Abstract
This investigation systematically evaluated the effect of low (10 mg/L) and high (50 mg/L) concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D), combined with 3 mg/L 2iP, on callus induction from shoot tip explants of Barhi date palm cv. The induced calli were used to establish an indirect somatic embryogenesis (ISE) system on MS medium supplemented with different plant growth regulator (PGR) formulations. Genetic stability was assessed using inter-simple sequence repeat (ISSR) markers. Callus induced with low 2,4-D (C1) concentration exhibited significantly (p < 0.05) superior morphogenic competence, yielding a higher fresh weight (FW) of callus at both the induction (0.290 g/jar) and regeneration (0.475 g/jar) stages, more somatic embryos (100% frequency, 8/explant), and enhanced regeneration capacity compared to callus induced by high 2,4-D (C2). Regenerants from C1 displayed superior bud germination (6.2/explant), shoot proliferation (7/explant) and elongation (6.7 cm), as well as root induction (5.6/shoot) and elongation (6.3 cm). Plantlets were successfully acclimatized, achieving an 88.5% survival rate. ISSR analysis confirmed 97.5% of genetic fidelity among all regenerants. These results indicate that a lower 2,4-D concentration (10 mg/L) enhances callus vigor and subsequent organogenesis, facilitating a highly efficient and genetically stable ISE protocol for date palm micropropagation.
Similar content being viewed by others
Introduction
Dates (Phoenix dactylifera L.) are highly esteemed fruits, recognized for their significant functional food properties. These properties are attributed to their rich nutritional composition and therapeutic benefits1. In numerous developing nations, dates serve as a dietary staple, particularly in mitigating malnutrition and food scarcity among vulnerable populations, including children. They provide a concentrated source of energy, essential minerals, and dietary fiber, rendering them a vital dietary component in resource-limited environments2. Furthermore, dates have historically been utilized for managing a diverse array of ailments, ranging from gastrointestinal disturbances to chronic conditions such as neurodegenerative and cardiovascular diseases3.
The Mediterranean region is home to hundreds of P. dactylifera cultivars, many of which are highly regarded for their exceptional nutritional and commercial value4. Among these, the Barhi cultivar (cv.) has garnered substantial recognition as a premium fruit crop due to its superior physicochemical properties and health-promoting characteristics. Comparative investigations have demonstrated that Barhi dates exhibit elevated fructose content, increased levels of essential micronutrients (e.g., potassium, magnesium, and iron), and enhanced antioxidant capacity when compared to other prominent cultivars5. Notably, in an assessment of 18 date varieties cultivated in the United Arab Emirates, Barhi cv. consistently ranked among the top performers owing to its favorable nutritional profile, including remarkably low levels of antinutritional factors4. Moreover, its therapeutic potential has been underscored in Saudi Arabia, where it constitutes one of the three most frequently consumed date varieties for addressing mineral deficiencies6.
The growing awareness of these health benefits has led to a steady rise in global demand for dates, particularly premium varieties such as Barhi. Consequently, market prices have surged in recent years, reflecting both consumer preference and constrained supply. However, this upward trend has simultaneously exposed critical challenges inherent in date palm cultivation. Date palm is conventionally propagated through offshoots; nevertheless, the mother plant produces only a limited number of offshoots7,8,9. Furthermore, this crop is highly susceptible to numerous diseases and pests, resulting in substantial yield reductions. Given these inherent limitations, the development of alternative propagation methodologies is imperative. Plant tissue culture emerges as an efficacious solution, facilitating the rapid production of disease-free, genetically uniform plants, thereby establishing itself as an indispensable tool for contemporary date palm cultivation 10,11,12.
Callus tissue is the foundational stage for initiating diverse in vitro culture systems, including indirect organogenesis, even under changing environmental conditions13. Somatic embryogenesis (SE), a pivotal biotechnological approach, enables the mass production of embryos either directly from explants or indirectly via callus in in vitro plant cultures14,15. Micropropagation through indirect somatic embryogenesis (ISE) is more efficient, yielding a greater productivity of in vitro plantlets. Additionally, a small quantity of induced callus can be cryopreserved for extended durations and subsequently regenerated in high quantities for further multiplication.
Various date palm cultivars, including Barhi cv., have been successfully propagated in vitro using both techniques (direct and indirect). These methodologies typically employ different explants, mainly as shoot tips16,17,18,19 and floral bud20, in conjunction with varied types of culture media, plant growth regulators (PGR) and controlled conditions. Consequently, micropropagated plants may exhibit phenotypic and genotypic variations. Therefore, a true-to-type genetic fidelity test is employed to ascertain the genetic stability of micropropagated plantlets, particularly those derived from callus21,22.
A primary factor implicated in somaclonal variation in callus-derived plants is the type and concentration of auxin utilized during the initial induction stage. 2,4-D, a synthetic herbicide, functions as an auxin mimic at low concentrations and demonstrates high stability during heat sterilization23. This unique characteristic has prompted its widespread application by researchers across a wide range of concentrations, with conflicting reports regarding its impact on the genomic stability of in vitro produced plantlets across diverse plant species24. In date palm, 2,4-D has been extensively applied at varying doses, from very low (1 mg/L) to very high (100 mg/L), to promote optimal callus formation and undifferentiated biomass growth, often in combination with different cytokinins (such as 2iP or BA)25,26,27,28,29,30,31. However, research findings have been inconsistent. Some studies reported that lower to moderate 2,4-D levels (1 mg/L)27 and 5 mg/L26 were most effective for maximizing callus biomass, as higher concentrations (> 20 mg/L) were found to suppress in vitro growth and cause tissue necrosis32. Additionally, doses exceeding 50 mg/L were linked to genetic instability and mutations in some date palm species25,33.
On the other hand, some studies found that low 2,4-D concentrations had negligible effects on callus induction, leading to the use of very high levels (100 mg/L) without observed somaclonal variations30,31. These conflicting results persist in recent literature19,25,27,30,31,33 with no experimental study conclusively determining a safe and effective 2,4-D range for in vitro date palm regeneration.
To address this gap, the current study investigated two previously suggested 2,4-D concentrations: 50 mg/L (the highest reported non-detrimental dose)25,33 and 10 mg/L (a concentration considered high in studies favoring lower hormone levels)34,35, both combined with 3 mg/L 2iP to induce callus from Barhi date palm shoot tips. The resulting callus was then used as explant material for plantlet regeneration through an ISE system, with genetic stability assessed via ISSR markers.
