Abstract
4-Hydroxynonenal (4-HNE), a product of lipid peroxidation, is recognized as a biomarker of oxidative stress. However, its relationship with the severity and prognosis of acute exacerbation in chronic obstructive pulmonary disease (AECOPD) remains unclear. This prospective cohort study aimed to investigate the associations between plasma 4-HNE levels and disease severity and prognosis in AECOPD patients. A total of 150 AECOPD patients, 80 stable COPD (SCOPD) patients, and healthy volunteers were enrolled. Plasma 4-HNE and inflammatory cytokines were measured using enzyme-linked immunosorbent assay (ELISA). Compared to healthy individuals, plasma 4-HNE levels were significantly elevated in both SCOPD and AECOPD patients, with progressively increasing alongside worsening pulmonary function and higher mMRC, CAT, and CCQ scores. In AECOPD patients, plasma 4-HNE was positively correlated with inflammatory cytokines, and linear regression analysis revealed that elevated plasma 4-HNE was associated with increased disease severity. Furthermore, higher plasma 4-HNE levels at admission were linked to prolonging hospital stays and AECOPD, indicating a poorer prognosis. Compared with several conventional biomarkers, plasma 4-HNE demonstrated superior predictive value for AECOPD and clinical outcomes. These findings suggest that plasma 4-HNE may be a useful biomarker for assessing severity and prognosis in AECOPD patients, potentially playing a role in the underlying pathophysiology of the disease.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the major causes of morbidity and death worldwide and is characterized by airflow limitation1. COPD has become the fourth leading cause of death, and 328 million people were diagnosed with COPD globally in 20152. In China, 13.7% of people over 40 years old suffer from COPD3. Tobacco smoke, ambient air pollution, underweight status and genetic factors are significant risk factors for COPD4. Symptoms of COPD include breathlessness, sputum production, and excessive mucus production. COPD decreases the ability to exercise in daily life and quality of life5. COPD is a chronic disease, and its course is characterized by acute exacerbation of respiratory impairment. Every acute exacerbation may promote disease progression and increase the risk of death and hospital admission for COPD patients6,7,8. Unfortunately, the prevalence of COPD is gradually increasing annually. Many studies have demonstrated that various clinical indicators have been broadly applied as prognostic factors for mortality in acute exacerbation of COPD (AECOPD) patients9,10. However, the predictive effectiveness of clinical indicators varies across studies. Therefore, it is essential to identify new biomarkers to monitor disease severity and guide the management of AECOPD patients.
The most important pathogeny for AECOPD is infection8. During the infection process, the body produces a series of antibacterial responses, including the generation of reactive oxygen species (ROS)11. ROS can react with cellular lipids and initiate lipid peroxidation12. 4-Hydroxynonenal (4-HNE) is an oxygen-containing unsaturated aldehyde originating from the lipid oxidation process of polyunsaturated fatty acids, including linoleic acid, linolenic acid and arachidonic acid13. 4-HNE is an important product of endogenous lipid peroxidation14. 4-HNE is maintained at a very low physiological level in cells or the body. Nevertheless, 4-HNE is evidently elevated when the body is subjected to oxidative stress15,16. As a significant product of oxidative stress in body, 4-HNE is also an important cell signaling molecule. Several studies have demonstrated that 4-HNE can react with enzymes and kinases in a variety of cell pathways, indicating that 4-HNE is involved in the physiological activities17,18. Lipid peroxidation induced by oxidative stress is related to many diseases in humans. There is growing evidence that oxidative stress is increased in patients with community-acquired pneumonia, airway inflammation, acute lung injury, and pulmonary fibrosis19,20,21,22,23. Moreover, oxidative stress plays a significant role in the pathogenesis of COPD21.
4-HNE is also considered a biomarker of oxidative stress24. Previous studies have revealed that 4-HNE is increased in airway epithelial cells, alveolar epithelial cells and endothelial cells in COPD rat models. Moreover, an in vivo study indicated that 4-HNE is increased in a mouse model of cigarette smoke-evoked COPD25. Immunohistochemistry has revealed that 4-HNE is located in the lung tissues of COPD patients and mice26,27. Moreover, a previous study revealed that 4-HNE is increased in the lung tissues of COPD patients26. Therefore, we assume that 4-HNE may participate in the pathogenesis of AECOPD. However, the function of 4-HNE in AECOPD has not been clarified. Consequently, the purpose of this study was to explore the associations of plasma 4-HNE levels with disease severity and prognosis in patients with AECOPD.
