Abstract
To assess the incidence and severity of delirium and subsequent neurological impairment in pediatric intensive care unit (PICU) patients with meningoencephalitis (ME) and sepsis. Prospective study enrolling PICU patients with sepsis or meningoencephalitis (ME) and healthy controls. Daily delirium assessment, repeated electroencephalography, blood sampling at days 1, 3, 5 and lumbar puncture, if clinically indicated, for cerebrospinal fluid (CSF) sampling were performed. Biomarkers of inflammation (CRP, PCT, IL-6), endothelial activation (NT-proCNP), neurodegeneration (NSE, tau), neuroaxonal damage (NfL, NfH, UCHL-1) and glial injury (GFAP, S100B) were measured. Neurological status was assessed using the Functional Status Scale (FSS), the pediatric overall performance category (POPC) and the pediatric cerebral performance scale (PCPC) before admission and after three months. Twenty-four PICU patients (ME n = 9, sepsis n = 15) and eleven controls were enrolled. The delirium incidence was not statistically higher in patients with sepsis (60%, n = 9/15) compared to ME (33%, n = 3/9, p = 0.105). Children with delirium (n = 12) had a higher FSS (10.0 [9.8, 12.0] vs. 7.5 [7.0, 9.0], p = 0.009) at day 1 than children without delirium (n = 12), without significant differences in blood and CSF biomarker levels. Patients with ME presented higher levels of blood NfL compared to sepsis patients at day 3 (16.8 [12.9, 77.5] vs. 10.7 [8.6, 12.2] pg/ml, p = 0.011) and day 5 (16.2 [6.8, 170.4] vs. 12.0 [7.9, 14.3] pg/ml, p = 0.005). Routine EEG results did not differ between the groups, but quantitative EEG parameters representing encephalopathy correlated with biomarkers of brain injury. FSS, POPC and PCPC after three months were identical between in all patients. Independent from the origin of inflammatory stimulation by sepsis or ME delirium is associated with transient neurological dysfunction in PICU patients, without neurological impairment after three months, as confirmed by biomarker levels.
Similar content being viewed by others
Background
Delirium is one of the most frequent neuropsychiatric complications in critically ill patients and can be triggered by multiple causes, including infection, trauma and critical illness itself. 1 Typically, delirium is characterized by an acute and transient change in behavior, awareness and other cognitive abilities, but neurocognitive impairments may persist for months, increasing morbidity and mortality4,5. In daily clinical routine delirium is challenging due to its complex presentation. Particularly in special populations like critically ill pediatric patients, delirium is often underdiagnosed6. Therefore, validated biomarkers of brain injury might help to close the gap between patient-related diagnostic short-comings and the detection of neurocognitive impairment in a highly vulnerable patient cohort. 2,7 It has been estimated, that delirium affects between 25 and 60% of patients in the pediatric intensive care unit (PICU)10,11. Similar to adults symptoms can persist with varying duration, worsening patient outcome11. Beyond the acute phase of illness, delirium in pediatric patients has also been associated with impairments in post-discharge cognition and quality of life, indicating persistent central nervous system (CNS) injury12. However, data regarding the extent of severe neurological impairment and consecutive deleterious long-term outcomes in pediatric patients are limited13,14.
In this proof-of-concept study, we hypothesized that critically ill pediatric patients with delirium exhibit clinical and laboratory features of structural brain injury resulting in subsequent neurological impairment compared to critically ill children without delirium. Furthermore, we hypothesized that the source of infection, represented by either systemic infection (sepsis) with secondary involvement of the brain and primary infection (ME) is relevant for the development of delirium as well as for structural brain injury. Finally, we hypothesized that routine electroencephalography (EEG) improves the detection of encephalopathy in pediatric patients with delirium and EEG results correlate with brain injury biomarker levels.
Materials and methods
Study design, setting, ethical approval and trial registration
We conducted a prospective observational single center cohort study in pediatric patients treated at a university hospital. The study was approved by the local ethics committee of Rostock University (identifier A 2020 − 0160) and was prospectively registered (clinicaltrials.gov: NCT04467762, 08-JUL 2020). The STROBE guidelines apply. Furthermore, all diagnostic and therapeutic procedures were performed in accordance with pediatric clinical guidelines and the Declaration of Helsinki.
Inclusion and exclusion criteria
Pediatric patients between one day and 17 years of age with an acute severe infection (symptom duration below 24 h) admitted to the PICU at the University Medical Center Rostock were eligible. Pediatric sepsis definition based on the International pediatric sepsis consensus conference 2005 criteria used at the time of patient recruitment15. These criteria comprised at least two SIRS criteria plus suspicion or evidence of infection15. Children with clinical signs of ME, e.g. fever, altered level of consciousness, abnormal behaviour, headache, meningeal irritation, new focal neurologic deficits or seizures and with pleocytosis in the CSF (with or without microbiological detection of pathogens), were defined to have ME16.