Materials and methods
The in vitro culture experiments were conducted at the Plant Tissue Culture Laboratory of the General Corporation for Agricultural Services in Sana’a, Republic of Yemen, from 2020 to 2022. DNA extraction and subsequent genetic stability analysis were performed at the Department of Botany and Microbiology, College of Science, King Saud University, Saudi Arabia.
Plant materials
Date palm (Barhi cv.) offshoots (3–4 years old), confirmed to be free from diseases and pests, were selected and collected from Bakil Thiba date palm farms located in Al-Jouf Province, in Khab and Alshaaf District, Republic of Yemen, in 2020.
Callus induction and ISE formation
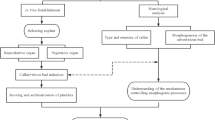
The detailed methodology for callus induction, callus proliferation, and somatic embryogenesis formation has been comprehensively described in our recently published work36. The culture media compositions are summarized in Table 1, and the in vitro culture stages of ISE are shown in Fig. 1. Briefly, to induce callus tissue, the apical meristem of the shoot tip was cut longitudinally into four explants (1.2–1.5 cm long). Then, the prepared explants were individually inserted in the MS (Murashige & Skoog)37 medium treated with different PGR formulations (Table1). Explants were placed on the medium with the basal end in contact and the abaxial side facing upward to ensure consistent orientation across replicates (Fig. 1). To establish indirect SE, approximately 0.5 g of fresh weight (FW) from two distinct samples of well-developed callus tissue, induced separately by fortified MS medium with either 10 mg/L 2,4-D + 3 mg/L 2iP (designated as Callus 1) or 50 mg/L 2,4-D + 3 mg/L 2iP (designated as Callus 2), were transferred to MS medium + 0.1 mg/L NAA to facilitate the formation of embryogenic callus (EC). Subsequently, both EC1 (derived from Callus 1) and EC2 (derived from Callus 2) were germinated through sub-culturing on MS medium containing NAA (0.1 mg/L) + BA (0.05 mg/L) for 12 weeks in darkness (with subculture every 4 weeks). Following this, the germinated buds from each treatment were counted and re-cultured on the same medium, but with incubation under an 8/16-h light/dark photoperiod to induce the development of green shoots/sprouts.
Shoot elongation
Well-developed sprouts (Sprouts1) originating from SE in EC1 and Sprouts2 developed from SE in EC 2 were transferred to an elongation medium fortified with gibberellic acid (GA3) at three different levels (0.2, 0.5, and 1 mg/L) for a period of 2 months under an 8/16-h light/dark photoperiod (Table 1). Subsequently, the shoot length (cm) was measured for each treatment group.
Root formation
Well-developed shootlets derived from Sprouts1 and Sprouts2 were transferred to a rooting 1/2 strength semi-liquid MS medium with NAA at three separated concentrations (0.5, 1, 1.5 mg/L) for 2 months under an 8/16-h light/dark photoperiod (Table 1). The number of roots formed and their respective lengths (cm) were quantified for each experimental trial.
Plantlet acclimatization
The plantlets were aseptically removed from their culture containers and meticulously washed with sterilized tap water to eliminate any remaining gel medium attached to the root system. To prevent fungal contamination, they were then immersed in a fungicidal solution. Subsequently, the plantlets were transplanted into plastic pots filled with a growth medium consisting of a 2:1(v/v) mixture of peat moss and sand. During the early acclimatization stage, a high-humidity environment (87.5%) was maintained by enclosing the plantlets within a polyethylene plastic tunnel and irrigating them with a half-strength MS macro-salt solution. To ensure successful acclimatization, the plantlets were gradually introduced to greenhouse conditions, and the plastic covering was incrementally removed after 3 weeks. For the first 2 weeks following cover removal, the plantlets were frequently misted with water to aid in their transition. After a 3-month acclimatization period in the greenhouse, the plantlets were transferred to larger nursery poly-bags or earthen pots containing a 2:1(v/v) blend of garden soil and farmyard manure, where they were further cultivated under nursery conditions. Survival rates were monitored during both the hardening and acclimatization phases, while the average leaf count and leaf length per plant were recorded as growth metrics.
DNA isolation and genetic fidelity evaluation
Genomic DNA was extracted from fresh leaf tissue (0.25 g per sample) of in vitro-regenerated plantlets and the donor plant using the Doyle and Doyle38 protocol. DNA purity and concentration were spectrophotometrically determined (Nanodrop 2000, Thermo Scientific, USA). Genetic homogeneity among regenerants was assessed using seven ISSR markers (UBC, Canada). PCR amplification was performed in 25 µL reactions containing 50 ng/µL genomic DNA (1.5 µL), ISSR primer (1.5 µL), GoTaq® Green Master Mix (12.5 µL, Promega, USA), and nuclease-free water (9.5 µL). Thermal cycling (Bio-Rad, USA) consisted of an initial denaturation (94 °C, 5 min); 34 cycles of denaturation (94 °C, 45 s), annealing (46–55 °C, 30 s), and extension (72 °C, 90 s); followed by a final extension (72 °C, 5 min). All reactions were performed in triplicate to ensure reproducibility. Amplification products were resolved by electrophoresis (1.5% agarose, 1X TAE, 75 V) and imaged (G: BOX F3, Syngene, UK).
Data analysis
The study employed ten replicates per treatment (each in vitro culture experiment was replicated ten times). Each individual culture tube was inoculated with a single explant, embryo cluster, or regenerated plantlet. A factorial randomized complete block design was implemented, and the collected data were analyzed using a one-way analysis of variance (ANOVA). Post-hoc mean comparisons were performed using a LSD test at a stringent significance level of 5%.
Results
Callus induction and proliferation
As depicted in Fig. 2, the supplemented culture medium with 10 mg/L 2,4-D + 3 mg/L 2iP significantly (p < 0.05) induced a higher callus fresh weight (Callus 1), recorded as 0.290 g/jar, compared to the fresh weight (0.223 g/jar) of callus induced by the medium augmented with 50 mg/L 2,4-D + 3 mg/L 2iP (Callus 2). These findings indicate that 2,4-D functions as a potent inducer for callus formation when applied at a lower concentration (10 mg/L), yielding a greater callus FW than at the higher dose (50 mg/L). Furthermore, Callus 1 exhibited faster proliferation, with a callus FW of 0.475 g/jar, representing a 70.25% increase, when regenerated on a medium containing 1 mg/L 2,4-D + 5 mg/L 2iP, in contrast to Callus 2, which demonstrated a lower proliferated callus FW (0.326 g/jar) with a 64.65% increase rate (p > 0.05). Both callus induction treatments resulted in a consistent compact texture but displayed distinct coloration (creamy for Callus1and brownish for Callus 2), whereas both regenerated calluses were observed to be creamy and friable (Fig. 1).