Materials and methods
Subjects
In this study, AECOPD and stable COPD (SCOPD) patients were all enrolled from September 2020 to April 2021 in the Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Anhui Medical University. Pulmonary function tests were conducted in all COPD patients. A pulmonary function test was performed when the patient’s condition was stable. We also recruited 150 healthy volunteers (CTRL) without respiratory diseases from the physical examination. Each healthy volunteer was matched with one AECOPD patient according to age, sex, and BMI. The diagnosis of COPD must meet the following criteria: forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) less than 0.7 and an FEV1% less than 80%28. The COPD patients were classified into three ranks according to pulmonary function: Grade 1–2, FEV1%>50; Grade 3, 30 < FEV1%<50; and Grade 4, FEV1%<5029. The exclusion criteria for patients were as follows: complicated with other respiratory diseases; serious complications and asthma; serious infection; malignant tumor patients; younger than 30 years; and organ transplants. All COPD patients were hospitalized for acute exacerbation of COPD. When AECOPD patients were admitted to the hospital before treatment on the first day, fasting blood samples were collected. SCOPD patients were recruited from the outpatients. Moreover, clinical information and demographic characteristics, including the length of hospital stay, history of smoking, number of exacerbations, and clinical laboratory results, were obtained from the electronic medical records system. The length of hospital stay was considered a prognostic outcome and was calculated from hospitalization. Additionally, a questionnaire survey was performed to evaluate disease severity in all COPD patients. This questionnaire included the modified British Medical Research Council (mMRC), COPD assessment test (CAT) score and Clinical COPD Questionnaire (CCQ)30,31. The study was approved by the Ethics Committee in The Second Affiliated Hospital of Anhui Medical University. All COPD patients and controls agreed to participate in this study and signed the informed consent form by themselves or their authorized children.
Enzyme-linked immunosorbent assay (ELISA)
Fasting blood samples were collected from all participants. Each blood sample was divided into two parts: one was placed in common blood collection tubes, and the other was placed in anticoagulant tubes containing EDTA-K2. After centrifugation (3500 g/min), the supernatant was collected from common blood collection tubes and anticoagulant tubes. Then, the plasma and serum were isolated. The samples were immediately stored at -80 °C32,33. 4-HNE-protein adducts were detected in plasma, and inflammatory cytokines were measured in serum through ELISA. Monocyte chemoattractant protein-1 (MCP-1) and 4-HNE ELISA kits were obtained from Cusabio, Wuhan, China (http://www.cusabio.com/). Tumor necrosis factor-α (TNF-α) ELISA kits (JYM0110Hu) were purchased from Wuhan Colorful Gene Biological Technology Co., Ltd. (http://www.jymbio.com/). The levels of 4-HNE in plasma, TNF-α and MCP-1 in serum were measured following the instructions with minor adjustments34. The standard 4-HNE in protein adducts was diluted, and a standard curve was established. The plasma samples and dilutions were added to each well. HRP-conjugated and enzyme-IgG antibodies were subsequently added to the ELISA plate and incubated. The ELISA plate was washed with wash buffer. Finally, the absorbance was measured at a wavelength of 450 nm35,36.
Statistical analysis
All the statistical analyses were conducted via SPSS 21.0. Demographic information and clinical characteristics are expressed as the mean (standard error) or median (interquartile range). Differences in continuous variables were compared with two independent samples t tests. Differences in categorical variables were compared via the chi-square test. Associations between the plasma 4-HNE level and the clinical characteristics of AECOPD patients were analyzed through Pearson correlation analysis or Spearman correlation analysis. Linear and logistic regression analyses were performed to analyze the associations between plasma 4-HNE and severity and prognosis in AECOPD patients. Statistical significance was regarded as P < 0.05.
RESULTS
Demographic information and clinical characteristics
The demographic information and clinical characteristics are summarized in Table 1. No significant differences in sex, age, or body mass index (BMI) were observed among the AECOPD patients, SCOPD patients and healthy controls. The results suggested that the level of pulmonary function was greater in SCOPD patients than in AECOPD patients. Moreover, the severity was evaluated by the CAT, mMRC, and CCQ scores in AECOPD patients. We also detected several inflammatory cytokines in the three groups. While there was no difference of interleukin-6 (IL-6) contents in three groups, the levels of tumor necrosis factor (TNF)-α, C-reactive protein (CRP), and monocyte chemotactic protein (MCP)-1 were significantly elevated in AECOPD patients compared to SCOPD patients and control subjects, particularly higher in AECOPD patients (Table 1).
