Abstract
The activation of neutrophils by cytokines and cell-free DNA might precede hemoconcentration in dengue. Then, the neutrophil extracellular traps (NETs) and the neutrophils in the peripheral blood mononuclear cell (PBMC) fraction, referred to as low-density granulocytes (LDGs), were explored. The blood samples at 5 days of fever from patients with dengue were collected and categorized into dengue without and with warning signs (DWoWS and DWWS). Meanwhile, the patients with hemoconcentration were excluded. The NET-associated parameters, as determined by serum cell-free DNA and the fluorescent staining in the polymorphonuclear (PMN) fraction, in the DWWS group (n = 31) were higher than in DWoWS (n = 40). Meanwhile, complete blood count, serum cytokines (IFN-α, TNF-α, and IL-6), and LDGs were not different. The extracellular traps (ETs), stained by neutrophil elastase (NE) and myeloperoxidase (MPO), in the PBMC fraction of the DWWS group were higher than in DWoWS, while the ETs were non-detectable in healthy volunteers. The recombinant IFN-α and cell-free DNA reduced the density of regular neutrophils isolated from healthy volunteers into LDGs (measured by gradient separation) and also induced NETs (evaluated by NE and MPO co-expression). Meanwhile, CD66b (an adhesion molecule) and apoptosis in these LDGs were more prominent than in regular neutrophils. The NETs and LDGs presented in blood samples of patients with dengue might be due to the activation by IFN-α and cell-free DNA. However, only NETs could differentiate DWWS from DWoWS. Further studies to use NETs and LDGs in clinical practice are interesting.
Similar content being viewed by others
Introduction
Dengue is an important mosquito-borne infection with a wide spectrum of manifestations ranging from benign febrile illness to a serious infection with multiple organ failure1,2. The dengue incubation period is approximately 4–5 days after the mosquito bites, and severe dengue with platelet dysfunction and vascular leakage commonly occurs between the 3rd and the 7th day from the fever onset1,2. Viremia usually begins before or at the onset of fever and peaks during the febrile phase (the 1st to 3rd day) before progressing into the critical phase with hemoconcentration (the 3rd to the 7th day) and recovering after the 7th day (the recovery phase)3. Hemoconcentration is a rise in hematocrit (Hct) of more than 20% (at least 15%) caused by the leakage of plasma from damaged blood vessels that frequently combines with thrombocytopenia and is used to differentiate severe from non-severe dengue4. Notably, hemoconcentration from low fluid intake and profuse vomiting is correctable by adequate rehydration without the evidence of plasma leakage by ultrasonography or radiography (ascites and pleural effusion)4. Although not all patients with dengue develop hemoconcentration, its presence indicates a critical phase of the illness that requires close monitoring and management5. The hemoconcentration, platelet dysfunction, and vascular leakage are associated with the warning signs, including abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation (ascites or clinical pleural effusion), mucosal bleeding, lethargy or restlessness, liver enlargement, and increasing Hct6. The concurrent increase in Hct with a rapid decrease in platelet count indicates the worsening vascular permeability and plasma leakage that are important clinical observations for severe dengue6. Although hemoconcentration with thrombocytopenia indicates severe dengue (prominent plasma leakage, shock, respiratory distress, and organ impairment)7, the prediction of severe hemoconcentration will be beneficial for a prompt preparation of the supportive care. Because the mechanisms of dengue-induced hemoconcentration and vascular injury are correlated with pro-inflammatory cytokines and innate immune cells (neutrophils and monocytes)8,9,10, innate immunity parameters are interesting as dengue biomarkers. Notably, data on innate immunity in dengue are still less different from the established impact of adaptive immunity (antibody-dependent enhancement9 and circulating immune complexes11).
Indeed, neutrophils are the most abundant innate immune cells in humans that are responsible for the control of viral abundance, especially at the early phase of infection12. A high percentage of neutrophils (neutrophilia) can be found in the early febrile phase of dengue, which predominates in the first 5 days of fever. Subsequently, a decrease in neutrophil count (neutropenia) with a corresponding increase in lymphocytes is demonstrated as the disease progresses into the critical and recovery phases13. However, activated neutrophils have been associated with severe dengue. Additionally, neutrophils neutralize viruses through several mechanisms, including phagocytosis, degranulation of antimicrobial proteins, respiratory burst, secretion of cytokines and reactive oxygen species (ROS), and neutrophil extracellular traps (NETs)12. Accordingly, NETs are the release of extracellular DNA networks that are initiated by activated citrullinated histone H3 through peptidyl arginine deiminase 4 (PAD4) enzyme14,15,16. As such, the formation of NETs in dengue has been previously reported17,18. On the other hand, low-density granulocytes (LDGs) are the neutrophils in the peripheral blood mononuclear cell (PBMC) fraction after the separation of the blood sample by the gradient separation procedure19. The density of LDGs is similar to lymphocytes and monocytes, while it is lower than regular neutrophils, resulting in the presence of LDGs in the PBMC part above the PMN fraction. Notably, LDGs are positively associated with the severity of coronavirus disease 2019 (COVID-19)20 and influenza21 in a similar characteristic of NETs and viral infection22,23. Both NETs and LDGs can be induced by type 1 interferon (an important cytokine from innate immunity) in dengue24 and systemic lupus erythematosus (a common autoimmune disease)25.
Although high IFN-α (an important type I interferon found in dengue) indicates severe dengue in some publications26, several evasion strategies of dengue reduce IFN-α production, possibly leading to the inconsistency of IFN-α as a dengue severity biomarker27. Moreover, the use of a single or a panel of cytokines for dengue severity biomarkers might be difficult due to the redundancy of cytokines (different cytokines induce a similar effect)28,29,30, and the non-cytokine parameters might be more interesting. As such, we hypothesized that LDGs and NETs in dengue are induced by a combination of cytokines that might be present before the hemoconcentration. Hence, we explored LDGs and NETs in patients with dengue at 5 days of the fever with the exclusion of patients with hemoconcentration and also performed in vitro experiments.