Fresh weight of callus induced from shoot tip explant; (C1) callus 1 induced on MS + 10 mg/L of 2,4-D + 3 mg/L of 2iP; (C2) callus 2 induced on MS + 50 mg/ L of 2,4-D + 3 mg/L of 2iP & fresh weight of callus 1 and 2 separately regenerated on MS + 1 mg/L 2,4-D + 5 mg/L of 2iP. Different superscript letters at the same colored column indicate the values are significantly different at 0.5 level.
Somatic embryogenesis formation, bud germination, and sprouting
Both friable calluses 1 and 2 were utilized as explants (0.5 g/tube) for the indirect development of somatic embryogenesis (SE) over a 6-month in vitro culture period. Both embryogenic calluses (EC1&2) initially manifested as small white granules, originating from the aggregation of cells within friable tissues (Fig. 1). These formed granules developed into many white globular stage somatic embryos (Fig. 3a) after culture on the MS medium supplemented with 0.1 mg/L NAA for 8 weeks in darkness. When the globular somatic embryos were cultured on the same medium and under the same incubation conditions for additional 12 weeks, the globular stage-embryo developed into heart (Fig. 3b), torpedo (Fig. 3c), and cotyledonary stage embryos (Fig. 3d), as well as a cluster of SE (Fig. 3e). After an additional 4 weeks of incubation in the light, somatic embryos successfully germinated on MS medium supplemented with 0.1 mg/L NAA + 0.05 mg/L BA (Fig. 3f) and developed into normal plantlets (Fig. 3g). This final stage of shoot and root formation is described in detail in the shoot and root sections.
Morphological developmental stages of SE of date palm Barhi cv.; (a) Globular-shaped SE induced on MS + 0.1 mg/L NAA; (b) Heart-shaped SE; (c) Torpedo- shaped SE; (d) Cotyledonary-shaped SE; (e) Cluster of SE developed on MS + 0.1 mg/L NAA + 0.05 mg/L BA; (f) Germinating SE; (g) Whole developed plant.
The results presented in Table 2 indicate that EC1 exhibited the highest EC formation rate (100%) with maximal embryo production (8 embryos/explant), whereas EC2 demonstrated a 90% EC formation rate with 7 embryos/explant. Somatic embryos in EC1 and EC2 germinated into small white buds following sub-culturing on MS medium containing 0.1 mg/L NAA + 0.05 mg/L BA for 12 weeks in darkness. Subsequently, approximately 6.2 buds/explant germinated from EC1 and 5 buds/explant from EC2. These germinated buds subsequently developed into Sprouts1 (7 sprouts) and Sprouts 2 (6 sprouts) through re-culturing on the identical medium but incubated under an 8/16-h; light/dark photoperiod to facilitate the formation of green shoots for 1 month.
Shoot elongation
Well-developed sprouts, each possessing a minimum of two sprouts, were isolated from the explant and transferred to a medium treated with three distinct formulations of GA3 (Table 1). As presented in Table 3, the highest mean shoot length was observed in both treated Sprouts 1 and 2 when were cultured on MS medium supplemented with the moderate concentration (0.5 mg/L) of GA3. Sprouts 1 yielded taller shoots (6.7 cm), while Sprouts 2 exhibited a slightly shorter shoot length (6.4 cm) on the same medium.
Figure 4 shows that both higher (1 mg/L) and lower (0.2 mg/L) concentrations of GA3 significantly (p < 0.05) resulted in reduced shoot lengths. Furthermore, abnormal growth was observed in shoot 6, which developed from Sprout 2, when cultured on the medium containing the higher concentration (1 mg/L) of GA3.
Root formation
Results presented in Table 4 and Fig. 5 demonstrated that roots developed most robustly on rooting 1/2 MS medium (semi-liquid) containing 0.5 mg/L NAA. A greater number of roots (5.6 roots/shoot) and increased root length (6.3 cm) were initiated from shoots developed from Sprout 1. In contrast, a significant reduced number of roots (4 roots/shoot) and slightly shorter root lengths (5 cm) were observed on the same medium from sprout 2 developed shoots.
In vitro plantlets acclimatization
The acclimatization process was successfully executed irrespective of the specific composition of the culture medium and the growth regulator employed. Well-elongated plantlets from all in vitro treatments were carefully removed from their culture medium and progressively hardened under greenhouse conditions, achieving a high survival rate 87.5%. After a 3-month period under greenhouse conditions, all surviving plants exhibited normal morphological development and uniform growth, with no observable abnormalities (Fig. 6). Acclimatized plants developed an average of 3–5 leaves per plant, with an approximate leaf length of 15–20 cm.
Genetic fidelity using ISSR markers
The results of ISSR analysis indicated that all seven ISSR primers utilized produced scorable, clear and reproducible bands following PCR amplification, yielding a total of 45 scorable bands with an average of 6.43 bands/ primer (Table 5). The primer UBC-827 generated the maximal bands (13), whereas both UBC-868 and UBC-880 primers each generated only a single band.
Results revealed that the most obtained bands (44 out of 45) of six ISSR primers were monomorphic and exhibited very high similarity (97.5%). Only one band generated by UBC-834 primer was polymorphic (2.5%) (Figs. 7 and 8).
Discussion
In vitro micropropagation of date palm via ISE system is a highly favored and widely implemented methodology, particularly for commercial production requiring large-scale regeneration of plantlets. Callus tissue, characterized by its undifferentiated cellular state, serves as a renewable source for sustained in vitro propagation. However, plantlets derived from callus tissues often exhibit somaclonal variations and genetic instability. These undesirable phenomena are frequently attributed to various factors employed during the callus induction process, the type and concentration of auxin, particularly 2,4-D, being a prominent determinant, especially when applied at elevated levels. In this study we addressed two ISE protocols established from calli induced by low and high levels of 2,4-D, as repeatedly suggested in previous studies, and implemented a genetic fidelity test.