The levels of plasma 4-HNE in different groups
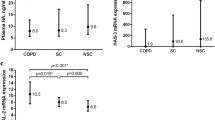
Plasma 4-HNE levels were measured in different groups. As shown in Fig. 1A, the level of plasma 4-HNE was significantly higher in AECOPD and SCOPD patients than those in healthy volunteers. Moreover, the levels of plasma 4-HNE were greater in AECOPD patients compared with SCOPD patients (Fig. 1A). Moreover, we compared the levels of plasma 4-HNE in patients with different grades of AECOPD. The levels of plasma 4-HNE gradually increased with elevating grades among AECOPD patients (Fig. 1B). Moreover, the levels of plasma 4-HNE were further compared in AECOPD patients with different scores. As shown in Fig. 1C, the levels of plasma 4-HNE gradually increased in line with the mMRC score. The CAT score revealed that the levels of plasma 4-HNE was lower in patients with scores ≤ 23 than in those with scores > 23 in patients with AECOPD (Fig. 1D). There was no difference in plasma 4-HNE among AECOPD patients with different CCQ scores (Fig. 1E).
Plasma 4-HNE concentrations in COPD patients and control subjects. Plasma 4-HNE was measured via ELISA and compared in different groups. (A) Plasma 4-HNE concentrations in the three groups (n = 150 for control subjects; n = 150 for AECOPD patients; n = 80 for SCOPD patients). (B) Plasma 4-HNE concentrations in AECOPD patients with different grades. (C) Plasma 4-HNE concentration in AECOPD patients with different mMRC scores. (D) Plasma 4-HNE concentrations in AECOPD patients with different CAT scores. (E) Plasma 4-HNE concentrations in AECOPD patients with different CCQ scores. *P < 0.05, **P < 0.01.
Associations of plasma 4-HNE with clinical characteristics in AECOPD patients
The associations between plasma 4-HNE levels and routine blood indices were analyzed in AECOPD patients via Pearson correlative analysis or Spearman correlative analysis. Pearson correlation analysis revealed that plasma 4-HNE was negatively associated with lymphocytes (r=-0.258, P = 0.031) (Table 2). Moreover, the associations of plasma 4-HNE with renal function, liver function and myocardial function were assessed in AECOPD patients. As shown in Table 2, Spearman correlative analysis found that plasma 4-HNE was positively associated with alanine aminotransferase (ALT) (r = 0.272, P = 0.022), aspartate aminotransferase (AST) (r = 0.261, P = 0.030), total bilirubin (TBIL) (r = 0.249, P = 0.040), direct bilirubin (DBIL) (r = 0.209, P = 0.007), and lactate dehydrogenase (LDH) (r = 0.354, P = 0.012) levels. In addition, the levels of plasma 4-HNE were positively associated with D-dimer (r = 0.209, P = 0.007), procalcitonin (PCT) (r = 0.331, P = 0.043), and fibrinogen (FIB) (r = 0.235, P = 0.045) in patients with AECOPD. Moreover, the association between plasma 4-HNE and pulmonary function was assessed in AECOPD patients. As shown in Fig. 2A and D, Pearson correlative analysis indicated that plasma 4-HNE was negatively correlated with FEV1% (r=-0.500, P < 0.001), FVC (r=-0.298, P < 0.001), FEV1 (r=-0.410, P < 0.001), and FEV1/FVC% (r=-0.259, P = 0.002) in AECOPD patients. Additionally, serum 4-HNE was positively associated with the levels of inflammatory cytokines (MCP-1, TNF-α, IL-6, and CRP) in AECOPD patients (Fig. 2E and H).
Associations among plasma 4-HNE concentration, pulmonary function and inflammatory cytokines in AECOPD patients. (A) Associations between plasma 4-HNE concentration and FEV1% in AECOPD patients. (B) Association between plasma 4-HNE concentration and FVC in AECOPD patients. (C) Association between plasma 4-HNE concentration and FEV1 in AECOPD patients. (D) Association between plasma 4-HNE concentration and FEV1/FVC% in AECOPD patients. (E) Association between plasma 4-HNE concentration and MCP-1 concentration in AECOPD patients. (F) Association between plasma 4-HNE concentration and TNF-α level in AECOPD patients. (G) Association between plasma 4-HNE concentration and the IL-6 concentration in AECOPD patients. (H) Association between plasma 4-HNE concentration and CRP level in AECOPD patients.
