Abstract
We developed a surface-electrode ion trap with a square hole measuring \(40\,\mathrm {\mu m}\) for atomic loading. The hole was fabricated using anisotropic etching of a silicon substrate and was designed to minimize potential distortion in the trapping region. By introducing the atomic beam through the hole, we achieved enhanced isotope selectivity and experimentally demonstrated the selective trapping of calcium isotope ions using an atomic oven. We successfully prepared isotope ion pairs directly from the oven via sympathetic cooling at a rate comparable to that achieved using ablation loading. The sympathetic cooling process occurred on the order of a few seconds. We demonstrated the direct generation of an ion chain above the through-hole. This approach can be applied for trapping a wide range of ion species using a remarkably simple experimental setup, making it desirable for several applications such as quantum-charge-coupled-device (QCCD) architectures and precision measurements of isotope shifts.
Similar content being viewed by others
Introduction
Surface-electrode ion traps are anticipated for the practical implementation of ion-trap quantum computers, particularly in the realization of a quantum-charge-coupled-device (QCCD) architecture1,2,3, because they enable realization of a variety of electrode layouts. One of the issues of surface-electrode ion traps is that atomic deposition on the electrodes induces patch potentials, generating electric-field noise that leads to ion heating4. This phenomenon presents a significant challenge for quantum computing and precision measurements. Furthermore, exposure to the atomic beam causes time-dependent degradation of the surface, leading to distortion of trapping potential and ion instability5. Therefore, suppressing trap surface contamination and mitigating time-dependent degradation induced by atomic deposition are significant objectives.
One approach to suppress surface contamination and time-dependent degradation involves placing the atomic source behind the trap and loading ions through a small aperture, a strategy proposed and demonstrated for surface-electrode ion traps6,7,8,9,10. In previous work10, a 2D magneto-optical trap (MOT) was used as a source of neutral atoms, and the loading time was made to be as fast as milliseconds for one ion. Such a loading method using MOT is sophisticated and requires several laser light sources and relatively large vacuum systems.
Another crucial aspect for realizing a QCCD architecture is the treatment of ion heating induced by transports. To avoid perturbing the internal states of ions used as qubits, a cooling mechanism that it does not rely on the internal transitions. Sympathetic cooling is well suited to this purpose because it does not involve qubit transition and has been successfully employed in various fields such as quantum operations and optical clocks11,12,13. These research efforts require specific isotope species, such as \(^{171}\textrm{Yb}^+\) and \(^{27}\textrm{Al}^+\). Therefore, isotope selectivity is a critical aspect of ion loading.
In this study, we developed a surface-electrode trap featuring a 40-\(\,\mathrm {\mu m}\) square through-hole. Surface-electrode traps with through-holes are generally used to prevent contamination of the trap surface caused by atomic deposition by introducing the atomic beam from the back side. The loading scheme can be exploited to enhance the isotope selectivity of resonant photoionization by illuminating the vertically propagating atomic beam with a horizontally directed ionization laser. We experimentally verified high isotope selectivity and enabled selective loading of different ion species. The hole was fabricated by anisotropic etching to create a structure that suppresses the electric field noise that arises from the build-up of charge on the sidewalls of the hole14,15.
An atomic oven, used as a source, is placed on the backside of the electrode behind the hole, thereby minimizing atomic contamination on the electrode surface. Atoms are loaded through this hole, ionized and cooled by laser beams covering the entire beam size. This loading method suppresses contamination and achieves a high selectivity of ion species to be loaded. The loading of initially hot ions is accomplished via sympathetic cooling with preloaded coolant ions, which significantly reduces the required experimental resources. The loading of initially hot ions via sympathetic cooling using an ablation laser and blade-type traps has been reported16,17. By contrast, we loaded initially hot ions via sympathetic cooling with an atomic oven and a surface-electrode trap. Although loading with a resistive oven generally requires more time than loading with an ablation laser, we find that we could load pairs of ions, with \(^{40}\textrm{Ca}^+\) (\(^{44}{\textrm{Ca}}^+\)) as the coolant ions and \(^{44}\textrm{Ca}^+\) (\(^{40}\textrm{Ca}^+\)) as the corresponding sympathetically cooled ions, in a comparable time with the ablation method17. This approach allows for simple and compact experimental apparatus, desirable for various applications such as QCCD architectures and precision measurements of isotope shifts18.
