Abstract
This study evaluated the diagnostic performance of a novel vessel tracing software that quantifies retinal vessel tortuosity in premature infants for assessing retinopathy of prematurity (ROP). Seventy-three retinal images of premature infants were subjectively classified as having “Normal” or “Abnormal” tortuosity based on arterial tortuosity of the posterior retina by seven clinicians. The software then semi-automatically traced the same arteries within circular regions of interest centered at the optic disc, with radii ranging from 2 to 20x the disc diameters. Vessel tortuosity was defined as the sum of external angles at vessel bend points. Tortuosity was significantly greater in Abnormal images within regions exceeding 4-disc diameters (p < 0.05). Diagnostic accuracy improved with larger regions: the area under the curve increased from 0.56 at 2 times to 0.90 at 8 times and 1.00 at 14 times the diameter. At 14 times, corresponding approximately to Zone I, the software achieved 100% sensitivity and specificity for detecting abnormal tortuosity. The software accurately quantified vessel tortuosity especially within Zone I of the retina, which may reduce inter-observer variability and aid clinicians, including non-specialists, in the evaluation of ROP eyes.
Similar content being viewed by others
Introduction
Retinopathy of Prematurity (ROP) is a leading cause of blindness in premature infants, characterized by abnormal development of retinal vessels, which could progress to retinal detachment if left untreated1. Plus disease is an important feature indicating the exacerbation of ROP, which manifests as marked vessel tortuosity and dilation particularly in Zone I of the posterior pole1. However, the assessment of vessel tortuosity often relies heavily on subjective evaluations by clinicians, leading to variability and sometimes disagreement among observers.
In recent years, several approaches have been introduced to reduce inter-observer variability in diagnosing plus disease by objectively quantifying morphological features of the retinal vasculature, with particular focus on vascular tortuosity2,3,4,5,6,7. Well-known semi-automated programs such as ROPTool can automatically calculate the tortuosity index of retinal vessels within an operator-defined circular area centered at the optic disc using a radius equal to the disc-to-fovea distance3. However, the efficiency of such program is constrained by the image quality of the fundus photograph and the evaluation of vessel tortuosity is recommended to be determined from vessels in Zone I, which involve a much larger area1.
This study proposes a new vessel tracing software that quantitatively evaluates vessel tortuosity in a pre-defined region on a retinal image. The software allows objective assessment of tortuosity over a broader region of interest, capturing both central and peripheral vascular changes which could be useful in evaluating ROP eyes.
Methods
Study design and data selection
Retinal images of pre-term infants were acquired from two sources:
-
1.
A publicly available HVDROPDB dataset intended for ROP research8.
-
2.
RetCam Envision (Natus Medical Incorporated, USA) images from the University of Tokyo Hospital NICU.
Fifty retinal images from the HVDROPDB were acquired, of which 44 images were selected based on clarity and sufficient visualization of peripheral retinal vessels. These images were taken from infants with gestational ages ranging from 26 to 36 weeks and birth weights from 760 to 3000 g.8 Additionally, 29 images with well-defined vessels were obtained using RetCam at the University of Tokyo Hospital from infants born earlier than 34 weeks gestation and/or with birth weights less than 1800 g, resulting in a total of 73 ROP retinal images for analysis.
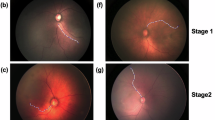
Seven ROP clinicians independently assessed the vasculature in each of the 73 images and categorized them as having either “Normal” or “Abnormal” tortuosity based on the arterial vessel tortuosity of the posterior retina. The image is classified as “Abnormal” if both arteries emerging directly from the optic disc in the upper and lower temporal quadrant had clinically significant vessel tortuosity. The classification followed the criteria for vascular tortuosity described in the International Classification of Retinopathy of Prematurity, Third Edition1. Due to the limited 130-degree view angle of the RetCam, only arteries in these two quadrants were evaluated. The same retinal arteries were then traced using the software to quantify vessel tortuosity.