PGR effect on callus initiation and multiplication
The results demonstrated a significant increase (p < 0.05) in the FW of callus induced and proliferated in the presence of a lower dose of 2,4-D in the medium. This indicates that a lower dose of 2,4-D exerted a more pronounced inductive effect on callus biomass accumulation than the higher dose applied. These results are consistent with previous reports in certain date palm cultivars. For example, elevated levels of 2,4-D inhibited in vitro cellular biomass accumulation in Shishi cv.27 and induced severe negative effects in Barhi cv. in vitro cultures 32,39, a finding also observed in other plant species such as rice40. The suppressive effect observed with higher concentrations of 2,4-D was attributed to necrosis in cultured tissues and subsequent browning of the medium, likely due to increased polyphenol production32,41. Furthermore, higher applications of 2,4-D are primarily responsible for somaclonal variations detected in in vitro regenerated plants 19,24,25.
2,4-D is commonly employed in callus induction/initiation, either alone or in combination with cytokinins such as 2iP or BA, across numerous date palm species, with varied concentrations of 2,4-D applied, including very low (1 mg/L)27, low (5 mg/L)26, moderate (10 mg/L) 34, high (50 mg/L)25, and very high (100 mg/L)28,29,30,31. These auxin levels are occasionally combined with low concentrations of cytokinins, such as 3 mg/L of 2iP34,35,42. The presence of PGR (auxin and cytokinin) is indispensable during callus induction and proliferation. This is owing to the synergistic effect exerted by these two types of PGR, where auxin plays a crucial role in cell dedifferentiation and biomass accumulation, whereas cytokinin exerts a considerable influence on cell division during callogenesis27,43. Our results indicate that a stronger synergistic effect, reflected in improved callogenesis growth and development, was observed when explants were treated with the combination containing the lower dose of 2,4-D. This superior effect was evidenced by higher callus FW, the absence of distinct brown spots, and more vigorous proliferation.
Somatic embryogenesis and bud formation
Somatic embryos were successfully formed from callus induced by either higher or lower levels of 2,4-D, with a higher formation rate observed in EC1. In this context, 2,4-D is widely used in the establishment stage of SE in date palm and is crucial for EC development in many cultivars, including Barhi23,30,35,44. In a recent study conducted by Othmani et al. (2024), 2,4-D at 1 mg/L successfully induced callus formation from the basal region of mature tetraploid female flowers of the Deglet Nour’ date palm cv.45. Although prolonged or high-dose 2,4-D treatments may induce somaclonal variation, including potential changes in ploidy, this study did not directly assess ploidy status. Future work could include flow cytometry or chromosome counts to confirm cytogenetic stability of regenerants. This limitation does not affect the conclusions on regeneration efficiency but outlines a direction for confirmatory analysis. In contrast to 2,4-D, NAA is preferred and extensively used in the culture medium for many in vitro post-initiation stages, such as EC induction, SE initiation and germination, and shoot/root development. In this study, the results indicated that both media used for EC induction and SE germination were fortified with NAA (0.1 mg/L) and NAA (0.1 mg/L) + 0.05 mg/L BA, respectively. The maximum germinated buds were observed in EC1. A similar level of NAA, but with a higher level of KIN (0.1), was applied to induce the highest multiplication and germination rate (88%) of buds in Barhi cv.30. Diverse combined levels of auxin and cytokinin are frequently applied in SE germination in many date palm species, as exemplified in Khalas cv. when 4 mg BA/L + 0.5 mg/L of NAA induced 4.50 buds, while 8.75 buds were achieved on medium had 2 mg BA/L + 1 mg/L NAA in Sagai cv.46.
In some instances, NAA alone successfully induced SE germination at 0.1 mg/L47. In other studies, a hormone-free MS medium was used for SE germination in several date palm species, such as Khalas, Ajwa, Jarvis48, Halawy and Khalas cvs49. Furthermore, different types of media, including solid/liquid, with various additives have been investigated. Resan et al.50 optimized liquid MS media with 50 mg/L phloroglucinol (PG) in combination with 0.5 mg/L TDZ for the highest buds germinated (23 buds) from SE of Barhi cv.
Sprouting and shoot elongation
Results indicated that the highest sprouting developed from EC1 on the germination medium containing 0.1 mg/L NAA + 0.05 mg/L BA. Previously, varying responses in sprouting or shoot formation have been reported across numerous date palm varieties, attributed to factors such as media composition, the type and concentration of PGR employed, and the specific cultivar under investigation. Different numbers and heights of shoots have been documented for various date palm in vitro propagations on MS medium augmented with diverse PGR formulations. For instance, a lower number of shoots (2.2) with maximal shoot length (8.8 cm) developed on MS medium containing 2.0 mg/L Kinetin + 1.5 IAA in Aseel cv.51. Also, 4 shoots formed on media containing 1 mg/L Kin + 1 mg/L BA in Jawzi cv.52, whereas Resan et al. 50 reported the highest average of shoots (41.0 shoots) in Barhi cv. formed on liquid media supplemented with 50 mg/L PG in combination with 0.5 mg/L TDZ. Additionally, Mazri et al.53 induced 30 shoot buds/explant of date palm cv. Al-Fayda on semi-solid 1/2 MS medium supplemented with 2.3 μM KIN + 2.4 μM NOA. These studies collectively indicate that MS medium fortified with auxin and cytokinin53 or with cytokinin alone 50 is commonly employed to induce and develop shoots in date palm in vitro culture.
It has also been posited that in vitro growth is highly dependent on the intricate balance between naturally occurring endogenous hormones within plant cells and the concentrations of analogous synthetic substances exogenously supplied to the culture nutrient medium54. The presence of appropriate auxin concentrations in conjunction with suitable cytokinin concentrations appears to be paramount for the successful micropropagation of most plants in vitro55,56. Low auxin concentrations in the presence of cytokinin have been shown to stimulate the multiplication of adventitious buds in date palm species 57.
Regarding shoot elongation, our results showed that the tallest shoots developed on the medium augmented with 0.5 mg/L GA3. Conversely, higher (1 mg/L) or lower (0.2 mg/L) concentrations of GA3 significantly (p < 0.05) resulted in reduced shoot lengths. Furthermore, abnormal growth was observed in some shoots developed from Sprouts 2 when cultured on the medium containing the higher concentration (1 mg/L) of GA3.