Experimental apparatus
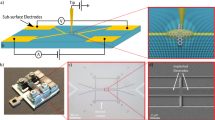
Overall structure of the trap. (a) Electrode arrangement and sizes are shown. Two RF electrodes, four End DC electrodes, six Middle DC electrodes, and one Center DC electrode are symmetrically arranged. (b) Image of the through-hole. The hole is located at the center of the trap and has a square shape with a side length of 40 \(\mathrm {\,\mu m}\). The Ca beam is introduced through this hole.
In this section, we detail the trap structure and experimental setup. The trap consists of two RF and eleven DC electrodes. The DC electrodes include four End, six Middle and one Center electrodes, named after their locations in the trap layout (Fig. 1a). A 40-\(\mathrm {\mu m}\) square hole for atom introduction is fabricated at the center of the Center electrode (Fig. 1b). All electrodes are gold plated and adhere to the Si substrate. The calcium oven is positioned 3 mm below the trap. The calcium source consists of a stainless steel tube with an outer diameter of 1.26 mm and an inner diameter of 0.9 mm. One end of the tube is sealed by threading a screw into it, and the opposite open end serves as the exit for the atomic beam. The screw overlaps with the tube by 2 mm, forming the base of the oven. The screw acts as the ground electrode, and a wire is welded to the opposite end of the stainless steel tube to apply a current ranging from 1.5–3.6 A. The estimated oven temperature, inferred from vacuum conditions, is in the range of 480–550 K.
Shaped through-hole
The hole placed at the center electrode had two functions: to introduce an atomic beam from the backside of the trap electrode to prevent accumulation of contamination on the electrode surface, and to collimate the atomic beam for obtaining high-resolution spectra in the photo-ionization transition. When determining the size of the through-hole, we performed numerical calculations of the trapping potential with the existence of a hole at the center electrode. We did not find any substantial potential distortion at the ion height of about \(200\,\mathrm {\mu m}\) above the electrode surface, provided that the hole size was less than 60-\(\mathrm {\mu m}\) square. In this case, the difference in the axial (z-direction) potential depth with and without the hole was suppressed to less than \(4\,\textrm{mV}\), corresponding to below \(0.6\%\) of the z-direction potential depth of \(0.67\,\textrm{V}\). On the other hand, if the size is too small, atoms cannot be loaded efficiently. Finally, we decided the hole size to be \(40\,\mathrm {\mu m}\) square.
Through the hole, the atomic beam is collimated, which allows us to obtain good isotope selectivity. Owing to the geometric relationship between the oven and through-hole, the atomic beam is collimated and expected to have a divergence angle of approximately 0.76\(^{\circ }\).
To avoid the effects of charging on the substrates, the trap structure must be designed such that the trapped ions are not exposed to the insulators15. Therefore, we designed a through-hole that has a square pyramid shape underneath the trap electrode. Owing to the characteristics of Si crystals, a \(54.7^\circ\)-sloped hole can be realized in a (100)-oriented Si substrate via anisotropic etching of the silicon substrate.
The trap was fabricated at the National Institute of Communications and Technology, Tokyo, Japan. The structure of the through-hole is shown in Fig. 2(a). For the trap, a 22-\(\mathrm {\mu m}\)-thick \(\hbox {SiO}_2\) layer was fabricated on the top of a silicon substrate using the direct bonding technique to ensure a flat surface. The surface electrodes were patterned using photolithography and electroplating. Anisotropic etching was then performed from the backside of the substrate using Tetramethylammonium Hydroxide (TMAH) solution (25%) with alkaline-resistant photoresist as a mask material. The \(\hbox {SiO}_2\) layer served as an etch stop. Finally, a hole was made in the \(\hbox {SiO}_2\) layer using the focused ion beam (FIB) method. Fig. 2(b) shows a scanning electron microscope (SEM) image of the hole taken from the backside of the trap electrode.
(a) Design of the through-hole. The Si base forms a square pyramid shape via anisotropic etching. The ionization beams comprise a 423-\(\textrm{nm}\) external cavity diode laser (ECDL) beam and a free-running 375-\(\textrm{nm}\) laser beam. After being collimated by the through-hole structure, the calcium beam crosses the ionization beams, which propagate parallel to the trap surface, thereby loading ions. (b) SEM image of the hole taken from the backside.