Association of plasma 4-HNE with severity in AECOPD patients
As shown in Table 3, although there was no association of plasma 4-HNE with the scores of CAT or mMRC, linear regression analysis indicated that plasma 4-HNE was positively associated with the CCQ score (β = 0.171, 95% CI: 0.018 ~ 0.398) and inversely associated with the FEV1% (β=-0.512, 95% CI: -0.899~-0.018) in AECOPD patients. To control for confounding factors, age, sex, BMI, comorbidities, and smoking status were adjusted. Multivariate linear regression analysis was carried out. The results revealed that there was a positive correlation between plasma 4-HNE and CCQ score (β = 0.146, 95% CI: 0.092 ~ 0.435), and inverse correlation of plasma 4-HNE with FEV1% (β=-0.478, 95% CI: -0.922~-0.026) in AECOPD patients.
Association of plasma 4-HNE with hospital stay in COPD patients
We further compared the levels of plasma 4-HNE in AECOPD patients with different durations of hospital stay. Plasma 4-HNE concentrations were significantly higher in patients with ≥ 13 hospital days compared with ≤ 8 and 8 ~ 13 days (Fig. 3). As shown in Table 4, logistic regression analysis confirmed the odds ratio (OR) of hospital day was evidently increased in cases with ≥ 13 hospital days (OR = 1.331, 95% CI: 1.078 ~ 1.732). After adjusting for age, sex, BMI, comorbidities, and smoking status, plasma 4-HNE levels were still positively correlated with the risk of ≥ 13 days of hospital stay (OR = 1.298, 95% CI: 1.047 ~ 1.712) in AECOPD patients.
The ability of plasma 4-HNE to predict prognosis in COPD patients
The predictive capacities of plasma 4-HNE and common biomarkers for prognosis were analyzed through the receiver operating characteristic (ROC) area under the curve (AUC). As shown in Fig. 4A, the AUCs of AECOPD were as follows: 4-HNE, 0.847 (95% CI: 0.780 ~ 0.914); PCT, 0.476 (95% CI: 0.385 ~ 0.567); neutrophil count, 0.722 (95% CI: 0.642 ~ 0.802); IL-6, 0.622 (95% CI: 0.533 ~ 0.710); and CRP, 0.670 (95% CI: 0.585 ~ 0.756). The optimal cutoff value of plasma 4-HNE for AECOPD patients was 1223.88 pg/mL, with a specificity of 82.5% and a sensitivity of 76.9%. Moreover, the predictive powers for longer hospital stays in AECOPD patients were as follows: 4-HNE, 0.814 (95% CI: 0.718 ~ 0.910); PCT, 0.484 (95% CI: 0.341 ~ 0.628); neutrophil count, 0.544 (95% CI: 0.414 ~ 0.674); IL-6, 0.566 (95% CI: 0.432 ~ 0.699); and CRP, 0.558 (95% CI: 0.420 ~ 0.697) (Fig. 4B). The optimal cutoff concentration of plasma 4-HNE was 2729.10 pg/mL. The specificity was 54.5%, and the sensitivity was 95.7%.
The predictive power for AECOPD and length of hospital stay. The predictive power for AECOPD and hospital stay duration was analyzed through receiver operating characteristic curves for different predictive biomarkers at admission. (A) The predictive power of plasma 4-HNE, IL-6, CRP, neutrophil count and PCT for AECOPD was analyzed. (B) The predictive power of plasma 4-HNE, IL-6, CRP, neutrophil count and PCT for hospital stay was analyzed.
DISCUSSION
The purpose of this study was to estimate the relationships between plasma 4-HNE and severity and prognosis in AECOPD patients via a prospective cohort study. The findings of this study were as follows:1 Plasma 4-HNE was increased in patients with AECOPD and SCOPD, particularly in patients with AECOPD2. Plasma 4-HNE gradually increased as pulmonary function decreased in AECOPD patients3. Plasma 4-HNE was inversely correlated with pulmonary function in AECOPD patients4. Increased plasma 4-HNE elevated the length of hospital stay in AECOPD patients during hospitalization5. Compared with several common COPD biomarkers, plasma 4-HNE levels were better at predicting AECOPD and prolonged hospital stays.