Algorithm for Vascular Tortuosity Analysis.
-
1.
Step 1: defining a circular region of interest
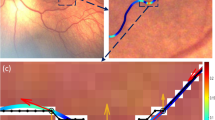
The operator manually selects the nasal and temporal edges of the optic disc on the retinal image as reference points, from which the program automatically calculates the diameter of the optics disc. The operator may select any multiplier of the disc diameter (ranging from 2x up to 20x) from which the program automatically generates concentric circles centered at the optic disc with a diameter equal to the selected multipliers of the disc diameter, marking the circular region of interest on the retina.
Multiple circular regions can be drawn simultaneously on a single retina image to facilitate broader vascular assessment (Fig. 1A). The disc diameter was used as the distance marker instead of disc-to-fovea distance, in contrast to ROPTool, as the fovea position was not sufficiently clear in some low-resolution images.
Algorithm for quantifying retinal vessel tortuosity using the software. (A) Circular regions of interest were automatically generated concentrically around the optic disc with diameters ranging from 2 to 20 times the optic disc diameter. (B) Retina arteries originating directly from the optic disc were semi-automatically traced within each region of interest in both the superior and inferior temporal quadrants. (C) Traced arteries were skeletonized to a single-pixel width and approximated into straight line segments, with bend points marked by red dots to indicate a change in vessel direction.
For landmarks, multiplier 8 approximated to a circular region with a radius of disc-to-fovea distance, while multiplier 14 approximated to Zone I of the retina, which has a radius of double the disc-to-fovea distance.
-
2.
Step 2: vessel selection and skeletonization
Retinal image from Step 1 was contrast-enhanced to better highlight the blood vessels in order to facilitate vessel selection and tracing9. The longest retinal artery emerging directly from the optic disc was traced from the disc to the edge of the circular region of interest (Fig. 1B). The tracing was terminated upon reaching the point where the arterial course became indistinct or extended beyond the photographic field. When branches of similar caliber were encountered, the branch going further temporally was selected to reduce intra-observer variability. The tracing was repeated for both upper and lower quadrants of the temporal retina. A semi-automated livewire segmentation algorithm was applied to assist accurate tracing of the blood vessels10.
-
3.
Step 3: curve approximation and Bend detection
Using a previously described method by Tan, which utilizes the Ramer-Douglas-Peuker algorithm to simplify curves into line segments, the traced vessels were “skeletonized” into one-pixel wide and then approximated into straight line segments with “bend” points marked as red dots (Fig. 1C)11.
-
4.
Step 4: vessel tortuosity calculation
The external angle was recorded at each major “bend points” (Fig. 2). Vessel tortuosity was defined as the sum of all recorded external angles (Θ) in a single traced vessel.
$${\text{Vessel Tortuosity }}\left( {{\text{degrees}}} \right)=\Theta _{{\text{1}}}+\Theta _{{\text{2}}}+\ldots+\Theta _{{\text{n}}}$$
The average vessel tortuosity was calculated from the mean tortuosity value of the two arteries traced in the upper and lower temporal quadrants of the retinal image. Average vessel tortuosity was calculated for each circular region of interest.
To assess the inter-grader repeatability of the proposed method, two independent graders (HPZ and TT) analyzed all 33 eyes which both the temporal vessels could be traced up to multiplier 14, which approximates to Zone I of the retina. The two graders used identical analysis protocols and the agreement between the two graders was evaluated using the interclass correlation coefficient, calculated using a two-way random effects model (added to lines of the revised “Methods” section).
Detailed step-by-step user instructions of the software, including screenshots, demonstration videos, and example output files, are provided in the Supplementary Material.
The executable binary and complete source snapshot (Windows 64-bit) of the software are publicly archived and freely available on Zenodo (v1.0, DOI: https://doi.org/10.5281/zenodo.15321286).
Statistical analysis
First, the average vessel tortuosity was compared between the Normal and Abnormal groups for each circular region of interest from 2x to 20x disc diameter. Secondly, the accuracy of the software in distinguishing between a Normal and Abnormal retina for each region of interest was evaluated. Finally, the threshold vessel tortuosity to define an Abnormal retina in each region of interest were determined.