Written informed consent was given by legal representatives before study inclusion. Exclusion criteria comprised preexisting CNS diseases (e.g. stroke, epilepsy or tumor), immunosuppression and participation in another study. Additionally, pediatric patients with hospital admission for minor elective surgery (e.g. material removal after osteosynthesis or surgery for hernia inguinalis) but without acute infection or above-mentioned exclusion criteria were recruited as healthy controls.
Study visits, sampling and data collection
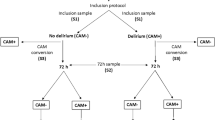
Study visits with neurological examinations and delirium assessment were performed daily within the first five days after study inclusion and at the time of hospital discharge by experienced study personnel (Fig. 1). Blood sampling and EEG recording were performed at study days 1 (day of enrolment), 3 and 5. Lumbar puncture and cerebrospinal fluid (CSF) sampling was performed once at study day 1, if clinically indicated. Three months after enrolment, patients received a detailed neurological follow-up examination or, if indisposed, standardized telephone interviews. Demographic and clinical parameters were collected using the in-house patient data management system. The Functional status scale (FSS), the Glasgow Coma Scale (GCS), the Richmond Agitation and Sedation Scale (RASS) and the pediatric Sequential Organ Failure Assessment (pSOFA) Score were documented at each study visit.
Healthy controls received the same clinical assessments like patients at day 1 during their preanesthetic visit (e.g. demographic data, neurofunctional tests, delirium screening tests, but no EEG). Blood sampling was performed in the operating room directly after placing the intravenous access before the induction of anesthesia.
Assessment of delirium and neurological outcome
Delirium was assessed using the pediatric Confusion Assessment Method (pCAM) for the ICU (for patients six years or older), the preschool (ps)CAM-ICU (between six months and five years) and the Sophia Observation Withdrawal Symptoms-scale for pediatric delirium (SOS-PD Scale) at all study visits once a day. Additionally, all patients including patients below six months of age were clinically assessed for signs of delirium by a neuropediatrician (AB). Furthermore, nursing reports were screened for signs of delirium outside the assessment procedures to reduce the risk of missed delirium events.
Three months after enrolment, patients underwent a comprehensive neurological examination including neurological status, POPC, PCPC, FSS and established developmental testing, which were compared to the pre-hospital developmental status17,18,19,20. For children aged 0 to 6 months, the Münchener Funktionelle Entwicklungsdiagnostik (MFED) was used, while children aged 6 months to 4 years were assessed with the Entwicklungstest für Kinder von 6 Monaten bis 6 Jahren (ET 6-6-R). For children older than 4 years, the Kaufman Assessment Battery for Children (KABC-II) was applied. For patients that were not available for in-house follow-up examination (due to restrictions in line with the COVID pandemia) a standardized telephone interview was performed. This consisted of detailed information provided by patients’ caregivers on the clinical and neurological development including potential residual symptoms as well as an assessment using the POPC, PCPC and FSS.
Electroencephalography
EEG was performed using the 10–20 system with bipolar montages and 20 min recording time to assess the severity of encephalopathy in the context of delirium (Neurofax EEG-1200, Nihon Kohden, Japan). If applicable, sedation was interrupted prior to recording. Normal EEGs were represented by physiological age-dependent background activity and the absence of pathological discharges. Abnormal EEGs were defined by either background activity deviating from the age norm and/or pathological discharges.