Materials and methods
Enrolled participants and study designs
A cross-sectional study was conducted between March and September 2024 with the approved Ethics Committee from the Faculty of Medicine, Chulalongkorn University (IRB number 0480/66), in accordance with the guidelines of the Declaration of Helsinki 1964 and STROBE guidelines with written informed consent from all participants. The diagnosis of dengue was supported by the NS1 antigen test (Platelia enzyme-linked immunosorbent assay (ELISA); Bio-Rad) and dengue immunoglobulin (Ig) M and IgG serology assays (Capture ELISA; Panbio), while the reverse-transcription polymerase chain reaction (RT–PCR) was used to identify the viral serotype. The laboratory-confirmed dengue was positive for RT–PCR, NS1 antigen, or IgM assays at the enrollment or the IgM seroconversion between the paired specimens according to the criteria from the World Health Organization (WHO, Geneva, Switzerland)31. The exclusion criteria were the known inducers of LDGs and NETs, including (i) severe dengue (plasma leakage, shock, respiratory distress, and organ impairment)32,33, (ii) end-organ damage34, and (iii) pregnancy35. The onset of fever was estimated from the patient information. The dengue assessment is based on the 2009 WHO guideline31 as follows: (i) The febrile and critical phases of infection are at the 1st to 3rd days and the 4th to 7th days of illness, (ii) the warning signs are abdominal pain or tenderness, and persistent vomiting, as well as clinical signs of fluid accumulation, mucosal bleeding, lethargy, and hepatomegaly, and (iii) severe dengue is plasma leakage, bleeding, or organ damage with high severity. Because our main objectives are (i) to explore the possible impact of cytokines on neutrophils, which usually occurs within the 1st week of infection, and (ii) to discover a possible biomarker at the critical phase to predict the disease severity, the blood samples were collected on the 5th day of fever to reduce the influence of the dengue-induced adaptive immunity toward LDGs and NETs.
Blood sample analyses
Complete blood count (CBC), serum creatinine, aspartate transaminase (AST), and alanine transaminase (ALT) were measured in the central laboratory of the King Chulalongkorn Memorial Hospital using the Sysmex XN9203 Analyzer (Kobe, Hyogo, Japan) for CBC and Cobas c502 (Roche Diagnostics, Basel, Switzerland) for other parameters. Serum cytokines (IFN-α, TNF-α, and IL-6) were measured by enzyme-linked immunosorbent assay (ELISA) (Invitrogen, Waltham, MA, USA). For the density gradient cell separation, the fractions of peripheral blood mononuclear cells (PBMC) and polymorphonuclear cells (PMN) were isolated from heparin-anticoagulated blood using Ficoll-Paque (Robbins Scientific Corporation, Sunnyvale, CA, USA) and Polymorphprep™ (Axis-Shield, Oslo, Norway) in a 1:1:1 ratio in a 50 mL sterile tube before 30 min centrifugation at 1900 rpm at room temperature as previously described25. The PMN layer, largely comprising neutrophils (> 95%), was collected and washed with RPMI 1640 (Thermo Fisher Scientific, Logan, Utah, USA) and was resuspended with RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS). Contaminating erythrocytes were removed by ammonium chloride lysis (ACK) buffer. Neutrophil preparations were at least 95% viable and pure, as confirmed by trypan blue exclusion (Sigma-Aldrich, Singapore) and Wright’s Giemsa staining (Biotech, Bangkok, Thailand), respectively. Low-density granulocytes (LDGs) in the PBMC fraction were stained by Wright’s Giemsa color before counting in a light microscope as the percentage of polymorphonuclear cells to the total cells (polymorphonuclear cells plus mononuclear cells). Serum cell-free DNA was measured by Quant-iT™ PicoGreen reagent according to the manufacturer’s instructions (Thermo Fisher Scientific, Paisley, UK) using 480/520 nm on a fluorescent microplate reader (Bio-Tek). For the detection of extracellular traps (ETs), the isolated cells were added onto the poly-l-lysine-coated glass coverslips (6 mm diameter) (Sigma-Aldrich), fixed with 4% (v/v) formaldehyde, permeabilized by Tris Buffered Saline (TBS) with 0.05% Tween 20 (Sigma-Aldrich), and blocked with TBS containing 2% (w/v) bovine serum albumin (BSA) (Sigma-Aldrich). The formation of ETs was detected by (i) nuclear morphology by 4′,6-diamidino-2-phenylindole (DAPI) color, (ii) the co-immunofluorescence staining of myeloperoxidase (MPO) and neutrophil elastase (NE) (Abcam, Cambridge, UK), or iii) citrullinated histone 3 (CitH3) (Abcam). The fluorescent images were analyzed with a confocal microscope (ZEISS LSM 980, Carl Zeiss, Germany). Then, the coverslips with fluorescent-tagged cells were mounted upside down using the ProLong Diamond Antifade Mountant (Invitrogen) before visualization by confocal microscopy. The percentage of cells with ETs represented the abundance of ET formation.
