compared to the ancestral variant, neutralizing efficacy against emerging variants has significantly decreased. Overall, hybrid immunity provides some degree of protection to university students, but its protective effect against the new Omicron variants is limited. In the context of coexisting new Omicron variants, it is essential for the student population to receive vaccination against the new Omicron variants when necessary. This will help maintain immunity against emerging SARS-CoV-2 variants, thereby reducing the additional risks posed by infections and preventing the occurrence of outbreaks.
Data availability
The data will be available from the corresponding author upon reasonable request.
References
Parsons, R. J. & Acharya, P. Evolution of the SARS-CoV-2 Omicron Spike. Cell. Rep. 42, 113444. https://doi.org/10.1016/j.celrep.2023.113444 (2023).
Zhan, H. et al. Booster shot of inactivated SARS-CoV-2 vaccine induces potent immune responses in people living with HIV. Journal of medical virology 95, e28428, (2023). https://doi.org/10.1002/jmv.28428
Medić, S. et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, serbia: A population-level observational study. Lancet Reg. Health Europe. 20, 100453. https://doi.org/10.1016/j.lanepe.2022.100453 (2022).
World Health, O. SARS-CoV-2 variant risk evaluation framework, 30 August 2023. vi, 26 pWorld Health Organization,. (2023).
Kozlov, M. Does Omicron hit kids harder? Scientists are trying to find out. Nature https://doi.org/10.1038/d41586-022-00309-x (2022).
Past, S. A. R. S. CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet (London England). 401, 833–842. https://doi.org/10.1016/s0140-6736(22)02465-5 (2023).
Boufidou, F. et al. SARS-CoV-2 reinfections and long COVID in the Post-Omicron phase of the pandemic. Int. J. Mol. Sci. 24 https://doi.org/10.3390/ijms241612962 (2023).
Bowe, B., Xie, Y. & Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 28, 2398–2405. https://doi.org/10.1038/s41591-022-02051-3 (2022).
Lu, W., Zeng, S., Yao, Y., Luo, Y. & Ruan, T. The effect of COVID-19 vaccine to the Omicron variant in children and adolescents: a systematic review and meta-analysis. Front. public. Health. 12, 1338208. https://doi.org/10.3389/fpubh.2024.1338208 (2024).
Munro, A. P. S. et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 22, 1131–1141. https://doi.org/10.1016/s1473-3099(22)00271-7 (2022).
Regev-Yochay, G. et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against Omicron. N. Engl. J. Med. 386, 1377–1380. https://doi.org/10.1056/NEJMc2202542 (2022).
Zou, J., Xie, X., Liu, M., Shi, P. Y. & Ren, P. Neutralization Titers in Vaccinated Patients with SARS-CoV-2 Delta Breakthrough Infections. mBio 13, e0199622, (2022). https://doi.org/10.1128/mbio.01996-22
Favresse, J. et al. Neutralizing antibody response to XBB.1.5, BA.2.86, FL.1.5.1, and JN.1 six months after the BNT162b2 bivalent booster. Int. J. Infect. Diseases: IJID : Official Publication Int. Soc. Infect. Dis. 143, 107028. https://doi.org/10.1016/j.ijid.2024.107028 (2024).
Wang, Q. et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 608, 603–608. https://doi.org/10.1038/s41586-022-05053-w (2022).
Wang, Q. et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 186, 279–286e278. https://doi.org/10.1016/j.cell.2022.12.018 (2023).
Arora, P. et al. Lung cell entry, cell-cell fusion capacity, and neutralisation sensitivity of Omicron sublineage BA.2.75. Lancet Infect. Dis. 22, 1537–1538. https://doi.org/10.1016/s1473-3099(22)00591-6 (2022).
Zhu, K. L. et al. Durability of neutralization against Omicron subvariants after vaccination and breakthrough infection. Cell. Rep. 42, 112075. https://doi.org/10.1016/j.celrep.2023.112075 (2023).
Zhou, S. et al. Estimating cumulative infection rate of COVID-19 after adjusting the dynamic zero-COVID policy in China. Infect. Disease Modelling. 10, 429–438. https://doi.org/10.1016/j.idm.2024.12.012 (2025).
Fu, D. et al. Effectiveness of COVID-19 vaccination against SARS-CoV-2 Omicron variant infection and Symptoms - China, December 2022-February 2023. China CDC Wkly. 5, 369–373. https://doi.org/10.46234/ccdcw2023.070 (2023).
Cohen, C. et al. SARS-CoV-2 incidence, transmission and reinfection in a rural and an urban setting: results of the PHIRST-C cohort study, South Africa, 2020–2021. MedRxiv: Preprint Serv. Health Sci. https://doi.org/10.1101/2021.07.20.21260855 (2021).
Guedes, A. R. et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci. Rep. 13, 712. https://doi.org/10.1038/s41598-022-25908-6 (2023).
Hansen, C. H., Michlmayr, D., Gubbels, S. M., Mølbak, K. & Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet (London England). 397, 1204–1212. https://doi.org/10.1016/s0140-6736(21)00575-4 (2021).
Song, X. D. et al. Prevalence of infection and reinfection among health care workers in a hospital of Northern China between BA.5/BF.7 and XBB.1.5 wave. Am. J. Infect. Control. https://doi.org/10.1016/j.ajic.2024.08.009 (2024).