GA3 positively influences shoot elongation in numerous plant species produced ex vitro or in vitro, although elevated levels may be associated with certain malformations. This observation was corroborated by Al-Najm et al.57, who reported that an increase in shoot length was associated with increasing GA3 concentration to 1.0 mg/L, but also noted some malformations such as thin growth and distortions of slender leaves, which subsequently hindered rooting and transplanting. Their results also indicated significantly improved elongation, with an optimized shoot length of 7.64 cm in date palm cultivated on medium containing 0.5 mg/L GA3, compared to other treatments. Our findings align with these results, as 0.5 mg/L of GA3 yielded normal and robust shoot elongation. This further confirms the well-established role of GA3 in plant cell elongation. Gibberellins promote elongation by stimulating sub-apical meristem cell proliferation and elongation, and by facilitating the hydrolysis of polysaccharides into simple sugars beneficial to plant tissue58.
Root formation
Results indicated a higher number and length of roots developed in semi-liquid 1/2 MS medium treated with 0.5 mg/L NAA compared to other treatments (p < 0.05). These findings are consistent with those reported by Alansi et al.46, who found that 0.5 mg/L NAA + MS medium induced more roots (4.14) in Sagai cv., whereas the longest root length (4.10 cm) was achieved at 1.5 mg/L of IAA. Conversely, Hassan et al.59 claimed that MS salt medium supplemented with 0.2 mg/l NAA and 0.1 mg/l indole-3-butyric acid (IBA) gave optimal root formation for Medjool cv. Also, Al-Najm et al.57 observed that optimal rooting (81%) was attained on MS medium + a lower concentration 0.2 mg/L NAA in six date palm cultivars, including Barhi. In vitro root induction and growth exhibit variability depending on numerous factors, primarily cultivar type, PGR formulations, and the physicochemical properties of the media. Furthermore, the physical state of the employed medium during rooting plays a vital role in root formation, with semi-solid/liquid media being preferred during the rooting stage. Mazri et al.53 observed that optimal rooting (3.90 roots/shoot) of Al-Fayda cv was successfully achieved on semi-solid 1/2 MS with 2.3 μM KIN + 2.4 μM NOA. Auxins are commonly incorporated into rooting media, having long been recognized for their active role in root formation60. The initial cell division for root initiation is primarily dependent on the type and concentration of auxin applied. NAA is considered the most effective auxin for root induction and is frequently utilized at various concentrations in numerous plant species61. Some studies suggest that high auxin levels may inhibit root formation and reduce root number. Al-Najm et al.57 demonstrated that the highest rooting rate and root number were achieved at lower concentrations (0.1–0.5 mg/L) of NAA in six date palm cultivars, while higher concentrations acted as rooting inhibitors.
Hardening and acclimatization of plantlets
Acclimatization is the physiological process of gradually transitioning plants from a heterotrophic (in vitro) to an autotrophic (ex vitro) mode of nutrition. The results exhibited a high survival rate (87.5%) for hardened plantlets after 6 weeks of ex vitro incubation. Normal phenotypic growth with no deformities was observed during 3 months of cultivation under greenhouse conditions. Similar results, with a higher survival rate (95%), were reported by Solangi et al.30 for micropropagated Barhi cv. plantlets acclimatized in a comparable manner. Conversely, a lower survival rate (70.32%) was recorded for hardened Barhi plantlets in a protocol implemented by Samiei et al.62. In other studies, an 80% survival rate was detected during the hardening of in vitro plantlets of six date palm species, including Barhi cv. Similar to the variations in culture conditions, PGR, and media types selected during in vitro culture, various soil mixtures are used in different ratios, and numerous uncontrolled conditions are imposed on plantlets during the acclimatization process. These variations inherently influence the growth and development of acclimatized plants. Therefore, this phase is critically important, as it directly determines the quality of preceding phases and the ultimate productivity of the extensive work invested.
Genetic conformity using ISSR markers
Establishing an in vitro regeneration system that preserves the quality traits of regenerants is highly valuable and strongly recommended, particularly for plants generated from callus61. In this regard, molecular markers have been extensively employed to assess the genetic conformity of plant species regenerated via in vitro culture, especially for economically important species such as date palm, prior to large-scale production49,63. Our results unequivocally demonstrated that the indirect organogenesis pathway established in this study effectively preserves the genetic stability of regenerants developed from callus induced by both low (10 mg/L) and high (50 mg/L) doses of 2,4-D PGR, maintaining 97.5% of genetic homogeneity between the regenerants and the donor plant. These findings corroborate previously reported genetic stability in in vitro plants initiated from SE derived from callus induced by 2,4-D concentrations below 50 mg/L in different date palm cultivars. Genetic stability has been confirmed in Barhi plantlets developed from callus induced on medium + 90.5 µM 2,4-D19, and no genotoxic effect of 2,4-D doses below 50 mg/L was observed in Hillawii date palm plantlets25. Furthermore, a high dose of 100 mg/L of 2,4-D is widely applied in inducing callus for establishing indirect in vitro plantlets from various date palm varieties, with no reported abnormalities or somaclonal variations28,29,30.
In contrast to these results, Baklouti et al.32 reported genetic variations by ISSR analysis during in vitro initiation of plantlets from callus induced by 2,4-D levels below 40 mg/L in Barhi cv. Similarly, Abass et al.25 detected genetic variations between Hillawii date palm plantlets using RAPD markers when 100 mg/L of 2,4-D was employed. These contrasting findings have been previously discussed; Mazri and Meziani7 review revealed that the genetic conformity of date palm plants micropropagated through SE has been a subject of controversial discussion. This discrepancy may be attributed to interactions among various factors during in vitro cultivation, such as indigenous and exogenous PGR, media composition, varied control conditions, plant species/cultivar, and the number of re/subcultures applied. These factors are frequently cited as the primary agents used to interpret the observed conflicting results regarding genetic conformity in date palm.
Numerous studies recommend strategies to mitigate the risk of somaclonal variation during in vitro date palm culture, including the use of low doses of auxins (primarily 2,4-D), reduced numbers of subcultures, and the utilization of juvenile explants 64,65,66,67,68. Although the present study demonstrated robust genetic stability in all obtained plantlets, those developed from callus treated with a low concentration (10 mg/L) of 2,4-D exhibited superior normality with enhanced in vitro organs development compared to plantlets derived from callus induced by a higher dose (50 mg/L) of 2,4-D, where some plants displayed dwarfism and abnormal leaves. These results corroborate and support studies that advocate for the use of lower doses of PGR over higher doses, even when genetic stability is ensured.