Experimental setup
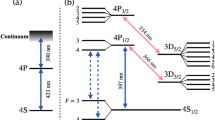
The experimental setup consists of four laser sources: 423-\(\textrm{nm}\) external cavity diode laser (ECDL), free-running 375-\(\textrm{nm}\) laser, 397-\(\textrm{nm}\) ECDL, and 866-\(\textrm{nm}\) ECDL (Fig. 3a). As described in Fig. 2(a), calcium atoms are ionized by the intersection of the 423-\(\textrm{nm}\) and 375-\(\textrm{nm}\) lasers above the through-hole.
Once the calcium ions are loaded, they are cooled via Doppler cooling. The 397-\(\textrm{nm}\) laser drives the \(^2S_{1/2} \longrightarrow \,^2P_{1/2}\) transition, and the 866-\(\textrm{nm}\) laser serves as a repumper for the \(^2D_{3/2} \longrightarrow \,^2P_{1/2}\) transition. During this process, all laser beams overlap and propagate along the z-axis. An additional 397-nm laser is sent at a 45\(^\circ\) angle to the z-axis using a half-mirror.
For observation, ion images in the trap are captured using an image intensifier and a Complementary Metal Oxide Semiconductor (CMOS) camera (Fig. 3b). The fluorescence intensity is measured with a photomultiplier tube (PMT) (Fig. 3a).
(a)Schematic of the ion fluorescence detection system and laser setup. Ions introduced into the trap are cooled by the 397-\(\mathrm {nm\,}\) and 866-\(\mathrm {nm\,}\) lasers incident along the z-axis, as well as by a 397-\(\mathrm {nm\,}\) laser incident at a 45\(^{\circ }\) angle to the z-axis. The 397-\(\mathrm {nm\,}\) lasers are split into two paths by a half mirror to irradiate from two directions. (b) Image of an ion trapped above the through-hole.
Experimental results
We trapped \(^{44}{\textrm{Ca}}^+\) and \(^{40}{\textrm{Ca}}^+\) ions selectively using naturally occurring calcium isotopes. The natural abundances of \(^{40}{\textrm{Ca}}\) and \(^{44}{\textrm{Ca}}\) are 96.9\(\%\) and 2.4\(\%\), respectively. We then loaded initially hot ions by sympathetic cooling. In our experiments, either of the two isotopes (\(^{44}{\textrm{Ca}}^+\) or \(^{40}{\textrm{Ca}}^+\)) could serve as the coolant and sympathetically cooled ion.
Isotope selective trap
Cross-beam spectroscopy. (a) Measurement results of the \(^{40}\textrm{Ca}^+\) spectrum. The red curve represents the best-fit Voigt function. (b) Measurement results of the \(^{44}\textrm{Ca}^+\) spectrum. The red curve shows a Voigt function fitted with physically reasonable parameter constraints. The isotope shift of \(^{44}\textrm{Ca}^+\) is approximately 757 MHz.
We measured the cross-beam spectrum of a calcium atomic beam using a 423-nm laser to investigate isotope selectivity. Based on the isotope shift values reported in19, we scanned the ionization laser from detunings of \(-250\) MHz to \(+950\) MHz, taking the resonance of \(^{40}\textrm{Ca}^+\) as the zero point. The resulting spectra near the \(^{40}\textrm{Ca}^+\) and \(^{44}\textrm{Ca}^+\) resonances are shown in Fig. 4. To analyze these data, we employed the Voigt function, which is the convolution of Lorentzian and Gaussian functions. The Gaussian component corresponds to Doppler broadening caused by the horizontal velocity of the atomic beam, enabling us to deduce the divergence angle of the beam. The Lorentzian component includes the natural linewidth of calcium (approximately 35.4 MHz20), as well as power broadening and transit-time broadening, both estimated from our experimental conditions21. The transit-time broadening depends on the length of time the atoms spend in the laser beam, which is determined by the beam velocity and diameter of the laser beam.In this experiment, the oven was supplied with a current of 3.54 A, and according to vapor pressure curve22, corresponding to a temperature of 530 K. Under these conditions, the most probable velocity of the atoms is roughly 573 m/s. The ionization laser used in the measurement had a power of 50 \(\mathrm {\mu }\)W with a beam diameter of 250 \(\mu\)m, while the saturation intensity of the transition is \(6.11\times 10^2\,\mathrm {W/m^2}\)21. From these parameters, we estimated the relative contributions of power broadening and transit-time broadening and then fitted the observed spectrum with the Voigt function. The Lorentzian and Gaussian widths were 60.1(1.17) MHz and 50.2(1.00) MHz, respectively, yielding a beam divergence angle of 2.54(0.05)\(^\circ\). This angle is greater than the predicted value (\(0.76^{\circ }\)) corresponding to the divergence angle from the center of the oven. The angle obtained from the measurement data is considerably larger than the predicted value, likely because atoms are emitted not only from the center but also from other regions of the oven. Nevertheless, based on the measured angle, the calcium atomic beam is emitted radially from an atomic source with a diameter of 93 \(\upmu\)m.