In vitro experiments
Because type I interferon and cell-free DNA might induce LDGs and NETs in dengue, recombinant IFN-α and the extracted cell-free DNA were incubated with isolated neutrophils from healthy volunteers. For cell-free DNA, 3.0 mL of whole blood from healthy volunteers was mixed with 12 mL of lysis buffer (Erythrocyte lysis buffer, Qiagen, Hilden, Germany), centrifuged at 400 × g for 5 min at 4 °C to collect white blood cells, and the DNA pellet was extracted by the QIAamp DNA Blood Kit (Qiagen). Subsequently, the isolated neutrophils of healthy volunteers (gradient separation) at 1 × 107 cells were incubated with recombinant human IFN-α2 (BioLegend, San Diego, CA, USA) at 25 ng/mL or cell-free DNA at 200 ng/mL before determination of cell density again by gradient separation to differentiate neutrophils with low density (LDGs) and regular density at the upper and lower parts of the Ficoll-Paque, respectively. In parallel, NETs were measured in the activated neutrophils using immunofluorescence with the antibodies against neutrophil elastase (NE) and myeloperoxidase (MPO) (Abcam), as mentioned above. To determine the differences between the stimulated neutrophils (IFN-α and cell-free DNA) with low-density granulocytes (LDGs) and regular-density granulocytes (RDGs), the cells (5 × 105 cells) were suspended in staining buffer and labeled with antibody panels, including fluorescein isothiocyanate (FITC)-conjugated anti-CD66b (BioLegend, San Diego, CA, USA) and APC Annexin V apoptosis detection with propidium iodide (PI) (Cat640932; BioLegend, San Diego, CA, USA) at 4 °C for 20 min in the dark before being fixed with 1% fixative buffer. Subsequently, at least 50,000 stained cells were examined by flow cytometry using the BD FACSLyric™ flow cytometry system (BD Biosciences, Franklin Lakes, NJ, USA), and the data were analyzed by FlowJo V10 (Ashland, Wilmington, DE, USA). Notably, the data for each dot of the ex vivo results was an average from the duplicated measurements that derived from a single patient (the number of patients is indicated in the figure legend).
Statistical analysis
Mean ± standard error of the mean (SEM) was presented using the one-way analysis of variance (ANOVA) followed by Tukey’s analysis for multiple group comparison. Analysis of the time-point data was determined by the repeated measures ANOVA. The statistical analyses were performed with the GraphPad Prism version 10.4.1 software (La Jolla, CA, USA), and a p value of < 0.05 was considered statistically significant. For the normality test of data distribution, the Shapiro–Wilk Test was performed using SPSS 11.5 (San Diego, CA, USA) and interpreted as a normal distribution with the value > 0.05. The representative pictures of the distribution of the data are demonstrated in Supplementary Fig. 1.
Results
LDGs and serum IFN-α of dengue with warning signs (DWWS) were not different from dengue without warning signs (DWoWS)
To explore LDGs and NETs in the critical phase of dengue (between the 4th and 7th day of fever)1, blood samples at the 5th day of fever were collected with the exclusion of severe dengue (an obvious hemoconcentration). The definite diagnosis of dengue was supported by NS1 and polymerase chain reaction (PCR), and the warning signs are abdominal pain, persistent vomiting, signs of fluid accumulation, mucosal bleeding, lethargy, and hepatomegaly. As a cross-sectional analysis, 19 healthy volunteers, 40 patients of dengue without warning signs (DWoWS), and 31 patients of dengue with warning signs (DWWS) were analyzed (Table 1). The DENV1 serotype was prominent in the DWoWS group, while other serotypes were similar between DWoWS and DWWS (Table 1). For the complete blood count (CBC), hemoglobin, Hct, total white blood cell count (WBC), neutrophils, lymphocytes, and monocytes were similar between DWoWS and DWWS (Fig. 1A–F). Although atypical lymphocytes in dengue were higher and platelet counts in patients were lower compared with healthy control, atypical lymphocytes in DWoWS and DWWS were not different (Fig. 1G–I). On the other hand, band-form neutrophils were elevated only in the DWWS group (Fig. 1G–I). Notably, the reduced platelets supported the critical phase in all recruited patients36, and the presence of band-form neutrophils in DWWS indicated severe dengue37. For liver enzymes, only alanine transaminase (ALT), but not aspartate transaminase (AST), in DWWS was higher than control (Fig. 1J, K). Additionally, serum IFN-α and IL-6 in dengue were higher than the control, and the levels were similar between DWoWS and DWWS (Fig. 1L–N). Meanwhile, serum TNF-α in DWWS, but not in DWoWS, was higher than in the control (Fig. 1L–N).
Characteristics of healthy control (n = 19), dengue without warning signs (DWoWS) (n = 40), and dengue with warning signs (DWWS) (n = 31), as indicated by complete blood count, including hemoglobin, hematocrit, white blood cells (WBC), neutrophils, lymphocytes, monocytes, atypical lymphocytes, band form neutrophils, and platelets (A–I), liver enzymes, including aspartate transaminase (AST) and alanine transaminase (ALT) (J, K), and serum cytokines, including interferon alpha (IFN-α), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6), are demonstrated. *, p < 0.05 between the indicated groups; #, p < 0.05 versus healthy control.