Statistical analyses were performed using Python (version 3.10.12). Welch’s t-test was used for group comparisons with p < 0.05 considered statistically significant. Receiver Operating Characteristic (ROC) analysis was performed, and the area under the curve (AUC) was calculated to evaluate diagnostic accuracy. The 95% confidence interval (CI) of the AUC was estimated using a stratified bootstrap procedure (2000 iterations). Positive and negative samples were resampled with replacement within their respective groups to generate bootstrap datasets. The AUC was computed for each bootstrap sample, and the 2.5th and 97.5th percentiles of the resulting AUC distribution were used as the 95% CI based on the percentile method. Youden’s J statistic was used to identify the threshold vessel tortuosity12.
Ethical considerations
This study was approved by the Institutional Review Board of the University of Tokyo Hospital (approval number: 2217). All methods were performed in accordance with the relevant guidelines and regulations. All patient data were anonymized, and the requirement for individual informed consent was waived due to the retrospective design and the use of anonymized data.
Results
A total of 73 retinal images were collected, consisting of 44 images from the HVDROPDB dataset and 29 RetCam images from our institution. Of these, 45 images were initially classified as having Normal vessel tortuosity agreed by at least 6 out of 7 human evaluators, whereas 14 images were initially classified as having Abnormal vessel tortuosity. The remaining 14 borderline images, where more than 2 out of 7 evaluators disagreed, were discussed and reviewed among the evaluators. Following the discussion, 7 images were classified into the Normal group and 7 images were classified into the Abnormal group. In total, 52 images were classified as Normal and 21 images were classified as Abnormal.
The average tortuosity of each circular region of interest in the Normal and Abnormal groups is summarized in Table 1. Among the re-analyzed 33 eyes, the measurements of vascular tortuosity by the two graders showed an excellent level of agreement at multiplier 14, with an interclass correlation coefficient of 0.94 (95% confidence interval: 0.84–0.97). As expected, the average vessel tortuosity increased with the diameter of the region of interest due to the accumulation of bend points along the traced vessels. Analysis at multiplier 20 for the Abnormal group was limited due to inadequate peripheral image resolution, thereby limiting the number of data collected (as the vessel could not be further traced into multiplier 20). Overall, average vessel tortuosity was significantly higher in the Abnormal group across all regions of interest with a multiplier of 4 or greater (p < 0.05). At multiplier 2, the difference was not statistically significant (p = 0.277) (FIGURE 3).
Comparison of average retinal vessel tortuosity between the Normal and Abnormal groups. The horizontal axis represents the diameter of the circular region of interest defined as a multiplier of the optic disc diameter. A multiplier of 8 approximately corresponds to a circular region with its radius as the disc-to-fovea distance, while a multiplier 14 corresponds approximately to the boundary of Zone I in retinopathy of prematurity (ROP). The vertical axis shows the average tortuosity measured in degrees. Statistically significant differences in the average tortuosity between the two groups were observed at multipliers and above (* p < 0.05). Data at multiplier 20 were insufficient for analysis due to insufficient peripheral vessel image resolution.
The accuracy of the software in differentiating between a Normal and Abnormal retinal image was assessed by constructing ROC curves for each region of interest (Fig. 4). Table 2 summarizes the corresponding AUC, sensitivity, specificity, and the threshold vessel tortuosity value for defining an abnormal retina in each region. Starting at multiplier 2, the AUC was 0.56 with specificity of 94% but a low sensitivity of 22%. As the size of the region of interest increased, the AUC improved substantially, reaching 0.90 at multiplier 8 with 82% sensitivity and 98% specificity. By multiplier 14, the AUC reached 1.00. Analysis at multiplier 20 was not available due to insufficient data.