An earlier study revealed that 4-HNE can upregulate the expression of transcription factors, such as nuclear factor-κB (NF-κB), which in turn regulates genes involved in cell proliferation and differentiation37. Previous studies have indicated that excessive NF-κB activation is closely correlated with many diseases, such as rheumatoid arthritis, cardiovascular and nervous system diseases38,39,40. In addition, 4-HNE can promote inflammation by stimulating the production of several inflammatory cytokines41. During the last decade, experimental evidence has indicated that 4-HNE is not only the product of oxidative stress but also a cell signaling molecule42,43. Previous results indicated that 4-HNE is increased in models of lipopolysaccharide-induced acute lung injury, bleomycin-evoked pulmonary fibrosis, and monocrotaline-induced pulmonary arterial hypertension44,45,46. Moreover, it is widely known that oxidative stress plays central roles in the occurrence and development of COPD21. Therefore, we speculate that 4-HNE may be involved in the pathogenesis of AECOPD. This study suggest that COPD patients have higher levels of plasma 4-HNE than healthy volunteers do, especially those with acute exacerbations. Pearson or Spearman correlative analyses found that plasma 4-HNE expression was closely associated with various clinical characteristics among AECOPD patients. In addition, linear regression analysis found plasma 4-HNE expression was positively related to the severity of AECOPD patients. So, we speculate that 4-HNE may be involved in the pathophysiology of AECOPD.
As shown in our previous studies, inflammation and oxidative stress are related to pulmonary function decline in COPD patients29,47,48. Increasing amounts of data have revealed that increased levels of inflammatory cytokines elevate the occurrence and development of COPD49. Moreover, an increasing number of studies have shown that redox imbalance and lipid peroxidation can serve as prognostic biomarkers in many diseases. An earlier retrospective cohort study suggested that serum 8-isoprostane and 8-hydroxydeoxyguanosine can predict the severity and prognosis of community-acquired pneumonia patients19,50. Serum malondialdehyde is positively correlated with adverse outcomes in patients with chronic heart failure51. The level of glutathione can reflect drug resistance and adverse effects in patients with lung cancer52. Some studies have shown that oxidative stress is one of the important causes of airway constriction and COPD53. Oxidative stress is a significant precursor of increased ROS in the human body54. ROS are produced by oxidative stress. It is correlated with lipid peroxidation and an imbalance in the redox system14. In fact, 4-HNE is also a biomarker of oxidative stress and lipid peroxidation. Moreover, we consider it to be one of the most powerful reactive aldehydes24. Therefore, the association between plasma 4-HNE and prognosis was evaluated in AECOPD patients.We found that the levels of plasma 4-HNE at admission were elevated in AECOPD patients with longer hospital stays, and were positively associated with the length of hospital stay in AECOPD patients. Additionally, the predictive power of plasma for AECOPD and hospital stay duration was greater than that of common biomarkers. This study demonstrated that the plasma 4-HNE at admission can predict the length of hospital stay among AECOPD patients.
Several studies have shown that increased 4-HNE may promote the progression of kidney and colon cancer55,56. In addition, 4-HNE can form adducts with enzymes in the electron transport chain complex, which promotes tumor metastasis57. 4-HNE or 4-HNE-protein adducts are elevated in some organs and tissues of diabetic patients and animal models of diabetes58. A recent study revealed that 4-HNE protein adducts are always found in vital organs and are particularly associated with inflammation, edema and tissue destruction in dead COVID-19 patients59. Because 4-HNE is increased in several diseases and disease complications, 4-HNE has been used as a therapeutic target in many diseases. ALDH2 (aldehyde dehydrogenase 2), an inhibitor of 4-HNE and a small molecule activator named Alda-1, can promote the production of ALDH260. An earlier study showed that pretreatment of alveolar epithelial cells with Alda-1 can prevent pulmonary ischemia‒reperfusion injury by reducing the production of 4-HNE61. Another study revealed that ARPE-19 cells exposed to quercetin display increased resistance to 4-HNE-mediated damage62. These data provide evidence that 4-HNE may be used as a therapeutic target for COPD in the future.