Conclusion
This study demonstrated a significant superiority of low 2,4-D concentration across different in vitro culture stages of date palm. The low concentration enhanced callus proliferation and organogenic efficiency, producing more robust regenerants compared to those from cultures treated with a high 2,4-D level. Critically, ISSR analysis confirmed that all regenerated plants were genetically uniform, and they were successfully acclimatized with a high survival rate (88.5%). The inhibitory effect of high 2,4-D concentration is thus confined to the phenotype, suppressing morphogenesis without inducing genetic variability. Based on these findings, two optimal concentration ranges of 2,4-D for date palm ISE can be recommended:
-
1.
From10 to 50 mg/L (effective for callus induction without genetic instability).
-
2.
From 0 to 10 mg/L (more favorable for maximizing morphogenic efficiency and ensuring genetic uniformity).
Data availability
The data analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Mssallem, M. Q. et al. Role of date palm to food and nutritional security in Saudi Arabia. In Food and nutrition security in the Kingdom of Saudi Arabia (eds Ahmed, A. E. et al.) 337–358 (Springer International Publishing Cham, Berlin, 2024).
Hussain, M. I., Farooq, M. & Syed, Q. A. Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.): A review. Food Biosci. 34, 100509. https://doi.org/10.1016/j.fbio.2019.100509 (2020).
Krueger, R. R. Date palm (Phoenix dactylifera L.) biology and utilization. In The date palm genome: Phylogeny Biodiversity and Mapping (eds Al-Khayri, J. M. et al.) 3–28 (Springer International Publishing, Berlin, 2021).
Habib, H. M. & Ibrahim, W. H. Nutritional quality of 18 date fruit varieties. Int. J. Food Sci. Nutr. 62, 544–551. https://doi.org/10.3109/09637486.2011.558073 (2011).
Rambabu, K. et al. Nutritional quality and physico-chemical characteristics of selected date fruit varieties of the United Arab Emirates. Processes 8, 256 (2020).
Perveen, K. & Bokahri, N. A. Comparative analysis of chemical, mineral and in-vitro antibacterial activity of different varieties of date fruits from Saudi Arabia. Saudi J. Biol. Sci. 27, 1886–1891. https://doi.org/10.1016/j.sjbs.2019.11.029 (2020).
Khanum, P., Khan, A. A., Khan, I. A., Ghaffar, A. & Khan, Z. Recent advances in date palm (Phoenix dactylifera L.) Biotechnology and breeding. In Breeding and biotechnology of leaf, fruit, and seed fiber crops advances in plant breeding strategies Vol. 11 (eds Salem, K. F. M. et al.) (Springer, Cham, 2025).
Mazri, M. A. & Meziani, R. Micropropagation of date palm: A review. Cell Dev. Biol. 4, 160 (2015).
Mullassery, B., Kurien, S., Umesh, C. & Kurup, S. Green house and field level problems of tissue cultured date palm (Phoenix dactylifera L.) plants: A review. Indian J Agric Res https://doi.org/10.18805/IJARe.A-6378 (2025).
Ali, A. S. A. & Hama, N. N. Integrated management for major date palm pests in Iraq. Emirates J. Food Agric. 28, 1. https://doi.org/10.9755/ejfa.2016-01-032 (2016).
Laaguidi, M., Meziani, R. & Sellam, K. In vitro culture of date palm: A review of challenges and solutions for managing endophytic bacteria contamination. Vegetos https://doi.org/10.1007/s42535-025-01287-x (2025).
Pandey, N., Tripathi, P., Pandey, N., Nakum, H., & Vala, Y. S. Advancements in Date palm genomics and biotechnology genomic resources to the precision agriculture: A comprehensive review. (2024).
Malabadi, R. B., Chalannavar, R. K. & Kolkar, K. P. Plant cell totipotency: Plant tissue culture applications—an updated review.. World J Adv Eng Technol Sci 16, 112–135. https://doi.org/10.30574/wjaets.2025.16.2.1262 (2025).
Nimavat, N. & Parikh, P. Innovations in date palm (Phoenix dactylifera L.) micropropagation: Detailed review of in vitro culture methods and plant growth regulator applications. Plant Cell Tissue Organ Cult. 159, 6. https://doi.org/10.1007/s11240-024-02866-7 (2024).
Zein Eldin, A. F. M. & Ibrahim, H. A. Some biochemical changes and activities of antioxidant enzymes in developing date palm somatic and zygotic embryos in vitro. Ann. Agric. Sci. 60, 121–130. https://doi.org/10.1016/j.aoas.2015.04.002 (2015).
Al-Asadi, A. Z., Al-Mayahi, A. M. & Awad, K. M. Effects of dicamba and casein hydrolysate on in vitro growth and growth and shoot regeneration of date palm (Phoenix dactylifera L.) cv. Barhee. Folia Oecol. 51, 56–65. https://doi.org/10.2478/foecol-2024-0006 (2024).
Eldawayati, M. M., Zayed, Z. E., Abdelaala, W. B. & Zayed, E. M. AgNPs: A superior alternative to AgNO3 for the optimal plantlets production by the indirect somatic embryogenesis protocol for date palm ‘Barhee’. RPCur. Tr. Agri. Env. Sci. 4, 25–31 (2025).
Al-Mayahi, A. M., Kalaf, Y. N., Abdul-Sahib, A. M., Abdul-Sahib, I. M. & Al-Sharifi, A. A. Anatomical study of adventitious bud regeneration from shoot tip of date palm (Phoenix dactylifera L.) cv Barhee in vitro. Basrah J. Date Palm Res. 23, 116–126 (2024).
AL-Mayahi, A. M. W. Effect of sodium nitroprusside with plant growth regulators on in vitro propagation and genetic stability of ‘Barhee’date palm (Phoenix dactylifera L.). J. Hortic. Res. 33, 1. https://doi.org/10.2478/johr-2025-0003 (2025).
Solangi, N. et al. Factors influencing somatic embryogenesis and plantlet regeneration of date palm using immature floral buds. Sarhad J Agric 39(2), 323–331. https://doi.org/10.17582/journal.sja/2023/39.2.323.331 (2023).