DWWS demonstrated more prominent NETs and ETs in the PMN and PBMC fractions than DWoWS
After the gradient separation, cell-free DNA was determined from serum, while LDGs and NETs were evaluated in the PBMC and PMN fractions, respectively (Fig. 2A, left). The percentages of LDGs, as determined by the Wright’s stain in the PBMC fraction, in dengue were higher than in the control, which were similar between DWoWS and DWWS (Fig. 2A, right). Serum cell-free DNA in dengue was higher than in the control, which was more prominent in DWWS than in DWoWS (Fig. 2B). Here, several methods for the evaluation of NETs in the PMN fraction16,38, including DAPI (nuclear morphology determination), anti-neutrophil elastase (anti-NE), anti-myeloperoxidase (anti-MPO), and anti-citrullinated histone 3 (anti-CitH3), were used (Fig. 2B–F). Interestingly, NETs as determined by all methods in dengue were higher than in the control, which were more prominent in DWWS than in DWoWS (Fig. 2B–F). The correlation between several pairs of the parameters was explored to determine the possible cause-effect. As such, the coefficient of determination (R squared or R2) of NETs (by DAPI) vs. LDGs, NETs (by DAPI) vs. cell-free DNA, and LDGs vs. cell-free DNA was low (Fig. 2G–I), indicating the limited correlation between groups. Because LDGs in the PBMC fraction can also produce NETs39, and NETs in Figs. 2C–E were evaluated only from the PMN fraction, the extracellular traps (ETs) in the PBMC fraction that consists of both LDGs and mononuclear cells25 were further explored. The ETs in the PBMC fraction (using NE and MPO staining) were not found in the healthy control and were more prominent in DWWS than in DWoWS (Fig. 3A, B). Because both NETs from the PMN fraction (neutrophils) and ETs from the PBMC fraction (neutrophils and mononuclear cells) were prominent in DWWS, the warning signs of dengue might be correlated with ETs in either the PMN (ETs of neutrophils) or the PBMC (ETs of LDGs and monocytes) fractions, and neutrophil-related parameters might be interesting biomarkers to determine dengue severity.
Characteristics of healthy control (n = 19), dengue without warning signs (DWoWS) (n = 40), and dengue with warning signs (DWWS) (n = 31) as indicated by percentage of low-density granulocytes (LDGs; the neutrophils in the peripheral blood mononuclear cell (PBMC) fraction after gradient separation) with a representative picture of blood sample to identify PBMC fraction and polymorphonuclear cell (PMN) fraction (A), cell-free DNA (B), neutrophil extracellular traps (NETs) as evaluated by string-like nuclear morphology using DAPI colour (C), immunofluorescent staining for neutrophil elastase (NE) and myeloperoxidase (MPO) (D), and anti-citrullinated histone 3 (CitH3) (E) with the representative pictures (F), and the correlation between several parameters (G–I) are demonstrated. *, p < 0.05 between the indicated groups; #, p < 0.05 versus healthy control.
Characteristics of healthy control, dengue without warning signs (DWoWS), and dengue with warning signs (DWWS) as indicated by the extracellular traps (ETs) in the peripheral blood mononuclear cell (PBMC) fraction after gradient separation using immunofluorescent staining for neutrophil elastase (NE) and myeloperoxidase (MPO) with a representative picture of the blood sample to identify the PBMC fraction and polymorphonuclear cell (PMN) fraction (A), and the representative immunofluorescent pictures (B) are demonstrated (n = 12/group). *, p < 0.05 between the indicated groups; #, p < 0.05 versus healthy control; white arrows, mononuclear cells; brown arrows, neutrophils.
The influence of IFN-α and cell-free DNA in NETs and LDGs
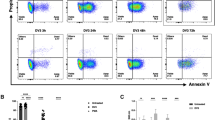
Because (i) cell-free DNA and NETs are the parameters that could differentiate DWoWS and DWWS (Figs. 1 and 2), (ii) IFN-α and cell-free DNA can activate neutrophils40,41, and (iii) IFN-α is important to control dengue28,29,30, both IFN-α and cell-free DNA might alter neutrophils into NETs and LDGs during the infection. Then, neutrophils from healthy controls were stimulated by IFN-α, cell-free DNA, and the combination of both factors before determining cell density (gradient separation) and immunofluorescent staining (NETs) (Fig. 4A). As such, IFN-α, cell-free DNA, and the combination similarly induced NETs at 1.5 h after stimulation, while the combination more prominently produced LDGs than the individual factor (Fig. 4B–D). Notably, the stimulations did not turn all regular-density granulocytes (RDGs) into LDGs, but maintained the density of the cells as RDGs, despite being stimulated (Fig. 5A). Subsequently, flow cytometry analysis was conducted in the LDGs and RDGs derived from the in vitro experiments (Fig. 5A). The higher expression of CD66b in LDGs and RDGs compared with the control indicated a prominent activity of the stimulated neutrophils (Fig. 5B, C). Meanwhile, the higher CD66b in LDGs than in RDGs implied more prominent LDG activities than RDG activities (Fig. 5B, C). Similarly, both IFN-α and cell-free DNA induced late apoptosis and necrosis (cell deaths) in LDGs and RDGs, while the more prominent cell deaths in LDGs over RDGs implied the more severe cell stress in LDGs (Fig. 5D–F). In parallel, both IFN-α and cell-free DNA similarly induced NETs in RDGs and LDGs (Fig. 6A, B). Hence, IFN-α and cell-free DNA prominently induced NETs in neutrophils regardless of the cell density (both LDGs and RDGs) (Fig. 6A, B). The activation by IFN-α and cell-free DNA might be responsible for the prominent NETs and ETs in the PBMC fraction in patients (Figs. 2 and 3).
Schema of the experiments demonstrates the use of neutrophils from the polymorphonuclear cell (PMN) fraction of the healthy volunteers after gradient separation to incubate with control media (Media) or IFN-α or cell-free DNA or the mixture of IFN-α and cell-free DNA (Mixed) before performing (i) The cell density determination (gradient separation) to separate low-density granulocytes (LDGs) and regular-density granulocytes (RDGs), and (ii) staining for neutrophil extracellular traps (NETs) using immunofluorescence for neutrophil elastase (NE) and myeloperoxidase (MPO) (A) is demonstrated. Characteristics of neutrophils after stimulation by IFN-α or cell-free DNA or the combination, as indicated by the percentage of LDGs (B) and NETs with the representative immunofluorescent pictures (C, D), are also demonstrated (n = 10/group). *, p < 0.05 between the indicated groups; #, p < 0.05 versus Media.