Further quantitative EEG (qEEG) processing was conducted using Brainwave (Version 0.9.165.57, freely available at http://home.kpn.nl/stam7883/brainwave.html). Analyses were stratified by age groups (1–11 months, 1–8 years, and 9–18 years) to account for developmental changes in qEEG features during brain maturation. Data was referenced to an average reference montage and power spectral analysis was performed using a Fast Fourier Transform. Power spectra and connectivity measures were calculated for delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz) and lower beta (13–20 Hz) bands. Band-pass filtering to lower beta frequencies was done to mitigate the impact of muscle activity21. The phase lag index (PLI) was used to calculate the functional connectivity strength22. The PLI is derived from the instantaneous phase differences obtained through the Hilbert transform, capturing the asymmetry between phase leading and lagging of two signals. PLI values range from 0 to 1, where 1 indicates perfect phase locking, and 0 signifies no phase synchronization or equal amounts of leading and lagging over the epoch. For each epoch, the PLI values across all channel pairs were averaged to compute a mean PLI for each frequency band per subject. Amplitude envelope correlation (AEC) was used to assess amplitude coupling between signals. This metric represents the Pearson correlation between the amplitude envelopes of the signals, which are derived via the Hilbert transform of the time series. In this study, we utilized the corrected version (AECc) to account for spatial leakage or volume conduction23. The correction for volume conduction is achieved by pairwise orthogonalization between signals X and Y (in both directions X to Y and Y to X) before calculation of the Pearson correlation coefficient, as described before24. The positive part of the correlation coefficient was used to estimate connectivity strength, which ranges between 0 and 1. 24
Body-fluid biomarkers
Eleven blood-based and five cerebrospinal fluid biomarkers were measured. Biomarkers of inflammation (C-reactive protein [CRP], Procalcitonin [PCT], Interleukin-6 [IL-6 ]), endothelial activation (N-Terminal pro C-type natriuretic neptide [NT-proCNP]), global neurodegeneration (Neuro-specific enolase [NSE], tau), neuroaxonal damage (Neurofilament light chains [NfL], Neurofilament heavy chains [NfH], Ubiquitin-Carboxyl-Terminal-Hydrolase [UCHL)-1]) and glial injury (glial fibrillary acidic protein [GFAP], S100 calcium-binding protein B (S100B)] were assessed.
Blood (plasma and serum) and CSF samples were centrifuged at 2,000 g (4 °C) for 15 min, aliquoted and stored at -80 °C until analysis. NfH (R-PLEX Human Neurofilament H Assay) was measured with electrochemiluminescence-based immunoassays (ECLIA) according to manufacturer’s recommendations using the MESO Quickplex SQ120 (Meso Scale Discovery, Rockville, MD, USA). GFAP, NfL, tau protein and UCHL-1 concentrations were measured using Single molecule array (Simoa, Neurology 4-Plex) assays on an HD-1 Analyzer (Quanterix, Billerica, MA, USA), as described before. 25 S100B (Cloud-Clone Corp., Houston, USA) and NT-proCNP (Biomedica Medizinprodukte GmbH, Vienna, Austria) were quantified using sandwich enzyme-linked immunosorbent assays (ELISA). NSE concentration was measured using ECLIA in the certified laboratory of the Rostock University Medical Center.
All biomarker samples were measured in duplicates. For samples with a coefficient of variation (CV) > 20%, we repeated the measurement. No results were excluded.
Statistical analysis
Data were analyzed using R studio built (R version 4.4.1, R Core Team 2013, Vienna, Austria). Categorical variables were compared with the Chi²-Test or the Fisher’s exact test, if the expected probability was < 5%. Continuous variables were compared by the Wilcoxon signed-rank test. Biomarker levels were logarithmically transformed and analyzed using linear mixed models (LMM). The LMM was fitted taking the group (delirium vs. no delirium, meningoencephalitis vs. sepsis, abnormal EEG vs. normal EEG), the time point (study visits 1, 3 and 5) and their interaction term (group x time point) as fixed effects and patient-specific intercepts as random. The interaction term was used to reveal differences in biomarker trajectories over time between the corresponding groups. The patient-specific intercept accounted for repeated measurements at the study visits 1, 3 and 5 within each patient. As bespoke biomarker levels can be confounded by age, we additionally performed a separate analysis including age as covariate into the LMM. Exploratory analyses were conducted to assess the correlation between qEEG parameters indicative of encephalopathic changes and the maximum or mean values of fluid biomarkers that showed alterations during treatment. Pearson’s correlation coefficient was used, with adjustments for multiple comparisons using the Benjamini-Hochberg false discovery rate procedure. A p < 0.05 (two sided) was considered statistically significant.
Results
Between July 2020 and December 2021, a total of 1044 patients were admitted to the PICU and were screened for participation in the study. Based on the in- and exclusion criteria 998 patients were not eligible for study participation. From 46 eligible patients, written informed consent from the legal representatives could not be achieved in 22 cases, resulting in 24 PICU patients and 11 healthy controls who were enrolled (Fig. 1; Table 1). Focused sub-group analyses have been performed between patients and healthy controls, PICU patients with delirium and without delirium, PICU patients with ME and sepsis as well as PICU patients with abnormal and normal EEG. All corresponding groups were comparable for age and sex. Patients with sepsis were significantly younger than patients with ME (17.0 [5.5, 55.0] months vs. 99.0 [64.0, 151.0] months, p = 0.032). Furthermore, septic patients (2.0 [1.0, 2.5] vs. 0.0 [0.0, 0.0], p = 0.001) and patients with delirium (1.5 [1.0, 2.0] vs. 0.0 [0.0, 1.2], p = 0.047) had higher pSOFA scores at day 1.