The atomic beam was confirmed to be collimated. The intensity peak at 750 MHz (Fig. 4b) was attributed to \(^{44}{\textrm{Ca}}\) because the ratio of the fluorescence peak to the natural abundance ratio is similar for Fig. 4(a) and (b). The Doppler broadening was significantly smaller than the isotope shift, indicating high isotope selectivity. From these results and other isotope shift values19, we trapped \(^{40}{\textrm{Ca}}^+\) and \(^{44}{\textrm{Ca}}^+\) selectively by appropriately detuning each laser. It took approximately 20 s to trap \(^{40}{\textrm{Ca}}^+\) and 30 s to trap \(^{44}{\textrm{Ca}}^+\) owing to the significant difference in their natural abundance.
Sympathetic cooling experiment
Guggemos et al.16 trapped and detected hot ions via sympathetic cooling in a linear Paul trap. In the same manner, we trapped hot isotope ions via sympathetic cooling using selectively trapped ions. The experimental setup is simplified owing to the use of isotope ions. The experimental procedure (Fig. 5) is as follows: i) We trap \(^{40}{\textrm{Ca}}^+\) as a coolant ion via Doppler cooling. ii) We detune the 423-\(\textrm{nm}\) laser by 750\(\mathrm {\,MHz}\) and introduce \(^{44}{\textrm{Ca}}^+\) above the trap. iii) We detune the 397-\(\textrm{nm}\) laser by 842\(\mathrm {\,MHz}\) and the 866-\(\textrm{nm}\) laser by \(-4495\mathrm {\,MHz}\) to identify the sympathetically cooled ion species. The \(^{44}{\textrm{Ca}}^+\) ions introduced in Step ii) require almost no cooling because the 397-\(\textrm{nm}\) and 866-\(\textrm{nm}\) lasers are tuned for cooling the \(^{40}{\textrm{Ca}}^+\) ions; therefore, if we can trap \(^{40}{\textrm{Ca}}^+\) ions, it implies that hot \(^{44}{\textrm{Ca}}^+\) ions are trapped by sympathetic cooling. Because the surface-electrode trap has a shallow potential, we thought that the atomic beam and coolant ions would collide and the coolant ions would escape; however, we could trap hot \(^{44}{\textrm{Ca}}^+\) ions by sympathetic cooling (Fig. 5).
Experimental procedure and results of the sympathetic cooling experiment. After loading the coolant ions and trapping the hot ions through sympathetic cooling, the species of the sympathetically cooled ions was identified via Doppler cooling. The upper panels show the schematics, the middle panels show the operational settings of the lasers, and the lower panels show the ion images. Only one 397-\(\textrm{nm}\) and one 866-\(\textrm{nm}\) laser were used. During the detuning stage before detection, it was observed that when \(^{40}{\textrm{Ca}}^+\) were the coolant ions, they escaped due to laser heating, whereas when \(^{44}{\textrm{Ca}}^+\) were the coolant ions, they were not affected by laser heating but often escaped during the few seconds while the 397-\(\textrm{nm}\) laser frequency was far from the resonance frequency of \(^{44}{\textrm{Ca}}^+\) and \(^{40}{\textrm{Ca}}^+\) ions. In the middle panels, the arrows indicate which isotope (\(^{40}{\textrm{Ca}}^+\) or \(^{44}{\textrm{Ca}}^+\)) the laser frequency targets.