Schema of the experiments using neutrophils from the polymorphonuclear cell (PMN) fraction of the healthy volunteers to incubate with control media (Media) or IFN-α or cell-free DNA or the mixture of IFN-α and cell-free DNA (Mixed) before the separation into low-density granulocytes (LDGs) and regular density granulocytes (RDGs) with the analysis by flow cytometry (A) is demonstrated. Characteristics of neutrophils after stimulation by IFN-α or cell-free DNA or the combination, as indicated by an activation marker (CD66b) with the representative flow cytometry patterns (B, C), late apoptosis (positive for both annexin V and propidium iodide (PI)), and necrosis (positive for PI) with the representative flow cytometry patterns (D–F) are also demonstrated (n = 10/group). *, p < 0.05 between the indicated groups; #, p < 0.05 versus Media.
Neutrophil extracellular traps (NETs) as determined by immunofluorescent staining of neutrophil elastase (NE) and myeloperoxidase (MPO) after stimulation by IFN-α or cell-free DNA or the combination (Mixed) in low-density granulocytes (LDGs) and regular density granulocytes (RDGs) as indicated by the graph presentation (A) and the representative pictures (B) are demonstrated (n = 10/group). #, p < 0.05 versus Media.
Discussion
At 5 days of dengue without hemoconcentration, only cell-free DNA and ETs (measured from either the PBMC or PMN fractions), but neither LDGs nor serum IFN-α, differentiated DWoWS from DWWS. Despite the differences in the warning signs between DWoWS and DWWS, all routine parameters (CBC and liver enzymes) and serum cytokines were not different between groups. Notably, both of the band form neutrophils and ALT in DWWS were higher than in healthy controls, but they were not different between DWoWS and DWWS. The elevated band-form neutrophils and increased serum cytokines (IFN-α, TNF-α, and IL-6) support innate immunity activation in dengue42. Although the combination of cytokines (IL-6, MIP-1, IL-17, TNF-α, IL-13, IL-1Ra, IFN-α, IFN-γ, IL-7, and IL-12) might predict dengue severity29,43, only selected serum cytokines could not differentiate dengue severity in our patients, supporting previous publications44,45. Because different cytokines might induce similar effects (cytokine redundancy) due to the sharing of common receptor subunits46, outcomes of the cytokine stimulation might be better biomarkers than the individual cytokine. Then, the activation of innate immune cells might be an outcome of cytokine stimulation, and neutrophils, the highest-abundance immune cells in humans14,47, were explored. Here, both LDGs and NETs were prominent in dengue when compared with controls; however, only NET parameters (cell-free DNA and ETs in the PBMC or PMN fractions) differentiated DWoWS from DWWS. As such, cell-free DNA is the fragmented DNA released from the cells into the blood circulation and other body fluids during cell damage or injury48,49. Some of the cell-free DNA is not derived from the host cells and mitochondria but might originate from the translocation of bacterial-free DNA from the gut permeability defect50. Nevertheless, cell-free DNA from any source might counteract dengue-induced coagulopathy51 through the enhanced thrombin and fibrinolysis inhibition48,52. Meanwhile, the nonstructural protein 1 (NS1) of dengue can directly induce NETs through the platelet activation, according to the laboratory-based experiments17. Despite the limited data in dengue, several activators, including cytokines, viral particles, cell-free DNA, and toxic substances25,32,53,54, can induce LDGs during the infection. Our data support that cytokines (IFN-α) and cell-free DNA might, at least in part, induce LDG transformation in dengue.
Although LDGs (neutrophils in the PBMC fraction) did not differentiate between DWoWS and DWWS, the extracellular traps (ETs) in the PMN and PBMC fractions differentiated both conditions. Notably, ETs occur in the neutrophils that are located in both the PMN fractions (regular-density cells) and PBMC fractions (LDGs). While ETs in the PMN fraction consist of only neutrophils (NETosis), ETs in the PBMC fraction might be a combination of NETosis and monocyte extracellular traps (METosis)55,56. Because both NETosis and METosis can induce cell-free DNA38,57, the determination of ETs only in the PMN fraction without the concern of ETs in the PBMC fraction might not be a good representative biomarker. In contrast, the cell-free DNA might be an interesting parameter because it is derived from the whole section of the blood sample. Although both cell-free DNA and ETs (in either the PMN or PBMC fractions) can differentiate DWWS from DWoWS, the measurement of cell-free DNA is more convenient due to the non-necessity for cell separation. From previous publications, a vicious cycle of LDGs and NETs (LDGs increase NETs, and NETs induce more LDGs) in systemic lupus erythematosus starts from LDGs-released IFN-α and nucleic acids58,59. Notably, LDGs in patients with autoimmune diseases also more prominently produce cytokines than regular neutrophils60,61,62. Due to the presence of both LDGs and NETs in dengue, the vicious cycle of injury might also be possible in dengue. Here, an additive effect of IFN-α and cell-free DNA on LDG induction was demonstrated in vitro, while there was no additive effect on NETosis. Perhaps the NET induction property of both IFN-α and cell-free DNA is too potent to see the additive effect on NETosis. As such, approximately 50% of the cells expressed ETs after activation by IFN-α or cell-free DNA (Figs. 4C and 6A). Regarding cell activities, LDGs were more active than regular-density granulocytes (RDGs), as indicated by the higher CD66b (an adhesion molecule), which might lead to the more prominent cell stress (late apoptosis and necrosis) in LDGs. However, LDGs and RDGs similarly produced NETosis after being induced by IFN-α and cell-free DNA, as more than 50% of both LDGs and RDGs produced NETs after 1.5 h of the activation (Fig. 6). Hence, the cytokine receptors63,64 and DNA recognition (TLR-4, TLR-9, and cytosolic receptors)65,66 of LDGs might still be intact and are capable of NET formation. Further studies on LDGs and ETs in the PBMC fraction in dengue are interesting.