Continuous analgosedation was present in one patient with sepsis receiving invasive mechanical ventilation, with successful weaning and extubation nine hours after admission at day 1. Periprocedural short-term analgosedation was used for lumbar puncture at day 1 in two children with ME and four children with sepsis at day 1.
After three months, 58.3% (n = 14/24) of PICU patients were available for clinical in-house follow-up examination, 41.7% (n = 10/24) were evaluated via standardized telephone interviews. All patients survived until three months after study inclusion without neurological impairment (Table 2).
Patients versus controls
Biomarkers of systemic inflammation [white blood count (WBC), CRP and PCT, IL-6] were significantly higher in patients than controls (eTable 1). NSE was significantly lower in patients at day 1 (781.2 [529.2, 991.7] pg/ml) compared to controls (1760.4 [1216.5, 2550.7] pg/ml, p < 0.001). UCHL-1 was significantly higher in patients at day 1 (22.2 [14.9, 37.8] ng/ml) than in controls (14.2 [6.0, 20.7] ng/ml, p = 0.032). All other biomarker levels did not differ significantly between patients and controls (eTable 1).
PICU patients with and without delirium
Tau protein at day 3 (15.3 [10.4, 25.1] pg/ml vs. 10.4 [5.2, 13.4] pg/ml, p = 0.019), as well as UCHL-1 at day 3 (25.2 [20.2, 35.7] pg/ml vs. 11.2 [9.3, 21.3] pg/ml, p = 0.034) and day 5 (20.7 [16.5, 27.2] pg/ml vs. 9.1 [6.8, 17.0] pg/ml, p = 0.023) were significantly higher in patients with delirium compared to non-delirious children. After adjustment for age, these significant differences disappeared. All other blood-based biomarkers showed similar levels in both groups (eTable 2).
No statistically significant differences were observed regarding CSF biomarker comparisons (eTable 3).
Mixed linear effect models revealed no significant trajectories between biomarker levels at the different time points and group comparisons.
The global test for differences between the qEEG model and intercept-only model was significant (χ2(14) = 39.3, p < 0.01). Yet, no single qEEG parameter significantly differed between delirious and non-delirious patients.
Patients with ME and sepsis
Inflammatory biomarkers CRP and PCT were significantly elevated in patients with sepsis compared to patients with ME at nearly all study days (eTable 1).
NfL concentrations were significantly higher in patients with ME at day 3 (16.8 [12.9, 77.5] pg/ml vs. 10.7 [8.6, 12.2] pg/ml, p = 0.011) and day 5 (16.2 [6.8, 170.4] pg/ml vs. 12.0 [7.9, 14.3] pg/ml, p = 0.005) compared to patients with sepsis (Fig. 2). In contrast to sepsis patients, NfL levels increased also significantly over time in patients with ME, even after correction for age (Fig. 2). All other blood-based biomarkers had comparable levels between both groups (eTable 1).
In CSF samples (eTable 3), only tau protein levels were higher in patients with sepsis (401.9 [243.4, 1461.6] pg/ml vs. 127.1 [97.6, 169.8] pg/ml, p = 0.013), but missed statistical significance after correction for age (p = 0.202).
PICU patients with and without abnormal EEG
Blood and CSF biomarker levels were mostly similar between patients with normal (n = 16) and abnormal EEG (n = 8) (eTable 2, eTable 3). Only UCHL-1 at day 3 was higher in patients with abnormal EEG when corrected for age (25.8 [14.6, 34.9] ng/ml vs. 18.7 [10.6, 26.8] ng/ml, p = 0.026).
Mixed linear effect models revealed no significant trajectories between biomarker levels at the different time points and group comparisons.
Exploratory analyses of qEEG parameters associated with encephalopathy revealed significant correlations with fluid biomarkers across age groups. The analyses focused on NfL, Tau, and UCHL-1, as these biomarkers exhibited clear changes over time. A detailed summary of the results is presented in Table 3. UCHL-1 and Tau showed significant positive correlations with the power of slow-wave activity in the delta band, while negative correlations were observed with the power of fast activity. Consistently, peak frequency was lower in the 1–8-year age group for higher UCHL-1 values. Connectivity measures predominantly correlated with fluid biomarkers of neuroinflammation and neuroaxonal damage in children under 1 year of age. These correlations indicated a positive association with excessive slow-wave phase coupling and elevated amplitude envelope fluctuations across all frequencies, indicating brain dysfunction.