Mirani, A. A., Harikrishna, J. A., Teo, C. H. & Solangi, N. Factors influencing somaclonal variation in date palm, detection and selection for application in the plantation. In Somaclonal variation: basic and practical aspects (ed. Sánchez-Romero, C.) (Springer, Cham, 2024).
Qahtan, A. A., Faisal, M., Alatar, A. A. & Abdel-Salam, E. M. Callus-mediated high-frequency plant regeneration, phytochemical profiling, antioxidant activity and genetic stability in Ruta chalepensis L.. Plants 11, 1614 (2022).
Gueye, B. et al. Acquisition of callogenic capacity in date palm leaf tissues in response to 2, 4-D treatment. Plant Cell Tissue Organ Cult. 99, 35–45 (2009).
Garcia, C. et al. Abnormalities in somatic embryogenesis caused by 2,4-D: an overview. Plant Cell Tissue Organ Cult. (PCTOC) 137, 193–212. https://doi.org/10.1007/s11240-019-01569-8 (2019).
Abass, M. H., Al-Utbi, S. D. & Al-Samir, E. A. Genotoxicity assessment of high concentrations of 2, 4-D, NAA and Dicamba on date palm callus (Phoenix dactylifera L.) using protein profile and RAPD markers. J. Genet. Eng. Biotechnol. 15, 287–295 (2017).
Abohatem, M., Al-Qubati, Y. & Abohatem, H. Effect of dark incubation in germination of indirect date palm somatic embryos and conversion into plantlets. J. Plant Biotechnol. 50, 267–274. https://doi.org/10.5010/JPB.2023.50.033.267 (2023).
Al-Khayri, J. M. & Naik, P. M. Influence of 2iP and 2,4-D concentrations on accumulation of biomass, phenolics, flavonoids and radical scavenging activity in date palm (Phoenix dactylifera L.) cell suspension culture. Horticulturae 8, 683. https://doi.org/10.3390/horticulturae8080683 (2022).
Al-Khayri, J. M. Somatic embryogenesis of date palm (Phoenix dactylifera L.) improved by coconut water. J. Biotechnol. 9, 477–484 (2010).
Bhati, A., Singh, D., Garg, S. & Sivalingam, P. N. Effect of 2, 4-D and NAA on callus induction in date palm cultivars Halawy and Medjool. Int. J. Farm Sci. 7, 132–136 (2017).
Solangi, N. et al. Developing micropropagation protocols of date palm (Phoenix dactylifera L.) cv Barhi using shoot tip explants. Proc Natl Acad Sci, India, Sect B Biol Sci. 93, 995–1004. https://doi.org/10.1007/s40011-023-01452-9 (2023).
Rathore, M. S., Patel, P. R. & Siddiqui, S. A. Callus culture and plantlet regeneration in date palm (Phoneix dactylifera L.): An important horticultural cash crop for arid and semi-arid horticulture. Physiol. Mol. Biol. Plants 26, 391–398. https://doi.org/10.1007/s12298-019-00733-w (2020).
Baklouti, E. et al. 2, 4-D induction of somaclonal variations in in vitro grown date palm (Phoenix dactylifera L. cv Barhee). Plant Cell Tissue Organ Cult. 150, 191–205 (2022).
Al-Samir, E. A. H., Al-Utbi, S. D. & Abass, M. H. Phytotoxic effect of 2, 4-D and dicamba on date palm (Phoenix dactylifera L.) tissue cultures at initiation stage. Adv. Agric. Bot. 7, 96–108 (2015).
Eldin, Z. A. F. M. & Ibrahim, H. A. Some biochemical changes and activities of antioxidant enzymes in developing date palm somatic and zygotic embryos in vitro. Ann. Agric. Sci. 60, 121–130 (2015).
Zein El Din, A. F. M. et al. Morpho-anatomical and biochemical characterization of embryogenic and degenerative embryogenic calli of Phoenix dactylifera L. Horticulturae 7, 393. https://doi.org/10.3390/horticulturae7100393 (2021).
Ammar, M. A. et al. Enhancing phytochemical content and bioactive aspects in somatic embryogenesis developed from callus of Phoenix dactylifera L. Notulae Bot. Horti Agrobotanici Cluj-Napoca 53, 14374–14374 (2025).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physio. Plantarum 15, 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x (1962).
Doyle, J. J. & Doyle, J. L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987).
Oliveira, M. S. et al. Negative effects of high 2,4-D in Barhi cultivar. J. Plant Biotechnol. 50, 234–245 (2023).
Islam, F. et al. 2,4-D attenuates salinity-induced toxicity by mediating anatomical changes, antioxidant capacity and cation transporters in the roots of rice cultivars. Sci. Rep. 7, 1–23. https://doi.org/10.1038/s41598-017-09708-x (2017).
Islam, F. et al. Combined herbicide and saline stress differentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol. Biochem. 107, 82–95. https://doi.org/10.1016/j.plaphy.2016.05.027 (2016).
Zein El Din, A. F. M. et al. Antioxidants application enhances regeneration and conversion of date palm (Phoenix dactylifera L.) somatic embryos. Plants 11, 2023. https://doi.org/10.3390/plants11152023 (2022).
Tuaimah, M. H., Jaffar, O. N. & Sabti, M. Z. Effect of media type, Cytokinins and Auxins on the formation of embryogenesis of date palm Phoenix dactylifera L. in vitro. J. Kerbala for Agric. Sci. 12(1), 84–95. https://doi.org/10.59658/jkas.v12i1.3243 (2025).
Sinta, M. M., Saptari, R. T., Riyadi, I. & Sumaryono, S. Callus induction and regeneration of date palm (Phoenix dactylifera L.) cv Zambli through somatic embryogenesis from four layers of young leaves explant. Menara Perkebunan 92, 2. https://doi.org/10.22302/iribb.jur.mp.v92i2.588 (2024).
Othmani, A. et al. In vitro initiation, regeneration, and characterization of plants derived from mature tetraploid floral explants of date palm (Phoenix dactylifera L.). Horticulturae 10, 1206. https://doi.org/10.3390/horticulturae10111206 (2024).
Alansi, S. A. L. E. H. et al. An efficient micropropagation protocol via indirect organogenesis from callus of economically valuable crop date palm (Phoenix dactylifera L.) cultivars “Sagai and Khalas”. Pak. J. Bot. 52, 2021–2030 (2020).