Although this study enhances the understanding of the role of 4-HNE in COPD, there are several limitations in this study. First, this was a single-center study with a small sample size. All selected samples were from a certain hospital, which inevitably introduces certain biases. Therefore, a larger sample from multiple centers is needed in the future. Second, this was a correlation analysis based on the hospital population, and the mechanism of 4-HNE elevation in AECOPD patients was unclear. Only animal experiments and cell experiments may help resolve this puzzle. Finally, 4-HNE is only measured in plasma via ELISA, and the concentrations of 4-HNE in lung tissues and bronchoalveolar lavage fluid of COPD patients are unknown.
Conclusion
In summary, this study analyzed the associations of plasma 4-HNE with the severity and prognosis of AECOPD patients. Our results revealed that plasma 4-HNE is elevated in COPD patients, especially in AECOPD patients, compared with control subjects. The plasma 4-HNE level increases with elevating severity in AECOPD patients. Additionally, plasma 4-HNE is inversely associated with pulmonary function in AECOPD patients. Higher plasma 4-HNE levels on admission elevates the risks of prolonging hospital stay and AECOPD patients. As substantial evidence shows, 4-HNE may play an important role in the pathophysiological process of COPD. Plasma 4-HNE may be a viable biomarker for predicting the severity and prognosis of AECOPD patients. Moreover, 4-HNE may also be a therapeutic target for treating AECOPD in future clinical work.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ritchie, A. I. & Wedzicha, J. A. Definition, causes, pathogenesis, and consequences of chronic obstructive pulmonary disease exacerbations. Clin. Chest Med. 41, 421–438 (2020).
Quaderi, S. A. & Hurst, J. R. The unmet global burden of COPD. Glob Health Epidemiol. Genom. 3, e4 (2018).
Fang, L. et al. Chronic obstructive pulmonary disease in china: a nationwide prevalence study. Lancet Respir Med. 6, 421–430 (2018).
Wang, C. et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (The China pulmonary health [CPH] study): a National cross-sectional study. Lancet 391, 1706–1717 (2018).
Zhu, B., Wang, Y., Ming, J., Chen, W. & Zhang, L. Disease burden of COPD in china: a systematic review. Int. J. Chron. Obstruct Pulmon Dis. 13, 1353–1364 (2018).
Wedzicha, J. A., Brill, S. E., Allinson, J. P. & Donaldson, G. C. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 11, 181 (2013).
Mayhew, D. et al. Longitudinal profiling of the lung Microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax 73, 422–430 (2018).
Wedzicha, J. A., Singh, R. & Mackay, A. J. Acute COPD exacerbations. Clin. Chest Med. 35, 157–163 (2014).
Soler-Cataluña, J. J., Martínez-García, M. A., Sánchez, L. S., Tordera, M. P. & Sánchez, P. R. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med. 103, 692–699 (2009).
Lu, H. H., Zeng, H. H. & Chen, Y. Early chronic obstructive pulmonary disease: A new perspective. Chronic Dis. Transl Med. 7, 79–87 (2021).
Soulage, C. O. et al. Two toxic lipid Aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), accumulate in patients with chronic kidney disease. Toxins 12, 567 (2020).
Alary, J., Guéraud, F. & Cravedi, J. P. Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways. Mol. Aspects Med. 24, 177–187 (2003).
Guéraud, F. 4-Hydroxynonenal metabolites and adducts in pre-carcinogenic conditions and cancer. Free Radic Biol. Med. 111, 196–208 (2017).
Ayala, A., Muñoz, M. F. & Argüelles, S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 360438 (2014). (2014).
Schaur, R. J., Siems, W., Bresgen, N. & Eckl, P. M. 4-Hydroxy-nonenal-A bioactive lipid peroxidation product. Biomolecules 5, 2247–2337 (2015).
Di Domenico, F., Tramutola, A. & Butterfield, D. A. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic Biol. Med. 111, 253–261 (2017).
Singhal, S. S. et al. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 289, 361–370 (2015).
Guo, J., Wang, J., Guo, Y. & Feng, J. Association of aspirin resistance with 4-hydroxynonenal and its impact on recurrent cerebral infarction in patients with acute cerebral infarction. Brain Behav. 10, e01562 (2020).
Zheng, L. et al. Serum 8-iso-PGF2α predicts the severity and prognosis in patients with Community-Acquired pneumonia: A retrospective cohort study. Front. Med. 8, 633442 (2021).
Montuschi, P. et al. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am. J. Respir Crit. Care Med. 162, 1175–1177 (2000).
Kirkham, P. A. & Barnes, P. J. Oxidative stress in COPD. Chest 144, 266–273 (2013).