Functional and neurocognitive status before hospital admission and after three months
Patients with and without delirium, as well as with ME and sepsis were comparable regarding their functional and neurological (POPC, PCPC) status before hospital admission (Tables 1 and 2). Patients with delirium had a higher FSS (10.0 [9.8, 12.0] vs. 7.5 [7.0, 9.0], p = 0.009) and a significantly lower GCS at day 1 (13.5 [13.0, 14.0] vs. 15.0 [15.0, 15.0], p < 0.001). Similar results were observed by comparing patients with ME and sepsis, with the sepsis cohort presenting with a higher FSS (10.0 [8.5, 11.5] vs. 7.0 [7.0, 9.0], p = 0.019) and a lower GCS (14.0 [13.0, 15.0] vs. 15.0 [15.0, 15.0], p = 0.045) only at day 1. No differences were observed in patients with and without EEG abnormalities. No severe neurological complications were observed during the study, regardless of group classification. All groups were comparable in their functional (FSS) and neurological (GCS, RASS, PCPC, POPC) status after three months (Table 2).
Discussion
In the present study, delirium was not associated with increased morbidity or mortality among PICU patients with acute infectious diseases. The overall delirium prevalence was 50%, which is comparable to recent data10,11. Interestingly, these results contrast the delirium prevalence of only 4% in SARS-CoV-2-infected children, suggesting a stronger inflammatory stimulus by ME or sepsis compared to SARS-CoV-2. 25 Despite slight differences in functional and neurological measures within the acute phase, the outcome was comparable between children with and without delirium after three months. In accordance with the clinical follow-up, biomarker levels of brain injury in blood and CSF were elevated compared to healthy controls, but similar between patients with and without delirium. No clinically relevant correlations between blood biomarkers and outcome parameters were observed. Our data confirm that delirium is frequent in pediatric patients and reflects a transient neurological dysfunction that is rather unlikely to persist as subsequent neurological impairment.
Biomarker levels of neuronal injury (NSE) were elevated in children after cardiac surgery, but with only moderate correlation between biomarker concentrations and delirium scores26,27. On the other hand, similar concentrations of IL-6 in children with influenza and either delirium or febrile seizures have been reported, indicating a mismatch between biomarker levels and the clinical status28. In this context, no association of elevated CRP and PCT values and delirium in septic children was observed29. Furthermore, levels of NSE and S100B were similarly elevated in children with and without sepsis-associated encephalopathy and full neurological recovery after three months, indicating preserved CNS integrity at the cellular level30.
Neurofilament light chains were extensively evaluated over the last decades and correlate with delirium severity in adults. 2,7,31 However, in children NfL levels remain normal, even in the presence of neurological symptoms34. In contrast, we observed significantly elevated NfL blood levels in children with ME, but without acute or persistent neurological impairments, which may reflect the potential of the pediatric brain to compensate for CNS affection to a certain degree.
Normal and abnormal EEG findings from intermittent EEG recordings did not correlate with changes in serum and CSF biomarker levels. Thus, intermittent EEG seems unfavorable for outcome prediction here. Exploratory qEEG patterns in pediatric delirium differed significantly compared to adult data, possibly reflecting discrepancies between the phenotypical and neurophysiological characteristics of delirium35,36. Increased delta power emerges as the most prominent finding in qEEG analyses of delirium, showing significant positive correlations with UCHL-1 and Tau protein, which indicates that encephalopathy severity corresponds to the extent of neuronal injury and neuroinflammation. Additionally, UCHL-1 showed strong positive correlations with AECc in the delta band, supporting the concept of excessive network inhibition33. Decreased peak frequency was also significantly associated with elevated Tau protein levels, aligning with established patterns in encephalopathy38. In summary, our results support an advanced characterization of delirium endotypes (e.g. using qEEG) beyond investigations of the binary phenotype (i.e. being delirious or not)39.
Strengths and limitations
The broad panel of eleven blood-based and five CSF biomarkers allowed us to assess a widespread range of compartments, including neuronal, axonal, glial, vascular as well as to quantify the impact of systemic infection and primary CNS infection. Although the sample size was small, patients underwent repeated comprehensive assessments with neuropediatric examinations, EEG as well as blood and CSF sampling for biomarker analysis. This multimodal diagnostic approach at different time points allowed us to assess different qualities of neurological impairment and to quantify the extent of neurological injury at the cellular level. Unfortunately, we were not able to perform routine magnetic resonance imaging (MRI), which would have enabled us to assess brain injury at an imaging level. Only three PICU patients were examined by MRI without pathological results. Due to the COVID pandemia and legal restrictions of hospital visits it was impossible to assess all pediatric patients in our outpatient department. Instead of in-house examination, we performed standardized telephone interviews in these children, which allowed for reliable neurological outcome assessment, but only to a certain degree for an evaluation of neurodevelopmental delays. A larger sample size and comprehensive long-term neurocognitive testing should be part of future studies to verify the results of the present study. Furthermore, a control group without infection is warranted to assess the clinical utility of blood-based biomarker results over the time course of PICU patients.