Zayed, Z. E. Enhanced indirect somatic embryogenesis from shoot-tip explants of date palm by gradual reductions of 2,4-D concentration. In Date palm biotechnology protocols Vol. 1 (eds Al-Khayri, J. M. et al.) 77–88 (Humana Press, New York, 2017).
Sinta, M. M., Saptari, R. T., Riyadi, I. Tissue culture of four varieties of date palm grown in Indonesia. In: IOP Conference Series: Earth and Environmental Science Vol. 1255, 012019. IOP Publishing (2023).
Kumar, K., Singh, D. & Saroj, P. L. Callus induction, somatic embryogenesis, in vitro plantlet development and ex vitro transplantation of two date palm (Phoenix dactylifera L.) cultivars. Int. J. Chem. Stud. 8, 758–763 (2020).
Resan, A. Z., Al-Mayahi, A. M. & Abdulwahid, A. H. Effect of medium type, TDZ, PG, and their interactions on in vitro regeneration (Phoenix dactylifera L.) cv Barhee. Basrah J. Date Palm Res. 22, 32–51 (2023).
Mangrio, G. S., Simair, A. A., Shumaila, S., Kumar, B. & Mangrio, N. Nutrient media optimization for date palm micropropagation (Phoenix dactylifera L.). Pak. J. Biochem. Biotechnol. 2, 87–96. https://doi.org/10.52700/pjbb.v2i2.48 (2021).
Ibrahim, A. M., Hameed, M. K. & Mohammed, A. In vitro propagation of date palm (Phoenix dactylifera L.) cultivar Jawzi using shoot tip. Basrah J. Agric. Sci. 36, 267–284. https://doi.org/10.37077/25200860.2023.36.2 (2023).
Mazri, M. A., Meziani, R., Elmaataoui, S., Alfeddy, M. N. & Jaiti, F. Assessment of genetic fidelity, biochemical and physiological characteristics of in vitro grown date palm cv. Al-Fayda. Vegetos 32, 333–344. https://doi.org/10.1007/s42535-019-00034-3 (2019).
Kahia, J., Kirika, M., Lubabali, H. & Mantell, S. High-frequency direct somatic embryogenesis and plantlet regeneration from leaves derived from in vitro-germinated seedlings of a Coffea arabica hybrid cultivar. Hort. Sci. 51, 1148–1152 (2016).
Moradi, F. Plant growth regulators past, present and future. Res. Achieve Field Hortic. Crops 5, 71–95 (2016).
Sidik, N. J., Agha, H. M., Alkamil, A. A., Alsayadi, M. M. S. & Mohammed, A. A. A Mini review of plant tissue culture: The role of media optimization, growth regulators in modern agriculture, callus induction and the applications. AUIQ Complement Biol Syst 1(2), 96–109 (2024).
Al-Najm, A., Brauer, S., Trethowan, R. & Ahmad, N. Optimisation of in vitro micropropagation of several date palm cultivars. Aust. J. Crop Sci. 12, 1937–1949 (2018).
Robil, J. M., Awale, P., McSteen, P. & Best, N. B. Gibberellins: Extending the green revolution. J Exper Bot. 76(7), 1837–1853. https://doi.org/10.1093/jxb/erae476 (2025).
Hassan, M. M., Allam, M. A., Shams El Din, I. M., Malhat, M. H. & Taha, R. A. High frequency direct somatic embryogenesis and plantlet regeneration from date palm immature inflorescences using picloram. J. Genet. Eng. Biotechnol. 19, 33. https://doi.org/10.1186/s43141-021-00129-y (2021).
Chawla, R., Guleria, T. & Thakur, A. Role of plant growth regulators in fruit crop production: A comprehensive review. Appl. Fruit Sci. 67(4), 294. https://doi.org/10.1007/s10341-025-01535-z (2025).
Pasternak, T. P. & Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 13, 327 (2024).
Samiei, L. et al. Acclimatization protocols for Barhi plantlets. Plant Biotechnol. Rep. 16, 345–356 (2022).
Al-Salim, A.-H., Suhaim, A. A. & Jaffer, O. N. Evaluation of genetic diversity uses SSR and ISSR markers on date palm cultivars that propagated through tissue culture and vegetative propagation. Basrah J. Agric. Sci. 38, 84–95. https://doi.org/10.37077/25200860.2025.38.sp.7 (2025).
El Hadrami, A., Daayf, F., Elshibli, S., Jain, S. M. & El Hadrami, I. Somaclonal variation in date palm. In Date palm biotechnology (eds Jain, S. M. et al.) (Springer, Dordrecht, 2011).
Abdelghaffar, A. M. et al. Genetic diversity assessment and in vitro propagation of some date palm (Phoenix dactylifera L.) varieties. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 51(4), 13449–13449 (2023).
Khan, S. A. et al. In vitro inhibitory effects on α-glucosidase and α-amylase level and antioxidant potential of seeds of Phoenix dactylifera L. Asian Pac. J. Trop. Biomed. 6, 322–329. https://doi.org/10.1016/j.apjtb.2015.11.008 (2016).
Sota, V. et al. Challenges in the micropropagation of economically important fruit species in Europe. Plant Cell Tissue Organ Cult. 162, 53. https://doi.org/10.1007/s11240-025-03165-5 (2025).
Zammouri, G. et al. Morphological and biochemical characterization and in vitro regeneration of an elite Tunisian date palm (Phoenix dactylifera L.) cultivar ‘Boufeggous’. J. OASIS Agricul. Sustain. Devel. 7(03), 1–11 (2025).
Acknowledgements
The authors extend their appreciation for the support of the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant No. KFU254298).
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. KFU254298].
Author information
Authors and Affiliations
Contributions
Conceptualization, A.M.A.A. and J.M.A.; methodology, A.M.A.A. and A.A.Q.; software, M.N.N.; validation, A.M.A.A. and J.M.A.; formal analysis, A.M.A.A. and A.A.Q.; investigation, A.M.A.A. and A.A.Q.; funding acquisition, J.M.A. and H.S.G.; resources, A.M.A.A. and A.A.Q.; data curation, J.M.A. and H.S.G.; writing manuscript, A.M.A.A; Revision, J.M.A. and H.S.G.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ali, A.M.A., Qahtan, A.A., Al-Khayri, J.M. et al. Effect of low 2,4-D concentration on enhancing indirect embryogenesis and genetic stability in date palm (Phoenix dactylifera L.). Sci Rep 15, 43865 (2025). https://doi.org/10.1038/s41598-025-29924-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-29924-0