Sympathetically cooled ions do not fluoresce; therefore, their presence was inferred from the displacement of the coolant ions. However, because a natural atomic source was used, other isotopic ions could get trapped as well. Therefore, in Step iii), we identified the species of the trapped ion. In the same manner, we were able to trap hot \(^{40}{\textrm{Ca}}^+\) ions via sympathetic cooling using \(^{44}{\textrm{Ca}}^+\) as the coolant ion. In this case, \(^{40}{\textrm{Ca}}^+\) ions were trapped via sympathetic cooling and detected, even though they were subjected to laser heating owing to a small isotope shift in the red direction (842\(\mathrm {\,MHz}\)). In both cases, we conducted control experiments without preparing the coolant ions and confirmed that hot ions could be trapped via sympathetic cooling.
We performed experiments in which \(^{40}\textrm{Ca}^+\) and \(^{44}\textrm{Ca}^+\) served as the coolant ions to trap \(^{44}\textrm{Ca}^+\) and \(^{40}\textrm{Ca}^+\), respectively. The time-lapse images of the results are shown in Fig. 6. We conducted an experiment to directly create isotope-ion chains on the through-hole by selectively trapping isotope ions solely via sympathetic cooling. Figure 7 shows the results of generating ion chains by loading \(^{40}\textrm{Ca}^+\) ions via sympathetic cooling when two \(^{44}\textrm{Ca}^+\) ions acted as coolant ions.
Time-lapse images obtained by performing the same experiment continuously. The experiment is the one shown in Fig. 5, as well as the case where the roles of the coolant ion (LC) and sympathetically cooled ion (SC) are reversed. Except for the initially loaded \(^{40}\textrm{Ca}^+\) ions, all ions were loaded via sympathetic cooling. Note that the positions of \(^{40}\textrm{Ca}^+\) and \(^{44}\textrm{Ca}^+\) ions hop.
Extended version of the experiment shown in Fig. 6, where two \(^{44}\textrm{Ca}^+\) ions were used as coolant ions (LC) and \(^{40}\textrm{Ca}^+\) ions were selectively loaded, thereby generating various isotope ion chains. Except for the initial two \(^{44}\textrm{Ca}^+\) ions, all ions were loaded via sympathetic cooling. The region labeled (1) indicates the maximum number of ions that can be crystallized. In region (2) and beyond, one of the \(^{44}\textrm{Ca}^+\) ions (LC) escaped, resulting in a state where multiple \(^{40}\textrm{Ca}^+\) ions (SC) were trapped with only a single \(^{44}\textrm{Ca}^+\) ion acting as the coolant ion.
From region (1) in Fig. 7, the maximum number of \(^{40}\textrm{Ca}^+\) ions that can crystallize with two \(^{44}\textrm{Ca}^+\) ions was found to be four. In region (2), more than four \(^{40}\textrm{Ca}^+\) ions are loaded together with two \(^{44}\textrm{Ca}^+\) ions, resulting in the dominance of modes that cannot be cooled by sympathetic cooling. Consequently, the crystal melts, and one of the \(^{44}\textrm{Ca}^+\) ions eventually escapes. Mao et al.23 demonstrated that by optimizing the positions of the coolant ions, two coolant ions can efficiently cool up to fourteen sympathetically cooled ions to the Doppler limit. Therefore, precise control of the coolant ion positions could enable the loading of a longer isotope ion chain. In our experiment, no intentional operations, such as swapping ions, were performed. Nonetheless, the ion fluorescence images in Fig. 7 indicate that unintended hopping occurred frequently, preventing control of the coolant ion positions. Consequently, the number of ions that could be cooled was fewer than that in Ref.23. We believe that our vacuum condition (\(8.67\times 10^{-8}\,\)Pa), which increases ion heating, was a major factor contributing to ion hopping. In addition, as described in Discussion and Conclusion, potential distortion caused by the through-hole may also play a role. A previous study on Paul linear traps reported that ion hopping became more pronounced when the potential anisotropy was high24.