Several limitations should be mentioned. First, the exclusion of patients with hemoconcentration (focusing only on the early phase) resulted in the comparison between only 2 groups (DWoWS and DWWS) that did not differ much in the clinical features. The inclusion of the patients with severe dengue might more clearly demonstrate benefits of some parameters, especially LDGs and serum cytokines. More studies with patients with severe dengue are interesting. Second, the cross-sectional analysis with the limited number of patients was performed. An increased number of time points of sample collection (the longitudinal study) with an adequate number of patients might detect the better parameters to predict hemoconcentration that might be developed in 15–20% of the patients with DWWS67. Third, the quantitative analysis of the virus was not determined. The results from the NS1 antigen and RT-PCR for dengue serotype were qualitatively reported, as the standard curve for viral load estimation in the RT-PCR was not routinely generated68. Because the viral loads can be detected for approximately 7 days after infection with an unclear correlation to the dengue severity69, more studies focusing on viral loads and neutrophil activation are interesting. Fourth, the direct effect of the dengue virus on neutrophils was not evaluated. While cytokines might be a common factor causing LDGs in several diseases (sepsis, autoimmune diseases, and renal failure)19,25,70, the direct viral-induced LDGs are reported (COVID and HIV)54,71. Further studies on the direct effect of the virus and neutrophils in dengue are interesting.
In conclusion, the activation of neutrophils, including LDGs, NETosis, and apoptosis (potentially stimulated by IFN-α or cell-free DNA) in dengue may correlate with the disease activity and possibly be used for the clinical applications. Thus, ETs in the PMN or PBMC fraction and serum cell-free DNA might serve as valuable indicators of disease activity in dengue to predict the warning signs before an occurrence of hemoconcentration. Although LDGs could not differentiate DWoWS from DWWS, the crude measurement of LDGs is feasible for resource-limited hospitals. As such, the simple determination of the percentage of neutrophils in the PBMC fraction (LDGs) can be roughly conducted by separation of the PBMC fraction using centrifugation with a density gradient protocol, followed by determination of the percentage of neutrophils in the PBMC fraction using Wright’s stain color. This rough determination of the percentage of LDGs requires only a centrifugation machine, without the necessity of the more expensive flow cytometry apparatus. Then, further studies are necessary to make use of LDGs as a novel biomarker for dengue in some aspects. More studies to explore clinical use of LDGs are warranted.
Data availability
The datasets analyzed during the current study are not publicly available due to ethics in human research but are available from the corresponding author on reasonable request.
References
Khan, M. B. et al. Dengue overview: An updated systemic review. J. Infect. Public Health 16, 1625–1642 (2023).
Thisyakorn, U. et al. Epidemiology and costs of dengue in Thailand: A systematic literature review. PLoS Negl Trop. Dis. 16, e0010966 (2022).
Izmirly, A. M., Alturki, S. O., Alturki, S. O., Connors, J. & Haddad, E. K. Challenges in dengue vaccines development: Pre-existing Infections and cross-reactivity. Front. Immunol. 11, 1055 (2020).
Srikiatkhachorn, A. Plasma leakage in dengue haemorrhagic fever. Thromb. Haemost. 102, 1042–1049 (2009).
Chew, N. et al. Assessing dehydration status in dengue patients using urine colourimetry and mobile phone technology. PLoS Negl Trop. Dis. 14, e0008562 (2020).
Htun, T. P., Xiong, Z. & Pang, J. Clinical signs and symptoms associated with WHO severe dengue classification: A systematic review and meta-analysis. Emerg. Microbes Infect. 10, 1116–1128 (2021).
Gleeson, T. et al. Dengue management in triage using ultrasound in children from Cambodia: A prospective cohort study. Lancet Reg. Health West Pac. 19, 100371 (2022).
Durbin, A. P. Dengue vascular leak syndrome: Insights into potentially new treatment modalities. J. Clin. Invest. 129, 4072–4073 (2019).
Teo, A., Chua, C. L. L., Chia, P. Y. & Yeo, T. W. Insights into potential causes of vascular hyperpermeability in dengue. PLoS Pathog. 17, e1010065 (2021).
Ong, S. P., Lee, L. M., Leong, Y. F., Ng, M. L. & Chu, J. J. Dengue virus infection mediates HMGB1 release from monocytes involving PCAF acetylase complex and induces vascular leakage in endothelial cells. PLoS ONE 7, e41932 (2012).
Khanam, A., Gutiérrez-Barbosa, H., Lyke, K. E. & Chua, J. V. Immune-mediated pathogenesis in dengue virus infection Viruses 14 (2022).
Ma, Y., Zhang, Y. & Zhu, L. Role of neutrophils in acute viral infection. Immun. Inflamm. Dis. 9, 1186–1196 (2021).
Chaloemwong, J. et al. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: A retrospective study. BMC Hematol. 18, 20 (2018).
Sae-Khow, K., Charoensappakit, A., Chiewchengchol, D. & Leelahavanichkul, A. High-dose intravenous ascorbate in sepsis, a pro-oxidant enhanced microbicidal activity and the effect on neutrophil functions Biomedicines 11 (2022).
Saisorn, W. et al. Acute kidney injury induced lupus exacerbation through the enhanced neutrophil extracellular traps (and apoptosis) in Fcgr2b deficient lupus mice with renal ischemia reperfusion injury. Front. Immunol. 12, 669162 (2021).
Saithong, S. et al. Candida administration worsens neutrophil extracellular traps in renal ischemia reperfusion injury mice: An impact of gut fungi on acute kidney injury. J. Innate. Immun. 14, 502–517 (2022).