Yang, H., Lv, H., Li, H. & Ci, X. Peng. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell. Commun. Signal. 17, 62 (2019).
Tong, B. et al. Tauroursodeoxycholic acid alleviates pulmonary Endoplasmic reticulum stress and epithelial-mesenchymal transition in bleomycin-induced lung fibrosis. BMC Pulm Med. 21, 149 (2021).
Dalleau, S., Baradat, M., Guéraud, F. & Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell. Death Differ. 20, 1615–1630 (2013).
Li, C. et al. Recuperating lung Decoction attenuates inflammation and oxidation in cigarette smoke-induced COPD in rats via activation of ERK and Nrf2 pathways. Cell. Biochem. Funct. 35, 278–286 (2017).
Rahman, I. et al. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med. 166, 490–495 (2002).
Takimoto, T. et al. 4-Hydroxy-2-nonenal induces chronic obstructive pulmonary disease-like histopathologic changes in mice. Biochem. Biophys. Res. Commun. 420, 84–90 (2012).
Fei, J. et al. Low vitamin D status is associated with Epithelial-Mesenchymal transition in patients with chronic obstructive pulmonary disease. J. Immunol. 203, 1428–1435 (2019).
Huang, S. J., Ding, Z. N., Xiang, H. X., Fu, L. & Fei, J. Association between serum S100A8/S100A9 heterodimer and pulmonary function in patients with acute exacerbation of chronic obstructive pulmonary disease. Lung 198, 645–652 (2020).
Wang, Y. et al. Associations of the serum KL-6 with severity and prognosis in patients with acute exacerbation of chronic obstructive pulmonary disease. Lung 202, 245–255 (2024).
Zheng, L. et al. Circulatory cadmium positively correlates with epithelial-mesenchymal transition in patients with chronic obstructive pulmonary disease. Ecotoxicol. Environ. Saf. 215, 112164 (2021).
Sun, J. et al. Association of blood cadmium concentration with chronic obstructive pulmonary disease progression: a prospective cohort study. Respir Res. 25, 91 (2024).
Liu, H. Y. et al. The associations of serum S100A9 with the severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study. BMC Infect. Dis. 21, 327 (2021).
Fu, L. et al. Reactive oxygen species-evoked Endoplasmic reticulum stress mediates 1-nitropyrene-induced epithelial-mesenchymal transition and pulmonary fibrosis. Environ. Pollut. 283, 117134 (2021).
Singh, A., Gupta, M. K. & Mishra, S. P. Study of correlation of level of expression of Wnt signaling pathway inhibitors sclerostin and dickkopf-1 with disease activity and severity in rheumatoid arthritis patients. Drug Discov Ther. 13, 22–27 (2019).
Yamada, S. et al. Cytokine expression profiles in the Sera of cutaneous squamous cell carcinoma patients. Drug Discov Ther. 10, 172–176 (2016).
Shoeb, M., Ansari, N. H., Srivastava, S. K. & Ramana, K. V. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr. Med. Chem. 21, 230–237 (2014).
Mitchell, J. P. & Carmody, R. J. NF-κB and the transcriptional control of inflammation. Int. Rev. Cell. Mol. Biol. 335, 41–84 (2018).
El Assar, M., Angulo, J. & Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic Biol. Med. 65, 380–401 (2013).
Ju Hwang, C., Choi, D. Y., Park, M. H. & Hong, J. T. NF-κB as a key mediator of brain inflammation in alzheimer’s disease. CNS Neurol. Disord Drug Targets. 18, 3–10 (2019).
Dianzani, M. U. 4-hydroxynonenal from pathology to physiology. Mol. Aspects Med. 24, 263–272 (2003).
Yang, Y., Sharma, R., Sharma, A., Awasthi, S. & Awasthi, Y. C. Lipid peroxidation and cell cycle signaling:4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim. Pol. 50, 319–336 (2003).
Uchida, K. et al. Activation of stress signaling pathways by the end product of lipid peroxidation 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J. Biol. Chem. 274, 2234–2242 (1999).
Liu, P. et al. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 25, 10 (2020).
Peng, L. et al. Chelerythrine ameliorates pulmonary fibrosis via activating the Nrf2/ARE signaling pathway. Cell. Biochem. Biophys. 79, 337–347 (2021).
Xu, T. et al. Aldehyde dehydrogenase 2 protects against oxidative stress associated with pulmonary arterial hypertension. Redox Biol. 11, 286–296 (2017).