In the studies by Guggemos et al. and Sun et al.16,17, atoms were introduced via an ablation laser, and owing to the large initial energy and incidence angle, the time required to cool a hot ion via sympathetic cooling was approximately 52 seconds. By contrast, by using an atomic oven, we were able to cool a hot ion via sympathetic cooling on the order of a few seconds. Although it takes about 30 s to trap \(^{44}\textrm{Ca}^+\) ions by Doppler cooling, it requires only 30–35 s to trap \(^{44}\textrm{Ca}^+\) ions via sympathetic cooling. Because the atoms of the oven are continuously supplied, precisely measuring the sympathetic cooling time is difficult. In any case, laser cooling allows ion loading within approximately 20 s for \(^{40}\textrm{Ca}^+\) and 30 s for \(^{44}\textrm{Ca}^+\) ions. By contrast, when using only sympathetic cooling, ions can be loaded within approximately 25 s for \(^{40}\textrm{Ca}^+\) and 35 s for \(^{44}\textrm{Ca}^+\) ions. Therefore, ion pairs can be prepared in about 1 min.
Discussion and conclusion
In this study, we conducted experiments on selective ion generation and sympathetic cooling of isotope ions using a surface-electrode ion trap with a 40-\(\mathrm {\mu m}\) square hole for atomic loading. Rotating the principal axis of the trapping potential enables efficient laser cooling using only horizontally propagating lasers25,26. In our experiment, we applied asymmetric DC voltages to rotate the principal axis. However, we found that even with symmetrically applied DC voltages, where the principal axis is theoretically not rotated, ions could still be trapped and cooled using only horizontally propagating lasers. One possible explanation for this phenomenon is that the principal axis of the potential locally rotates near the through-hole. To investigate this, we measured the ion position and trapping frequencies using forced oscillation in the vicinity of the hole. The results showed that both the ion position and trapping frequencies exhibited linear variations up to the top of the hole. However, directly above the hole, while \(\omega _z\) continued to change continuously, the forced oscillation of \(\omega _x\) became undetectable within a certain region. This suggests that despite our design considerations to minimize the influence of the hole on the trapping potential, the presence of the hole introduced potential disturbances. These disturbances likely caused a localized rotation of the principal axis. Iqbal and Nizamani et al.25 reported that if the principal axis rotates by more than 6 degrees within the trapping region, laser cooling can still be sufficiently effective. Our numerical calculations confirmed that, in the absence of hole-induced local axis rotation, an asymmetric DC voltage application could tilt the principal axis by more than 6 degrees, making stable ion trapping feasible even if the hole did not induce local rotation.
We investigated the selective trapping of isotope ions and found that collimating the atomic beam through the through-hole effectively suppressed Doppler broadening. The fluorescence spectrum shown in Fig. 4 confirms high isotope selectivity. In an experiment using a conventional horizontal atomic oven without an aperture, where a 423-nm ionization laser was tuned to \(^{44}\textrm{Ca}^+\) resonance, we frequently observed \(^{40}\textrm{Ca}^+\) ions getting trapped instead. By contrast, when using the through-hole, only about 14% of the cases resulted in \(^{40}\textrm{Ca}^+\) ions getting trapped first. This was attributed to the slight overlap between the tail of the \(^{40}\textrm{Ca}^+\) fluorescence spectrum and the resonance frequency of \(^{44}\textrm{Ca}^+\) ions, as shown in Fig. 4(a), combined with the high natural abundance of \(^{40}\textrm{Ca}\) (96.9%).
Based on the selective trapping experiment, it took approximately 30 s to trap \(^{44}\textrm{Ca}^+\) ions. Therefore, in the sympathetic cooling experiment, we kept the oven on for 30 s to load the ions. As a result, \(^{44}\textrm{Ca}^+\) ions were trapped via sympathetic cooling, and a few seconds after turning off the oven, the position fluctuations of \(^{40}\textrm{Ca}^+\) stabilized. This suggests that the cooling time of \(^{44}\textrm{Ca}^+\) ions was on the order of a few seconds, which is one to two orders of magnitude longer than the theoretical estimate based on the numerical calculations by Guggemos et al.16. The main factors contributing to this discrepancy are likely the nonisotropic nature of the trapping potential and the higher-than-expected initial ion energy. Guggemos et al. reported cases where the experimental cooling time was approximately three orders of magnitude longer than the simulation results, concluding that the difference in the initial energy was a significant factor.