Conclusions
Pediatric delirium is unlikely to result in sustained brain injury and subsequent neurological impairment, despite transient brain dysfunction and corresponding transient elevations of biomarkers in specific subgroups. ME as a primary CNS infection and sepsis with secondary brain involvement trigger delirium to a comparable extent. Future studies might also focus on even more severely ill children to validate our observations. Intermittent or continuous qEEG monitoring might represent a clinically useful instrument to assess delirium in the future.
Data availability
The data underlying this article are available in the article and in its online supplementary material. Request for additional information can be made to the corresponding author.
Abbreviations
- AEC:
-
amplitude envelope correlation
- AECc:
-
corrected amplitude envelope correlation
- CNS:
-
central nervous system
- CRP:
-
C-reactive protein
- CSF:
-
cerebrospinal fluid
- CV:
-
coefficient of variation
- ECLIA:
-
electrochemiluminescence-based immunoassays
- EEG:
-
electroencephalogramm
- ELISA:
-
enzyme-linked immunosorbent assays
- ET 6-6-R:
-
Entwicklungstest für Kinder von 6 Monaten bis 6 Jahren
- FSS:
-
Functional Status Scale
- GCS:
-
Glasgow Coma Scale
- GFAP:
-
glial fibrillary acidic protein
- IL-6:
-
Interleukin-6
- KABC-II:
-
Kaufman Assessment Battery for Children
- LMM:
-
linear mixed model
- ME:
-
meningoencephalitis
- MFED:
-
Münchener Funktionelle Entwicklungsdiagnostik
- MRI:
-
magnetic resonance imaging
- NfH:
-
neurofilament heavy chains
- NfL:
-
neurofilament light chains
- NSE:
-
neuro-specific enolase
- NT-proCNP:
-
N-Terminal pro C-type natriuretic peptide
- pCAM:
-
pediatric Confusion Assessment Method
- PCPC:
-
pediatric cerebral performance scale
- PCT:
-
Procalcitonin
- PICU:
-
pediatric intensive care unit
- PLI:
-
phase lag index
- POPC:
-
pediatric overall performance category
- psCAM-ICU:
-
preschool CAM-ICU
- pSOFA:
-
pediatric Sequential Organ Failure Assessment
- qEEG:
-
quantitative electroencephalogramm
- RASS:
-
Richmond Agitation and Sedation Scale
- S100B:
-
S100 calcium-binding protein B
- SOS-PD:
-
Sophia Observation Withdrawal Symptoms-scale for pediatric delirium
- UCHL-1:
-
Ubiquitin carboxy-terminal hydrolase L1
References
Petzold, A., Downie, P. & Smith, M. Critical illness brain syndrome (CIBS): an underestimated entity? Crit. Care Med. 33 (6), 1464. https://doi.org/10.1097/01.ccm.0000166866.47020.e5 (2005).
Ehler, J., Petzold, A., Sharshar, T., Ely, E. W. & Saller, T. Biomarker panel to differentiate brain injury from brain dysfunction in patients with Sepsis-Associated encephalopathy. Crit. Care Med. 48 (5), e436–e7. https://doi.org/10.1097/CCM.0000000000004266 (2020).
Stollings, J. L. et al. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 47 (10), 1089–1103. https://doi.org/10.1007/s00134-021-06503-1 (2021).
Wilson, J. E. et al. Delirium Nat. Rev. Dis. Primers ;6(1):90. (2020).
Hshieh, T. T. et al. Trajectory of functional recovery after postoperative delirium in elective surgery. Ann. Surg. 265 (4), 647–653. https://doi.org/10.1097/SLA.0000000000001952 (2017).
Flaigle, M. C., Ascenzi, J. & Kudchadkar, S. R. Identifying barriers to delirium screening and prevention in the pediatric ICU: evaluation of PICU staff knowledge. J. Pediatr. Nurs. 31 (1), 81–84. https://doi.org/10.1016/j.pedn.2015.07.009 (2016).
Bircak-Kuchtova, B., Chung, H. Y., Wickel, J., Ehler, J. & Geis, C. Neurofilament light chains to assess sepsis-associated encephalopathy: are we on the track toward clinical implementation? Crit. Care. 27 (1), 214. https://doi.org/10.1186/s13054-023-04497-4 (2023).
Shahim, P. et al. Cerebrospinal fluid brain injury biomarkers in children: a multicenter study. Pediatr Neurol. 49 (1), 31 – 9 e2. (https://doi.org/10.1016/j.pediatrneurol.2013.02.015 2013).