Garishah, F. M. et al. Neutrophil extracellular traps in dengue are mainly generated NOX-independently. Front. Immunol. 12, 629167 (2021).
Chua, C. L. L., Morales, R. F., Chia, P. Y., Yeo, T. W. & Teo, A. Neutrophils–An understudied bystander in dengue?. Trends Microbiol. 32, 1132–1142 (2024).
Ning, X., Wang, W. M. & Jin, H. Z. Low-density granulocytes in immune-mediated inflammatory diseases. J. Immunol. Res. 2022, 1622160 (2022).
Dean, L. S. et al. Phenotypic alteration of low-density granulocytes in people with pulmonary post-acute sequalae of SARS-CoV-2 infection. Front. Immunol. 13, 1076724 (2022).
Cole, S. L. et al. M1-like monocytes are a major immunological determinant of severity in previously healthy adults with life-threatening influenza. JCI Insight 2, e91868 (2017).
Saithong, S. et al. A synergy between endotoxin and (1→3)-beta-D-glucan enhanced neutrophil extracellular traps in candida administered dextran sulfate solution induced colitis in FcGRIIB-/- Lupus Mice, an impact of intestinal fungi in lupus. J. Inflamm. Res. 14, 2333–2352 (2021).
Saithong, S., Worasilchai, N., Saisorn, W., Udompornpitak, K., Bhunyakarnjanarat, T. & Chindamporn, A. et al. Neutrophil extracellular traps in severe SARS-CoV-2 infection: A possible impact of LPS and (1→3)-β-D-glucan in blood from gut translocation Cells 11 (2022).
Gandini, M. et al. Dengue virus activates membrane TRAIL relocalization and IFN-α production by human plasmacytoid dendritic cells in vitro and in vivo. PLoS Negl. Trop. Dis. 7, e2257 (2013).
Saisorn, W. et al. Extracellular traps in peripheral blood mononuclear cell fraction in childhood-onset systemic lupus erythematosus. Sci. Rep. 14, 23177 (2024).
Castillo Ramirez, J. A. & Urcuqui-Inchima, S. Dengue virus control of type I IFN responses: A history of manipulation and control. J. Interferon. Cytokine Res. 35, 421–430 (2015).
Usuda, J. N. et al. Interferome signature dynamics during the anti-dengue immune response: A systems biology characterization. Front. Immunol. 14, 1243516 (2023).
De La Cruz Hernández, S. I. et al. A strong interferon response correlates with a milder dengue clinical condition. J. Clin. Virol. 60, 196–199 (2014).
Becquart, P. et al. Acute dengue virus 2 infection in Gabonese patients is associated with an early innate immune response, including strong interferon alpha production. BMC Infect. Dis. 10, 356 (2010).
Kurane, I. et al. High levels of interferon alpha in the sera of children with dengue virus infection. Am. J. Trop. Med. Hyg. 48, 222–229 (1993).
WHO Guidelines Approved by the Guidelines Review Committee. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization. Copyright © 2009, World Health Organization 2009.
Sun, R. et al. Dysfunction of low-density neutrophils in peripheral circulation in patients with sepsis. Sci. Rep. 12, 685 (2022).
Zhu, S. et al. Neutrophil extracellular traps contribute to immunothrombosis formation via the STING pathway in sepsis-associated lung injury. Cell Death Discov. 9, 315 (2023).
Nakazawa, D., Marschner, J. A., Platen, L. & Anders, H. J. Extracellular traps in kidney disease. Kidney Int. 94, 1087–1098 (2018).
Aslanian-Kalkhoran, L. et al. The role of neutrophils and neutrophil extracellular traps (NETs) in stages, outcomes and pregnancy complications. J. Reprod. Immunol. 163, 104237 (2024).
Rigau-Pérez, J. G. et al. Dengue and dengue haemorrhagic fever. Lancet 352, 971–977 (1998).
Rawat, S., Vrati, S. & Banerjee, A. Neutrophils at the crossroads of acute viral infections and severity. Mol. Aspects Med. 81, 100996 (2021).
Sae-Khow, K. et al. Syk inhibitor attenuates lupus in FcγRIIb(-)(/-) mice through the Inhibition of DNA extracellular traps from macrophages and neutrophils via p38MAPK-dependent pathway. Cell Death Discov. 11, 63 (2025).
Carmona-Rivera, C. & Kaplan, M. J. Low-density granulocytes in systemic autoimmunity and autoinflammation. Immunol. Rev. 314, 313–325 (2023).
Benguigui, M. et al. Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell 42, 253-265.e12 (2024).
Korabecna, M. et al. Cell-free DNA in plasma as an essential immune system regulator. Sci. Rep. 10, 17478 (2020).
Opasawatchai, A. et al. Neutrophil activation and early features of NET formation are associated with dengue virus infection in human. Front. Immunol. 9, 3007 (2018).
Kittigul, L., Temprom, W., Sujirarat, D. & Kittigul, C. Determination of tumor necrosis factor-alpha levels in dengue virus infected patients by sensitive biotin-streptavidin enzyme-linked immunosorbent assay. J. Virol. Methods 90, 51–57 (2000).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Zhai, G. H. et al. Diagnostic value of sIL-2R, TNF-α and PCT for sepsis infection in patients with closed abdominal injury complicated with severe multiple abdominal injuries. Front. Immunol. 12, 741268 (2021).
Nicola NA. Cytokine pleiotropy and redundancy: a view from the receptor. Stem Cells 1994; 12 Suppl 1:3–12; discussion 12–4.