Future research directions include evaluating the cooling temperature of the ion trapping via sympathetic cooling. One critical issue is that when attempting to observe \(^{44}\textrm{Ca}^+\), adjusting the cooling laser wavelength causes \(^{40}\textrm{Ca}^+\) ions to escape, preventing the simultaneous observation of both isotope ions. This issue could be resolved by introducing an additional laser capable of exciting and detecting both \(^{44}\textrm{Ca}^+\) and \(^{40}\textrm{Ca}^+\) ions simultaneously. Future prospects include trapping isotope ions other than \(^{44}\textrm{Ca}^+\), designing a trap with an X-junction structure27, and implementing low-heating transport methods that combine sympathetic cooling with transport techniques. Unintended hopping within ion chains during quantum operations is a significant challenge24. Addressing this issue will require improvements in trapping potential anisotropy, laser parameters, and other experimental conditions.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Wineland, D. J. et al. Experimental issues in coherent quantum-state manipulation of trapped atomic ions. J. Res. Natl. Inst. Stand. Technol. 103, 259–328. https://doi.org/10.6028/jres.103.019 (1998).
Kielpinski, D., Monroe, C. & Wineland, D. J. Architecture for a large-scale ion-trap quantum computer. Nature 417, 709–711. https://doi.org/10.1038/nature00784 (2002).
Pino, J. M. et al. Demonstration of the trapped-ion quantum ccd computer architecture. Nature 592, 209–213. https://doi.org/10.1038/s41586-021-03318-4 (2021).
Turchette, Q. A. et al. Heating of trapped ions from the quantum ground state. Phys. Rev. A 61, 063418. https://doi.org/10.1103/PhysRevA.61.063418 (2000).
DeVoe, R. G. & Kurtsiefer, C. Experimental study of anomalous heating and trap instabilities in a microscopic \(\small ^{137}\rm Ba\) ion trap. Phys. Rev. A 65, 063407. https://doi.org/10.1103/PhysRevA.65.063407 (2002).
Amini, J. M. et al. Toward scalable ion traps for quantum information processing. New J. Phys. 12, 033031. https://doi.org/10.1088/1367-2630/12/3/033031 (2010).
Moehring, D. L. et al. Design, fabrication and experimental demonstration of junction surface ion traps. New J. Phys. 13, 075018. https://doi.org/10.1088/1367-2630/13/7/075018 (2011).
Doret, S. C. et al. Controlling trapping potentials and stray electric fields in a microfabricated ion trap through design and compensation. New J. Phys. 14, 073012. https://doi.org/10.1088/1367-2630/14/7/073012 (2012).
Guise, N.D. et al. Ball-grid array architecture for microfabricated ion traps. Journal of Applied Physics 117, 174901, https://doi.org/10.1063/1.4917385 (2015). https://pubs.aip.org/aip/jap/article-pdf/doi/10.1063/1.4917385/19760134/174901_1_online.pdf.
Moses, S. A. et al. A race-track trapped-ion quantum processor. Phys. Rev. X 13, 041052. https://doi.org/10.1103/PhysRevX.13.041052 (2023).
Wang, P. et al. Single ion qubit with estimated coherence time exceeding one hour. Nat. Commun. 12, 233, https://doi.org/10.1038/s41467-020-20330-w (2021).
Pogorelov, I. et al. Compact ion-trap quantum computing demonstrator. PRX Quantum 2, 020343. https://doi.org/10.1103/PRXQuantum.2.020343 (2021).
Brewer, S. M. et al. \(\small ^{27}\rm {Al}^{+}\) quantum-logic clock with a systematic uncertainty below \(\small {10}^{-18}\). Phys. Rev. Lett. 123, 033201. https://doi.org/10.1103/PhysRevLett.123.033201 (2019).
Hong, S. et al. A new microfabrication method for ion-trap chips that reduces exposure of dielectric surfaces to trapped ions. J. Microelectromech. Syst. 27, 28–30. https://doi.org/10.1109/JMEMS.2017.2772310 (2018).