Xiang, Y. et al. Correlations among pulmonary DJ-1, VDR and Nrf-2 in patients with chronic obstructive pulmonary disease: A Case-control study. Int. J. Med. Sci. 18, 2449–2456 (2021).
Fu, L. et al. Low vitamin D status is associated with inflammation in patients with chronic obstructive pulmonary disease. J. Immunol. 206, 515–523 (2021).
Barnes, P. J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 138, 16–27 (2016).
Cao, L. F. et al. Serum 8-Hydroxydeoxyguanosine is a potential indicator for the severity and prognosis in patients with Community-Acquired pneumonia: A prospective cohort study. J. Immunol. 208, 321–327 (2022).
Romuk, E. et al. Malondialdehyde and Uric Acid as Predictors of Adverse Outcome in Patients with Chronic Heart Failure. Oxid Med Cell Longev 9246138 (2019). (2019).
Yang, P., Ebbert, J. O., Sun, Z. & Weinshilboum, R. M. Role of the glutathione metabolic pathway in lung cancer treatment and prognosis: a review. J. Clin. Oncol. 24, 1761–1769 (2006).
Låg, M., Øvrevik, J., Refsnes, M. & Holme, J. A. Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases. Respir Res. 21, 299 (2020).
Lee, S., Kim, S. M. & Lee, R. T. Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid. Redox Signal. 18, 1165–1207 (2013).
Lei, L., Zhang, J., Decker, E. A. & Zhang, G. Roles of lipid Peroxidation-Derived electrophiles in pathogenesis of colonic inflammation and colon cancer. Front. Cell. Dev. Biol. 9, 665591 (2021).
Segura-Aguilar, J. et al. The levels of Quinone reductases, superoxide dismutase and glutathione-related enzymatic activities in diethylstilbestrol-induced carcinogenesis in the kidney of male Syrian golden hamsters. Carcinogenesis 11, 1727–1732 (1990).
Zhong, H. & Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 4, 193–199 (2015).
Dham, D. et al. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: challenges and opportunities. Free Radic Res. 55, 547–561 (2021).
Zarkovic, N. et al. Post-mortem findings of inflammatory cells and the association of 4-Hydroxynonenal with systemic vascular and oxidative stress in lethal COVID-19. Cells 11, 444 (2022).
Perez-Miller, S. et al. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat. Struct. Mol. Biol. 17, 159–164 (2010).
Ding, J. et al. Alda-1 attenuates lung Ischemia-Reperfusion injury by reducing 4-Hydroxy-2-Nonenal in alveolar epithelial cells. Crit. Care Med. 44, e544–e552 (2016).
Hytti, M. et al. Quercetin alleviates 4-hydroxynonenal-induced cytotoxicity and inflammation in ARPE-19 cells. Exp. Eye Res. 132, 208–215 (2015).
Acknowledgements
We thank all patients and their families involved in this research.
Funding
This study was supported by the National Natural Science Foundation of China (81670060), National Natural Science Foundation Incubation Program of the Second Affiliated Hospital of Anhui Medical University (2020GQFY05) and Scientific Research of Health Commission in Anhui Province (AHWJ2021b091),Applied Medical Research Project of Hefei Municipal Health Commission (Hwk2023zd012), Scientific Research Projects of Higher Education Institutions in Anhui Province(2024AH050703), The Second People’s Hospital of Hefei City hospital-level topics (2024ykc010).
Author information
Authors and Affiliations
Contributions
Lin Fu conceived the study; Lin Fu designed the study; Lin Fu, Dong-Mei Su, Chen Zhang, Meng-Die Li, Peng Cao performed the research; Lin Fu conducted the statistical analyses of all the data. Chen Zhang and Xiaoqiong Wang drafted the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
The authors declare that they have no conflicts of interest.
Ethics declarations
This study was supported by the Ethics Committee of Anhui Medical University and met the principles expressed in the Declaration of Helsinki. All the COPD patients and controls agreed to participate in this study and signed the informed consent form by themselves or their authorized children.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
* These authors contributed equally to this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, C., Su, DM., Wang, XQ. et al. Plasma 4-hydroxynonenal estimates severity and prognosis in patients with acute exacerbation of chronic obstructive pulmonary disease. Sci Rep 16, 758 (2026). https://doi.org/10.1038/s41598-025-30368-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-30368-9