Receiver operating characteristic curves for diagnostic performance of the software on vessel tortuosity at each region of interest. Curves closer to the top-left corner indicate better diagnostic performance (sensitivity 100%, specificity 100%). Diagnostic accuracy was lower at multiplier 2 (AUC = 0.56), but substantially improved from multiplier 8 (AUC = 0.90) onwards, reaching ideal performance (AUC = 1.00) at multipliers of 14 and above. Data at multiplier 20 were insufficient for analysis due to insufficient peripheral vessel image resolution.
The threshold tortuosity value used to define an abnormal retina within each circular region of interest is shown in Table 2; Fig. 5. The results agreed with the ROC analysis, with a threshold vessel tortuosity value of 158.99 degrees at multiplier 8 (82% sensitivity and 98% specificity) and 253.71 degrees at multiplier 14 (100% sensitivity and specificity). Overall, the threshold tortuosity value increased with broader area of interest (Fig. 5). Analysis at multiplier 20 was not available due to insufficient data.
Threshold values for determining abnormal retinal vessel tortuosity in each regions of interest. The vertical axis represents the threshold tortuosity (degrees) distinguishing Normal and Abnormal groups, while the horizontal axis indicates the multiplier defining the diameter of the circular region of interest. Data at multiplier 20 were insufficient for analysis due to insufficient peripheral vessel image resolution.
Additionally, the 14 borderline images were re-evaluated by the software using the calculated threshold tortuosity value of 253.71 which defined an Abnormal image for the region 14x the disc diameter (corresponding to Zone I). Since many of the borderline images lacked image clarity of the peripheral retina, the operator was not able to trace the temporal retinal arteries up to 14x the disc diameter in 7 out of 14 images. When additional analysis was performed on the remaining 7 images, all results agreed with the human observers Table 3). The borderline images are available in the Supplementary Material.
Discussion
In this study we implemented a new vessel tracing software for quantifying vessel tortuosity in order to objectively assist the evaluation of ROP eyes.
Pre-existing programs such as ROPTool can also semi-automatically calculate vessel tortuosity within a smaller circular area around the optic disc with a radius of disc-to-fovea distance3. However, the latest revision of the International Classification of Retinopathy of Prematurity (ICROP) emphasizes the importance of a broader evaluation within Zone I, highlighting the need for techniques that can capture more peripheral changes beyond the posterior pole1.
Meanwhile, deep learning–based systems such as i-ROP and ROP.AI have demonstrated high accuracy in automated detection and classification of ROP5,6,7. However, artificial intelligence-based tools face multiple challenges, including the need for large-scale datasets, high-end computational resources, regulatory approvals, and seamless clinical integration.
Our software implements a different algorithm which the traced vessels are approximated into straight line segments and vessel tortuosity was defined as the sum of the external angles at each major “bend” points. The software also allows operators to expand the region of interest on a retinal image beyond Zone I, provided that peripheral vessels remain traceable.
Overall, our results indicated that smaller regions of interest, particularly up to multiplier 8, were less effective in detecting abnormal vessel tortuosity. This was likely due to the limited curvature of the arteries in these areas, where they have only just emerged from the optic disc. On the other hand, in broader regions of interest, particularly beyond multiplier 8, the software was more accurate in detecting abnormal tortuosity with better sensitivity and specificity. The region of interest used in the previously reported ROPTool corresponds approximately to the area covered by multiplier 8 of our software—a circular region centered at the optic disc with a radius equal to the fovea-to-disc distance3. Our results have shown that our software was equally effective in evaluating vessel tortuosity in this region compared to ROPTool. Moreover, both the sensitivity and specificity of the software reached 100% beyond multiplier 14, which correspond approximately to Zone I of the retina with a radius equal to two times the fovea-to-disc distance. In other words, our software was able to accurately identify abnormal vessel tortuosity up to the outer borders of Zone I, as recommended by ICROP1. Moreover, the high level of agreement observed between the two graders also suggests that the proposed method is less influenced by subjective judgment and provides a reproducible index for quantifying vascular tortuosity.