Charoensappakit, A., Sae-Khow. K. & Leelahavanichkul. A. Gut barrier damage and gut translocation of pathogen molecules in lupus, an impact of innate immunity (macrophages and neutrophils) in autoimmune disease. Int. J. Mol. Sci. 23 (2022).
Charoensappakit, A. et al. Cell-free DNA as diagnostic and prognostic biomarkers for adult sepsis: A systematic review and meta-analysis. Sci. Rep. 13, 19624 (2023).
Saisorn, W., Phuengmaung, P., Santiworakul, C., Doi, K., Rianthavorn, P. & Leelahavanichkul, A. Endotoxaemia in childhood-onset systemic lupus erythematosus induces low-density granulocytes and extracellular traps of innate immune cells Lupus Sci. Med. 12 (2025).
Kaewduangduen, W., Visitchanakun, P., Saisorn, W., Phawadee, A., Manonitnantawat, C. & Chutimaskul, C. et al. Blood bacteria-free DNA in septic mice enhances LPS-induced inflammation in mice through macrophage response. Int. J. Mol. Sci. 23 (2022).
Wang, H. et al. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target Ther. 9, 235 (2024).
Medeiros, S. K. et al. Does cell-free DNA promote coagulation and inhibit fibrinolysis in patients with unprovoked venous thromboembolism?. Thromb. Res. 186, 13–19 (2020).
Sae-Khow, K., Charoensappakit, A. & Leelahavanichkul, A. Neutrophil diversity (immature, aged, and low-density neutrophils) and functional plasticity: Possible impacts of iron overload in β-thalassemia. Int. J. Mol. Sci. 25 (2024).
Cabrera, L. E. et al. Characterization of low-density granulocytes in COVID-19. PLoS Pathog. 17, e1009721 (2021).
Doster, R. S., Rogers, L. M., Gaddy, J. A. & Aronoff, D. M. Macrophage extracellular traps: A scoping review. J. Innate. Immun. 10, 3–13 (2018).
Ibrahim, N. et al. Human monocyte subsets differ in their capacity to form extracellular traps. Cell Death Discov. 10, 281 (2024).
Granger, V. et al. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 102, 775–781 (2017).
Tay, S. H., Celhar, T. & Fairhurst, A. M. Low-density neutrophils in systemic lupus erythematosus. Arthritis Rheumatol. 72, 1587–1595 (2020).
Garcia-Romo, G. S., Caielli. S., Vega, B., Connolly, J., Allantaz, F. & Xu, Z. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3:73ra20 (2011).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Denny, M. F. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297 (2010).
Skrzeczynska-Moncznik, J. et al. Differences in staining for neutrophil elastase and its controlling inhibitor SLPI reveal heterogeneity among neutrophils in psoriasis. J. Invest. Dermatol. 140, 1371-1378.e3 (2020).
Peng, Y. et al. The potential roles of type I interferon activated neutrophils and neutrophil extracellular traps (NETs) in the pathogenesis of primary Sjögren’s syndrome. Arthritis Res. Ther. 24, 170 (2022).
Gul, E. et al. Type I IFN-related NETosis in ataxia telangiectasia and artemis deficiency. J. Allergy Clin. Immunol. 142, 246–257 (2018).
Suksawad, N., Udompornpitak, K., Thawinpipat, N., Korwattanamongkol, P., Visitchanakun, P. & Phuengmaung, P. et al. Cyclic GMP-AMP synthase (cGAS) deletion reduces severity in bilateral nephrectomy mice through changes in neutrophil extracellular traps and mitochondrial respiration. Biomedicines 11 (2023).
Chen, T. et al. Receptor-mediated NETosis on neutrophils. Front. Immunol. 12, 775267 (2021).
Wisanuvej, K. et al. Comparison between blood hemoglobin concentration determined by point-of-care device and complete blood count in adult patients with dengue. PLoS Negl Trop. Dis. 15, e0009692 (2021).
Bhatt, P., Jayaram, A., Varma, M. & Mukhopadhyay, C. Kinetics of dengue viremia and its association with disease severity: An ambispective study. Virusdisease 35, 250–259 (2024).
Dayarathna, S. et al. Are viral loads in the febrile phase a predictive factor of dengue disease severity?. BMC Infect. Dis. 24, 1248 (2024).
Chancharoenthana, W. et al. Significance of neutrophil extracellular traps and low-density granulocytes in advanced chronic kidney disease (stage 5D) cases with COVID-19. Ren. Fail 47, 2559093 (2025).
Cloke, T., Munder, M., Taylor, G., Müller, I. & Kropf, P. Characterization of a novel population of low-density granulocytes associated with disease severity in HIV-1 infection. PLoS ONE 7, e48939 (2012).
Funding
This research has received funding support from the NSRF via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation (B48G6600112) with Rachadapisek Sompote Matching Fund (RA-MF-02/67 and RA-MF-01/68), and the Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University (The Exchange Faculty Travel Grant; Grant No. CTG168008).
Author information
Authors and Affiliations
Contributions
W.S. performed the experiments, analyzed data, and approved the manuscript. P.P. performed the experiments, performed clinical data collection, and analyzed data. W.K. and T.B. performed the experiments and performed clinical data collection. K.D. and W.C. supervised to conception and study design. A.L. designed and coordinated all the experiments, analyzed the data, and wrote and approved the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study involves human participants and was approved by the research ethics board of the Chulalongkorn University Faculty of Medicine’s Institutional Review Board (IRB No. 0480/66). Participant gave informed consent to participate in the study before taking part.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Saisorn, W., Phuengmaung, P., Kaewduangduen, W. et al. Low-density granulocytes and neutrophil extracellular traps in dengue, impacts of interferon alpha and cell-free DNA. Sci Rep 16, 262 (2026). https://doi.org/10.1038/s41598-025-29640-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29640-9