Favresse, J. et al. Vaccine-induced binding and neutralizing antibodies against Omicron 6 months after a homologous BNT162b2 booster. J. Med. Virol. 95, e28164. https://doi.org/10.1002/jmv.28164 (2023).
Grant, M. C. et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PloS One. 15, e0234765. https://doi.org/10.1371/journal.pone.0234765 (2020).
Brasso, C., Bellino, S., Blua, C., Bozzatello, P. & Rocca, P. The impact of SARS-CoV-2 infection on youth mental health: A narrative review. Biomedicines 10 https://doi.org/10.3390/biomedicines10040772 (2022).
Ali, A. M., Ali, K. M., Fatah, M. H., Tawfeeq, H. M. & Rostam, H. M. SARS-CoV-2 reinfection in patients negative for Immunoglobulin G following recovery from COVID-19. New. Microbes new. Infections. 43, 100926. https://doi.org/10.1016/j.nmni.2021.100926 (2021).
Löfström, E. et al. Dynamics of IgG-avidity and antibody levels after Covid-19. J. Clin. Virology: Official Publication Pan Am. Soc. Clin. Virol. 144, 104986. https://doi.org/10.1016/j.jcv.2021.104986 (2021).
Franco-Luiz, A. P. M. et al. Longitudinal study of humoral immunity against SARS-CoV-2 of health professionals in brazil: the impact of booster dose and reinfection on antibody dynamics. Front. Immunol. 14, 1220600. https://doi.org/10.3389/fimmu.2023.1220600 (2023).
Gonzales, M. et al. Durability and extent of protection of SARS-CoV-2 antibodies among patients with COVID-19 in metro Manila, Philippines. Front. Immunol. 14, 1190093. https://doi.org/10.3389/fimmu.2023.1190093 (2023).
Muik, A. et al. Omicron BA.2 breakthrough infection enhances cross-neutralization of BA.2.12.1 and BA.4/BA.5. Sci. Immunol. 7, eade2283. https://doi.org/10.1126/sciimmunol.ade2283 (2022).
Angyal, A. et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 3, e21–e31. https://doi.org/10.1016/s2666-5247(21)00275-5 (2022).
Jiang, X. L. et al. Omicron BQ.1 and BQ.1.1 escape neutralisation by Omicron subvariant breakthrough infection. Lancet Infect. Dis. 23, 28–30. https://doi.org/10.1016/s1473-3099(22)00805-2 (2023).
Wang, H. et al. Neutralization against Omicron subvariants after BA.5/BF.7 breakthrough infection weakened as virus evolution and aging despite repeated prototype-based vaccination(1). Emerg. Microbes Infections. 12 (2249121). https://doi.org/10.1080/22221751.2023.2249121 (2023).
Zhao, X. J. et al. Epidemiological characteristics and antibody kinetics of elderly population with booster vaccination following both Omicron BA.5 and XBB waves in China. J. Med. Virol. 96, e29640. https://doi.org/10.1002/jmv.29640 (2024).
Meherali, S. et al. Mental health of children and adolescents amidst COVID-19 and past pandemics: A rapid systematic review. Int. J. Environ. Res. Public Health. 18 https://doi.org/10.3390/ijerph18073432 (2021).
Aparicio, C. et al. Risk factors for pediatric critical COVID-19: A systematic review and Meta-Analysis. J. Pediatr. Infect. Dis. Soc. 13, 352–362. https://doi.org/10.1093/jpids/piae052 (2024).
Zinszer, K. et al. Seroprevalence of SARS-CoV-2 Antibodies Among Children in School and Day Care in Montreal, Canada. JAMA network open 4, e2135975, (2021). https://doi.org/10.1001/jamanetworkopen.2021.35975
Acknowledgements
We thank all participants for providing blood samples.
Funding
This work was supported by grants from The Science and Technology Program of Shijiazhuang (grant no. 231200243), Hebei Provincial Traditional Chinese Medicine Scientific Research Project – Study on the Effect of Integrated Traditional Chinese and Western Medicine Rehabilitation Program on Health Management of COVID-19 Recovery Patients (grant no. 2023382), the Key R&D Project of Hebei Province (grant no. 22377744D), and the Science and Technology Program of Shijiazhuang (grant no. 231200103 A). The funders had no role in the study design, data collection and interpretation, or decision to submit this manuscript for publication.
Author information
Authors and Affiliations
Contributions
E.-H. D., A.-D. F conceived and supervised the study. Y.-C. X. extracted data, performed software analyzes, and visualized graphs and tables. Y.-C. X. wrote and revised the paper. X.-D. S supervised data analyzes and revised the manuscript. Y.-C. X., C. Y., Y.-Z. S., H.-X. G., Y. L., X.-N. D., F.-M. F., and E.-H. D. collected the questionnaire and blood samples. Y.-C.X., Y. L., X.-D. S., C.-M. Z. performed the experiments. All authors are accountable for all aspects of the study, and attest to the accuracy and integrity of the results. And all the authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was conducted following the Declaration of Helsinki and approved by the Ethics Committee of the Fifth Hospital of Shijiazhuang (Number: 2022-001). All the participants provided written informed consent.
Disclosure statement
We declare no potential conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, Y., Chen, Y., Shi, Y. et al. Humoral immunity and clinical characteristics of Chinese college students experiencing a BA.5/BF.7 and XBB.1.5 wave. Sci Rep 15, 45363 (2025). https://doi.org/10.1038/s41598-025-29058-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29058-3