The software is reasonably accessible as the source codes and user instructions are provided. It is compatible with retinal images captured by any retinal camera, provided the image display sufficient vascular details, making it easy to integrate into clinical settings. The software can also be used by non-specialists, including neonatologists, to evaluate plus disease from a fundus image.
Limitations of this study include the relatively small sample size and variability in image quality. As the performance of the proposed software depends on image quality, its diagnostic utility may be limited in eyes with media opacity or poor-quality fundus photographs. Consequently, variability in image quality considerably affected the number of data points collected within each circular region of interest. Furthermore, due to the limited 130-degree field of view of the RetCam images, only the temporal retinal arteries were evaluated. Consequently, an image classified as “Abnormal” in the context of this study does not correspond directly to plus or pre-plus disease in clinical practice. Similarly, future updates should also include modules that assesses the venous dilation, another key feature of plus disease1.
Additionally, while the diagnostic accuracy of the software was promising, reproducibility in broader clinical settings, such as on other retinal diseases, has not yet been evaluated. Given its flexible design, future validation studies could expand its use beyond ROP to include not only other retinal vascular disorders, but also quantifying curvature of non-vascular structures such as retinal layers on an optical coherence tomography scan.
A novel vessel tracing software for quantifying vessel tortuosity in ROP eyes was implemented, aiming to reduce inter-observer variability in the evaluation of ROP. The software demonstrated high accuracy in detecting abnormal vessel tortuosity, especially within Zone I of the retina, which may support the diagnosis of plus disease. This tool may enhance diagnostic reliability and assist even non-specialists in the evaluation of ROP eyes.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chiang, M. F. et al. International classification of retinopathy of prematurity, third edition. Ophthalmology 128, e51–e68 (2021).
Abbey, A. M. et al. Evaluation of screening for retinopathy of prematurity by roptool or a Lay reader. Ophthalmology 123, 385–390 (2016).
Wallace, D. K., Zhao, Z. & Freedman, S. F. A pilot study using roptool to quantify plus disease in retinopathy of prematurity. J. AAPOS. 11, 381–387 (2007).
Zhang, J., Liu, Y., Mitsuhashi, T. & Matsuo, T. Accuracy of deep learning algorithms for the diagnosis of retinopathy of prematurity by fundus images: a systematic review and meta-analysis. J. Ophthalmol 8883946 (2021).
Brown, J. M. et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 136, 803–810 (2018).
Tan, Z. et al. Deep learning algorithm for automated diagnosis of retinopathy of prematurity plus disease. Transl Vis. Sci. Technol. 8, 23 (2019).
Bai, A. et al. Multicenter validation of deep learning algorithm ROP.AI for the automated diagnosis of plus disease in retinopathy of prematurity. Transl Vis. Sci. Technol. 12, 13 (2023).
Agrawal, R. et al. HVDROPDB datasets for research in retinopathy of prematurity. Data Brief. 52, 109839 (2024).
Pizer, S. M. et al. Adaptive histogram equalization and its variations. Comput. Vis. Graphic Image Process. 9, 355–368 (1987).
Barrett, W. A. & Mortensen, E. N. Interactive live-wire boundary extraction. Med. Image Anal. 1, 331–341 (1997).
Tan, J. H., Acharya, U. R., Chua, K. C., Cheng, C. & Laude, A. Automated extraction of retinal vasculature. Med. Phys. 43, 2311 (2016).
Akobeng, A. K. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 96, 644–647 (2007).
Funding
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number: 22K16963). The funder had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
Toyama and Zhou conceptualized and designed the work, performed the analysis, interpreted the data. Toyama implemented the new software used in the work. Zhou drafted the manuscript. Mihara, Nakajima, Fujino, Nagahara, Hayashi, and Sugimoto acquired the data used. Ueta conceptualized and supervised the work. All authors reviewed and approved the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, H.P., Toyama, T., Mihara, G. et al. A novel method of quantifying retinal vascular tortuosity in retinopathy of prematurity. Sci Rep 15, 45005 (2025). https://doi.org/10.1038/s41598-025-29065-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29065-4