Shahim, P., Mansson, J. E., Darin, N., Zetterberg, H. & Mattsson, N. Cerebrospinal fluid biomarkers in neurological diseases in children. Eur. J. Paediatr. Neurol. 17 (1), 7–13. https://doi.org/10.1016/j.ejpn.2012.09.005 (2013).
Siegel, E. J. & Traube, C. Pediatric delirium: epidemiology and outcomes. Curr. Opin. Pediatr. 32 (6), 743–749. https://doi.org/10.1097/MOP.0000000000000960 (2020).
Traube, C. All delirium May not be created equal: consideration of differential effects of delirium based upon underlying etiology. Pediatr. Crit. Care Med. 19 (10), 1009–1010. https://doi.org/10.1097/PCC.0000000000001704 (2018).
Silver, G. et al. Association between pediatric delirium and quality of life after discharge. Crit. Care Med. 48 (12), 1829–1834. https://doi.org/10.1097/CCM.0000000000004661 (2020).
Meyburg, J. et al. Risk factors for the development of postoperative delirium in pediatric intensive care patients. Pediatr. Crit. Care Med. 19 (10), e514–e21. https://doi.org/10.1097/PCC.0000000000001681 (2018).
Meyburg, J. et al. Cognitive and behavioral consequences of pediatric delirium: A pilot study. Pediatr. Crit. Care Med. 19 (10), e531–e7. https://doi.org/10.1097/PCC.0000000000001686 (2018).
Goldstein, B., Giroir, B., Randolph, A. & International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 6 (1), 2–8. https://doi.org/10.1097/01.PCC.0000149131.72248.E6 (2005).
Deutsche Gesellschaft für Pädiatrische Infektiologie DGPI. DGPI Handbuch Infektionen bei Kindern und Jugendlichen, 7. Auflage. 154–168 (Thieme, 2018).
Hellbrugge, T. Münchener Funktionelle Entwicklungsdiagnostik: Zweites Und Drittes Lebensjahr (4., korr. u.erw. Aufl) (Munchen:Dienstleistungsgesellschaft fur gemeinnutzige Institution, 1994).
Hellbrugge, T., Lajosi, F., Menara, D., Schamberger, R. & Rautenstrauch, T. Münchener Funktionelle Entwicklungsdiagnostik (erstes Lebensjahr) (6., unverand. Aufl) (Hansisches Verlagskontor, 2001).
Macha, T. The ET 6–6: a method for developmental assessment for German-speaking countries. Z. für Psychologie/Journal Psychol. 216 (3), 154–160 (2008).
Lichtenberger, E. O., Sotelo-Dynega, M. & Kaufman, A. S. The Kaufman Assessment Battery for Children – Second Edition. In: Naglieri JA, Goldstein S, editors. Practitioner’s guide to assessing intelligence and achievement. New Jersey, USA; John Wiley & Sons; (2009).
Whitham, E. M. et al. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 hz are contaminated by EMG. Clin. Neurophysiol. 118 (8), 1877–1888. https://doi.org/10.1016/j.clinph.2007.04.027 (2007).
Stam, C. J., Nolte, G. & Daffertshofer, A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 28 (11), 1178–1193. https://doi.org/10.1002/hbm.20346 (2007).
Hipp, J. F., Hawellek, D. J., Corbetta, M., Siegel, M. & Engel, A. K. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci. 15 (6), 884–890. https://doi.org/10.1038/nn.3101 (2012).
Fleischmann, R. et al. Amplitude coupling is altered in delirium of various etiologies: results from a retrospective multi-center case-control EEG study. Clin. Neurophysiol. 2025 May :173,132–137. https://doi.org/10.1016/j.clinph.2025.02.266
Ehler, J. et al. No substantial neurocognitive impact of COVID-19 across ages and disease severity: a multicenter biomarker study of SARS-CoV-2 positive and negative adult and pediatric patients with acute respiratory tract infections. Infect. 2024 Oct. 1. https://doi.org/10.1007/s15010-024-02406-7
Kuhn, J. E. et al. Utility of brain injury biomarkers in children with congenital heart disease undergoing cardiac surgery. Pediatr. Neurol. 148, 44–53. https://doi.org/10.1016/j.pediatrneurol.2023.06.024 (2023).
Matsuishi, Y. et al. Pediatric delirium is associated with increased brain injury marker levels in cardiac surgery patients. Sci. Rep. 12 (1), 18681. https://doi.org/10.1038/s41598-022-22702-2 (2022).
Fukumoto, Y. et al. Serum levels of cytokines and EEG findings in children with influenza associated with mild neurological complications. Brain Dev. 29 (7), 425–430. https://doi.org/10.1016/j.braindev.2006.12.005 (2007).