Jung, C. et al. A microfabricated ion trap chip with a sloped loading slot to minimize exposing trapped ions to stray charges. Quantum Sci. Technol. 6, 044004. https://doi.org/10.1088/2058-9565/ac107d (2021).
Guggemos, M., Heinrich, D., Herrera-Sancho, O. A., Blatt, R. & Roos, C. F. Sympathetic cooling and detection of a hot trapped ion by a cold one. New J. Phys. 17, 103001. https://doi.org/10.1088/1367-2630/17/10/103001 (2015).
Sun, C. et al. Efficiently loading \(\small ^{40}\rm {Ca}^+\)–\(\small ^{27}\rm {Al}^+\) ion crystal using sympathetic cooling and pulsed laser ablation. Rev. Sci. Instrum. 93, 113201–113201 (2022).
Knollmann, F. W., Patel, A. N. & Doret, S. C. Part-per-billion measurement of the \(\small 4^{2}{S}_{1/2}\rightarrow 3^{2}{D}_{5/2}\) electric-quadrupole-transition isotope shifts between \(\small ^{42,44,48}{\rm Ca}^{+}\) and \(\small ^{40}{\rm Ca}^{+}\). Phys. Rev. A 100, 022514. https://doi.org/10.1103/PhysRevA.100.022514 (2019).
Lucas, D. M. et al. Isotope-selective photoionization for calcium ion trapping. Phys. Rev. A 69, 012711. https://doi.org/10.1103/PhysRevA.69.012711 (2004).
Kramida, A., Yu. Ralchenko, Reader, J. & and NIST ASD Team. NIST Atomic Spectra Database (ver. 5.11), [Online]. Available: https://physics.nist.gov/asd [2017, April 9]. National Institute of Standards and Technology, Gaithersburg, MD. (2023).
Foot, C.J. Atomic physics. Oxford master series in atomic, optical, and laser physics Oxford University Press, Oxford, (2004).
Honig, R. E. & Kramer, D. A. Vapor pressure data: The solid and liquid elements. RCA Rev. 30, 285–305 (1969).
Mao, Z.-C. et al. Experimental realization of multi-ion sympathetic cooling on a trapped ion crystal. Phys. Rev. Lett. 127, 143201. https://doi.org/10.1103/PhysRevLett.127.143201 (2021).
Liang, J. & Haljan, P. C. Hopping of an impurity defect in ion crystals in linear traps. Phys. Rev. A 83, 063401. https://doi.org/10.1103/PhysRevA.83.063401 (2011).
Iqbal, Q. & Nizamani, A. H. Scalable surface ion trap design for magnetic quantum sensing and gradiometry. Phys. Open 19, 100208. https://doi.org/10.1016/j.physo.2024.100208 (2024).
Romaszko, Z. D. et al. Engineering of microfabricated ion traps and integration of advanced on-chip features. Nat. Rev. Phys. 2, 285–299. https://doi.org/10.1038/s42254-020-0182-8 (2020).
Lekitsch, B. et al. Blueprint for a microwave trapped ion quantum computer. Sci. Adv. 3, e1601540. https://doi.org/10.1126/sciadv.1601540 (2017).
Acknowledgements
This work was supported by JST Moonshot R & D (Grant Number JPMJMS2063).
Funding
This work was supported by JST [Moonshot R&D][Grant Number JPMJMS2063].
Author information
Authors and Affiliations
Contributions
M.M. contributed equally to the conceptualization and methodology, led the data curation and validation, and took the lead in writing the original draft and in reviewing and editing the manuscript. K.F. and N.S. fabricated the ion trap used in this research, contributed to writing the section on the shaped through-hole, and participated in the review and editing process. T.H. contributed equally to software development and was involved in reviewing and editing the manuscript. K.H. was equally involved in developing the methodology, providing resources, and software development, and also took part in reviewing and editing. U.T. contributed equally to the conceptualization, methodology, and resource provision, led project administration and supervision, and participated in funding acquisition and manuscript review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Miyamoto, M., Higuchi, T., Furusawa, K. et al. Isotope-selective Ion trapping via sympathetic cooling using a surface-electrode trap with a hole for collimated atomic loading. Sci Rep 16, 300 (2026). https://doi.org/10.1038/s41598-025-29512-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29512-2