Dechnik, A., Mauer, E. A., Gerber, L. M. & Traube, C. C-Reactive protein and procalcitonin levels May not predict delirium in critically ill children. Pediatr. Crit. Care Med. 21 (11), e967–e71. https://doi.org/10.1097/PCC.0000000000002412 (2020).
de Araujo, B. E. S. et al. Clinical features, electroencephalogram, and biomarkers in pediatric sepsis-associated encephalopathy. Sci. Rep. 12 (1), 10673. https://doi.org/10.1038/s41598-022-14853-z (2022).
Khalil, M. et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 14 (10), 577–589. https://doi.org/10.1038/s41582-018-0058-z (2018).
Ehler, J. et al. The prognostic value of neurofilament levels in patients with sepsis-associated encephalopathy - A prospective, pilot observational study. PLoS One. 14 (1), e0211184. https://doi.org/10.1371/journal.pone.0211184 (2019).
Krogseth, M. et al. Delirium, neurofilament light chain, and progressive cognitive impairment: analysis of a prospective Norwegian population-based cohort. Lancet Healthy Longev. 4 (8), e399–e408. https://doi.org/10.1016/S2666-7568(23)00098-3 (2023).
Geis, T. et al. Serum neurofilament light chain (sNfL) values in a large cross-sectional population of children with asymptomatic to moderate COVID-19. J. Neurol. 268 (11), 3969–3974. https://doi.org/10.1007/s00415-021-10554-1 (2021).
Boord, M. S. et al. Investigating how electroencephalogram measures associate with delirium: A systematic review. Clin. Neurophysiol. 132 (1), 246–257. https://doi.org/10.1016/j.clinph.2020.09.009 (2021).
Slooter, A. J. C. et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten societies. Intensive Care Med. 46 (5), 1020–1022. https://doi.org/10.1007/s00134-019-05907-4 (2020).
Fleischmann, R. et al. Delirium is associated with frequency band specific dysconnectivity in intrinsic connectivity networks: preliminary evidence from a large retrospective pilot case-control study. Pilot Feasibility Stud. 5, 2. https://doi.org/10.1186/s40814-018-0388-z (2019).
van Dellen, E. et al. Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology 121 (2), 328–335. https://doi.org/10.1097/ALN.0000000000000329 (2014).
Bowman, E. M. L. et al. Advancing specificity in delirium: the delirium subtyping initiative. Alzheimers Dement. 20 (1), 183–194. https://doi.org/10.1002/alz.13419 (2024).
Acknowledgements
The authors gratefully thank Anja Rahn and Susanne Wettengel for their support with the handling of blood and CSF. Furthermore, the authors thank Regina Seiler for assistance with EEG recordings and Maximilian Rühlmann for preparation of figure 2.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JE, JH and AB conceptualized the study and performed the clinical and electrophysiological investigations. Laboratory assessments and biomarker measurements were made by LD, RB, HZ and AH. AP, RF, EW and HS assisted in the data interpretation. FK performed the statistical analysis and wrote the initial manuscript. HPS assisted in the statistical analysis and data interpretation. DCF, CS, GK and AKG reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
JH, LD, AP, DCF, FK, RB, HABS, EWE, RF and CS declare that they have no conflicts of interest. AB reports grants from UCB Pharma GmbH and honoraria for speaking engagements from Biogen GmbH, Desitin Arzneimittel GmbH, Eisai GmbH, GW Pharma GmbH, Jazz Pharmaceuticals, Neuraxpharm GmbH, Shire/Takeda GmbH, UCB Pharma GmbH, and ViroPharma GmbH. AH has served as a consultant for Quanterix. GK reports travel grants and honoraria for speaking engagements from Cytosorb Europe GmbH, Artcline GmbH, serves as President for Scientific Working Group Albunet and extended board member of the European Society for Artificial Organs. HPS reports KOLFF grant from the Dutch Kidney Foundation (grant no 19OK009). HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Enigma, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). HZ is a Wallenberg Scholar and a Distinguished Professor at the Swedish Research Council. JE received travel support for attending study meetings by B. Braun Melsungen AG and Novartis, has leadership as second spokesperson of the Scientific Working Group on Neuroanesthesia (WAKNA) of the German Society for Anaesthesiology and Intensive Care Medicine (DGAI) and is Research Committee Member of the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).
Ethics approval and consent to participate
Approved by the local ethics committee of Rostock University (identifier A 2020 − 0160). Written informed consent was given by legal representatives before study inclusion.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klawitter, F., Hackenberg, J., Danckert, L. et al. Impact of meningoencephalitis and sepsis on delirium and subsequent neurological impairment in pediatric patients: a prospective proof-of-concept biomarker and EEG study. Sci Rep 15, 43492 (2025). https://doi.org/10.1038/s41598-025-31058-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-31058-2